Radiomodulating Properties of Superparamagnetic Iron Oxide Nanoparticle (SPION) Agent Ferumoxytol on Human Monocytes: Implications for MRI-Guided Liver Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cell Proliferation Assay
2.3. Annexin V/PI Apoptosis Assay
2.4. Flow Cytometry
2.5. Cytokine Secretion
2.6. Experimental Design
2.7. Statistical Analysis
3. Results
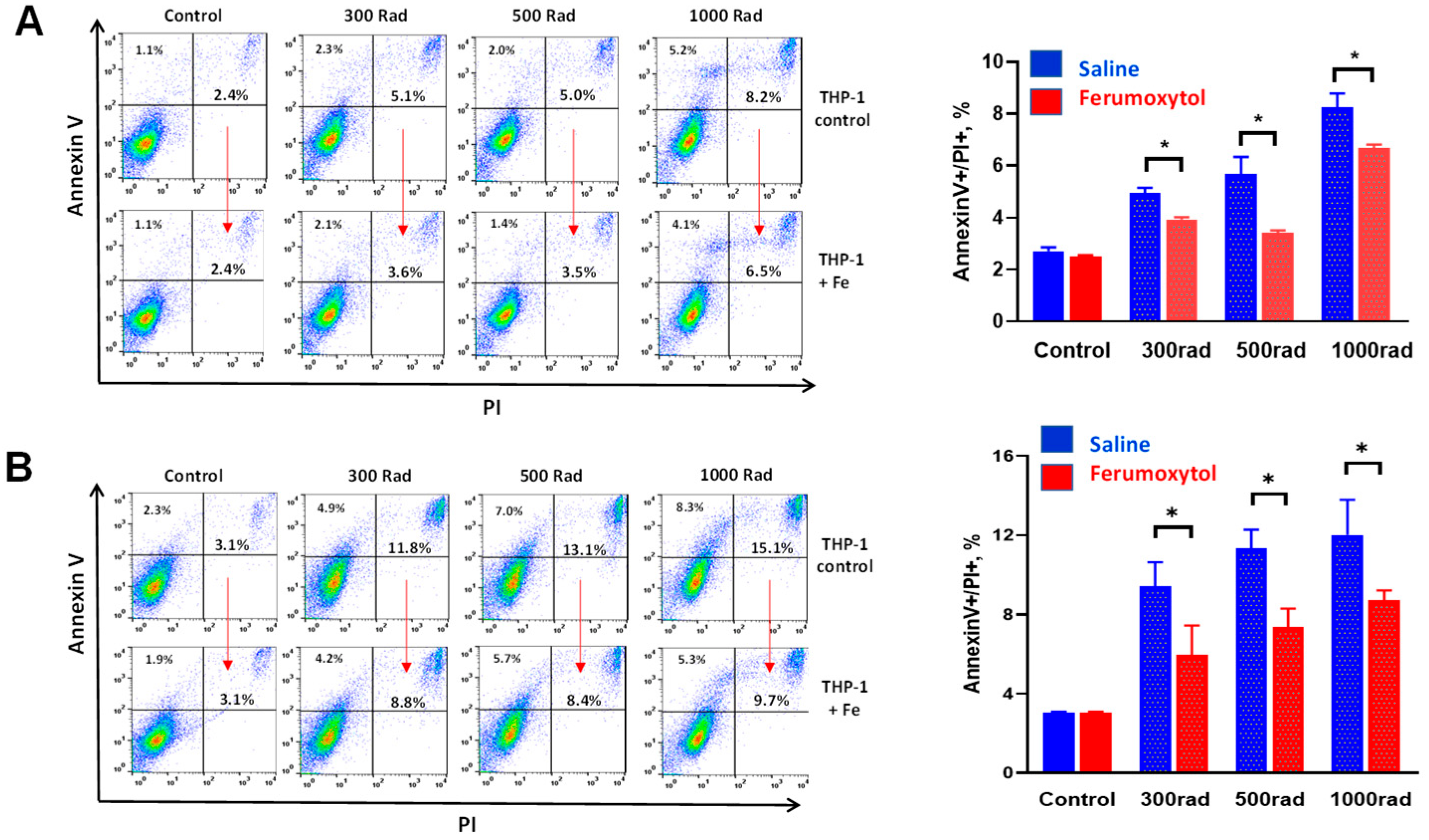
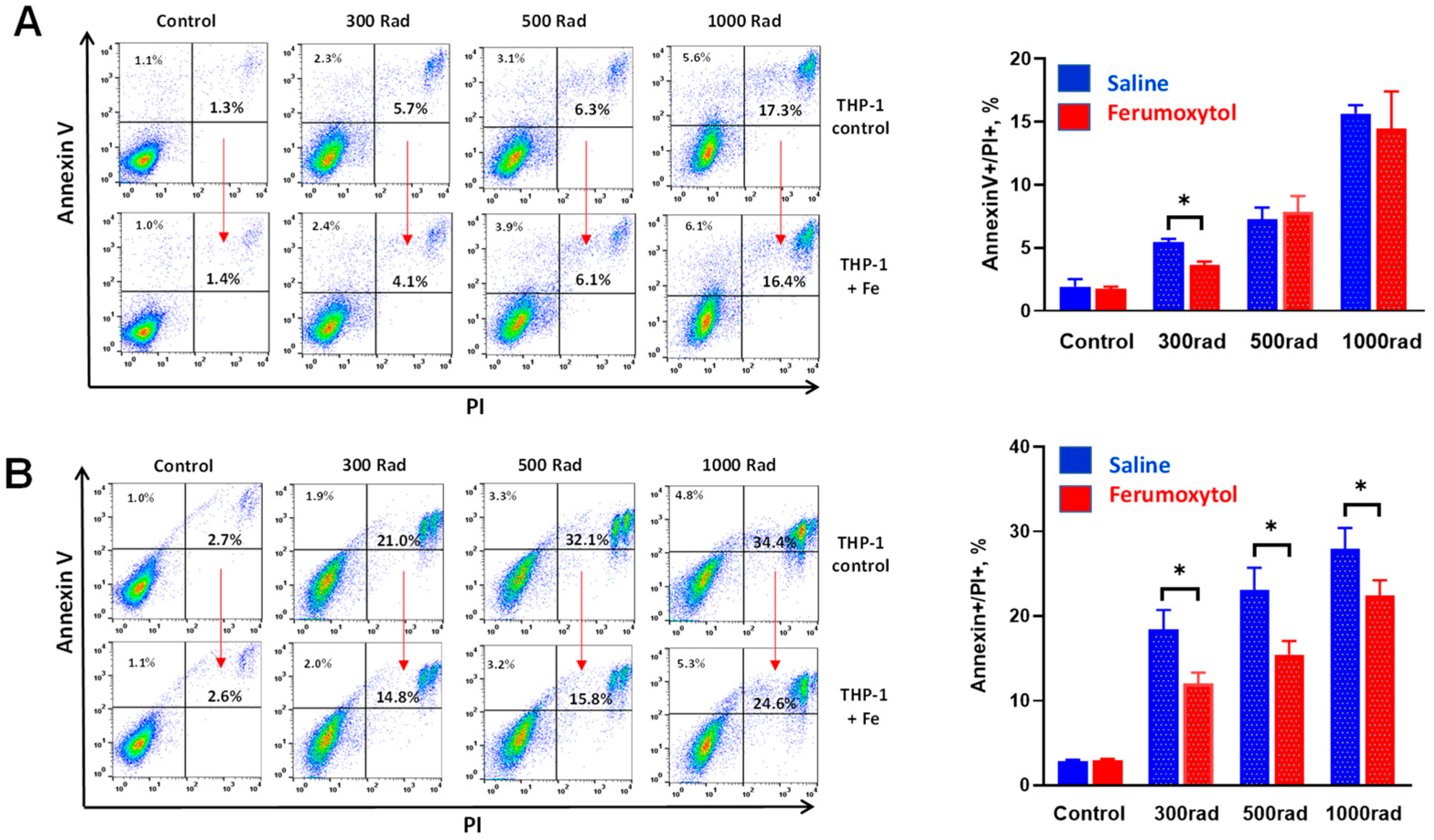
3.1. Ferumoxytol Decreases Radiation-Induced Cell Death of Human Monocytes In Vitro
3.2. Ferumoxytol Prevents Radiation-Induced Inhibition of Monocyte Proliferative Activity In Vitro
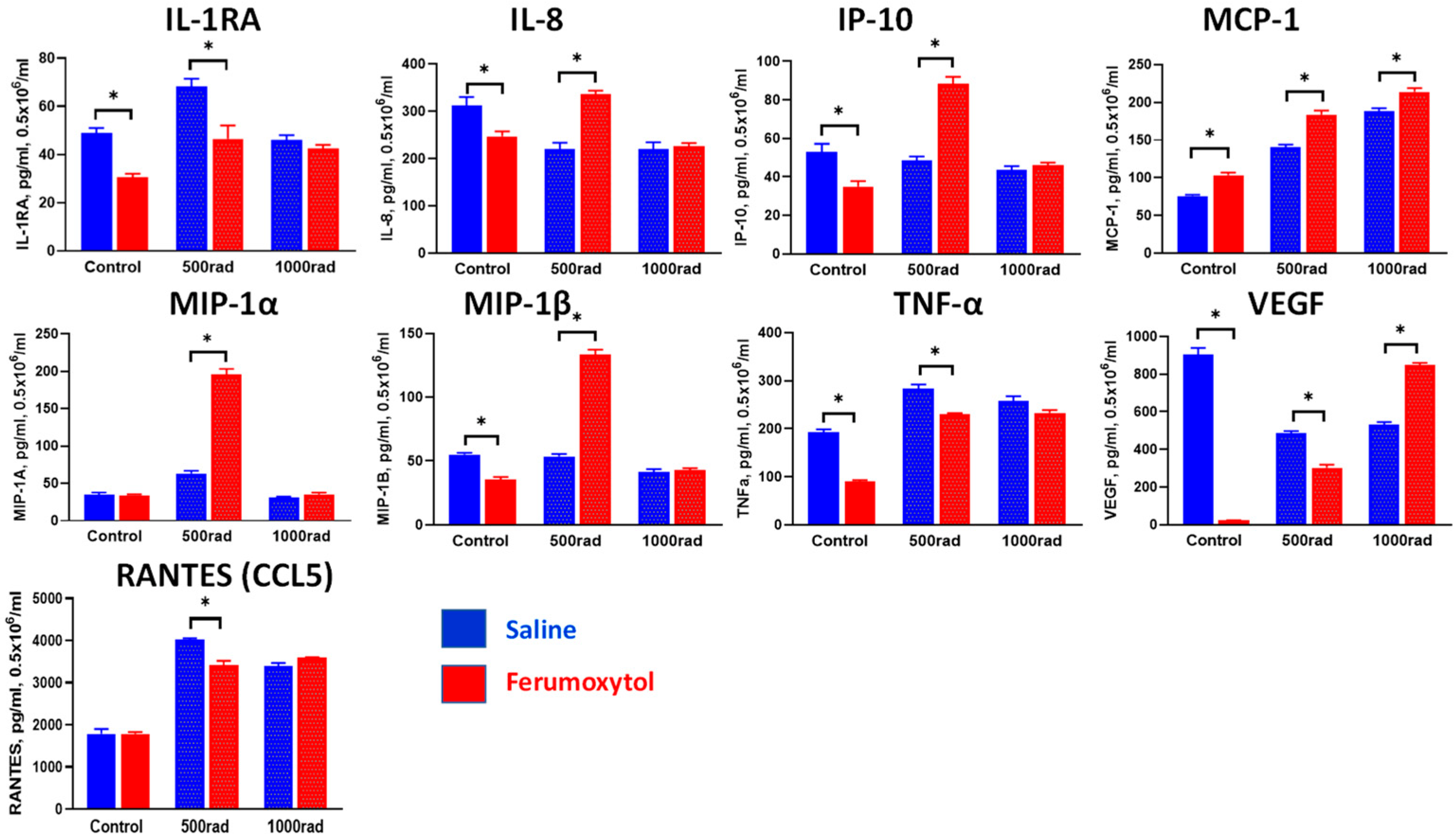
3.3. Modulation of Cytokine Production in Monocytes and Macrophages by SPION and Irradiation
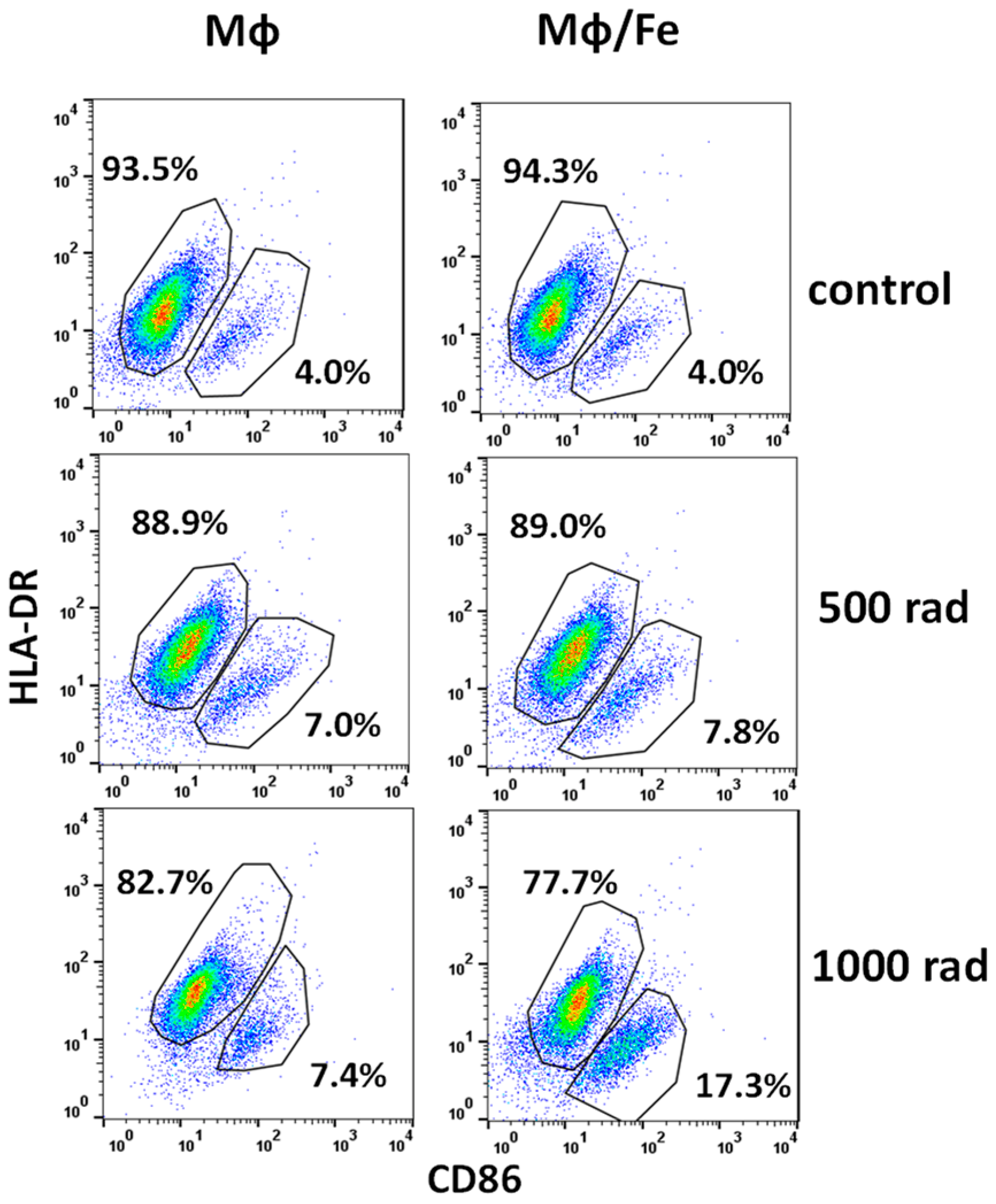
3.4. Ferumoxytol Changes the Phenotype of Monocytes/Macrophages Altered by Radiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Landaverde, C.; Jennings, L.; Goldstein, R.M.; Davis, G.L. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin. Gastroenterol. Hepatol. 2011, 9, 700–704.e1. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Ikai, I.; Matsuyama, Y.; Yamaoka, Y.; Makuuchi, M. Staging of hepatocellular carcinoma: Assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann. Surg. 2007, 245, 909–922. [Google Scholar] [CrossRef]
- Makuuchi, M.; Sano, K. The surgical approach to HCC: Our progress and results in Japan. Liver Transplant. 2004, 10, S46–S52. [Google Scholar] [CrossRef]
- Fortune, B.E.; Umman, V.; Gilliland, T.; Emre, S. Liver transplantation for hepatocellular carcinoma: A surgical perspective. J. Clin. Gastroenterol. 2013, 47, S37–S42. [Google Scholar] [CrossRef]
- Yao, F.Y.; Bass, N.M.; Nikolai, B.; Davern, T.J.; Kerlan, R.; Wu, V.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transplant. 2002, 8, 873–883. [Google Scholar] [CrossRef]
- Maddala, Y.K.; Stadheim, L.; Andrews, J.C.; Burgart, L.J.; Rosen, C.B.; Kremers, W.K.; Gores, G. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: Outcome with chemoembolization. Liver Transplant. 2004, 10, 449–455. [Google Scholar] [CrossRef]
- Decaens, T.; Roudot-Thoraval, F.; Hadni-Bresson, S.; Meyer, C.; Gugenheim, J.; Durand, F.; Bernard, P.H.; Boillot, O.; Sulpice, L.; Calmus, Y.; et al. Impact of UCSF criteria according to pre- and post-OLT tumor features: Analysis of 479 patients listed for HCC with a short waiting time. Liver Transplant. 2006, 12, 1761–1769. [Google Scholar] [CrossRef]
- Rusthoven, K.E.; Kavanagh, B.D.; Cardenes, H.; Stieber, V.W.; Burri, S.H.; Feigenberg, S.J.; Chidel, M.A.; Pugh, T.J.; Franklin, W.; Kane, M.; et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 2009, 27, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.H.; Dawson, L.A.; Mesci, A. Do We Have a Winner? Advocating for SBRT in HCC Management. Clin. Transl. Radiat. Oncol. 2024, 45, 100740. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, A.; Gayou, O.; Parda, D.; Kudithipudi, V.; Tom, K.; Khan, A.; Abrams, P.; Szramowski, M.; Oliva, J.; Monga, D.; et al. Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB 2016, 18, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Cardenes, H.R.; Price, T.R.; Perkins, S.M.; Maluccio, M.; Kwo, P.; Breen, T.E.; Henderson, M.A.; Schefter, T.E.; Tudor, K.; Deluca, J.; et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin. Transl. Oncol. 2010, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Yagi, H.; Uemura, M.T.; Hatanaka, Y.; Yamakado, H.; Takahashi, R. Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 2018, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Gayou, O.; Day, E.; Mohammadi, S.; Kirichenko, A. A method for registration of single photon emission computed tomography (SPECT) and computed tomography (CT) images for liver stereotactic radiotherapy (SRT). Med. Phys. 2012, 39, 7398–7401. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Chen-Zhao, X.; Hernando, O.; Flamarique, S.; Fernández-Letón, P.; Campo, M.; López, M.; Zucca, D.; Martínez, D.; Sánchez-Saugar, E.; et al. SBRT-SG-01: Final results of a prospective multicenter study on stereotactic body radiotherapy for liver metastases. Clin. Transl. Oncol. 2024; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Du, Y.; Tang, P.; Ma, G.; Jia, Z.; Zhou, X.; Mu, D.; Shen, Y.; Lu, Y.; Mao, Y.; et al. Unveiling the next generation of MRI contrast agents: Current insights and perspectives on ferumoxytol-enhanced MRI. Natl. Sci. Rev. 2024, 2024, nwae057. [Google Scholar] [CrossRef]
- Bae, S.H.; Chun, S.-J.; Chung, J.-H.; Kim, E.; Kang, J.-K.; Jang, W.I.; Moon, J.E.; Roquette, I.; Mirabel, X.; Kimura, T.; et al. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Meta-Analysis and International Stereotactic Radiosurgery Society Practice Guidelines. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 337–351. [Google Scholar] [CrossRef]
- Smith, L.; Byrne, H.L.; Waddington, D.; Kuncic, Z. Nanoparticles for MRI-guided radiation therapy: A review. Cancer Nano 2022, 13, 38. [Google Scholar] [CrossRef]
- Long, M.; Li, Y.; He, H.; Gu, N. The Story of Ferumoxytol: Synthesis Production, Current Clinical Applications, and Therapeutic Potential. Adv. Healthc. Mater. 2023, 13, e2302773. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kozaka, K.; Kobayashi, S.; Yoneda, N.; Yoshida, K.; Inoue, D.; Kitao, A.; Ogi, T.; Minami, T.; Kouda, W.; et al. Pathology and images of radiation-induced hepatitis: A review article. Jpn. J. Radiol. 2018, 36, 241–256. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Liang, S.; Tan, C.H.; Luo, B.; Xu, X.; Saw, P.E. Differently Charged Super-Paramagnetic Iron Oxide Nanoparticles Preferentially Induced M1-Like Phenotype of Macrophages. Front. Bioeng. Biotechnol. 2020, 8, 537. [Google Scholar] [CrossRef]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef]
- Sharkey, J.; Starkey Lewis, P.J.; Barrow, M.; Alwahsh, S.M.; Noble, J.; Livingstone, E.; Lennen, R.J.; Jansen, M.A.; Carrion, J.G.; Liptrott, N.; et al. Functionalized superparamagnetic iron oxide nanoparticles provide highly efficient iron-labeling in macrophages for magnetic resonance-based detection in vivo. Cytotherapy 2017, 19, 555–569. [Google Scholar] [CrossRef]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutiérrez, L.; Pérez-Yagüe, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1127–1138. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Liu, X.; Pan, Y.; Sun, Z.; Xue, Y.; Wang, T.; Dou, H.; Hou, Y. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int. J. Nanomed. 2019, 14, 6779–6797. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Corot, C.; Robert, P.; Idée, J.-M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Xue, Y.; Wang, G.; Cheng, Y.; Pan, Y.; Zhao, S.; Hou, Y. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics 2018, 8, 6307–6321. [Google Scholar] [CrossRef] [PubMed]
- Ariza de Schellenberger, A.; Poller, W.C.; Stangl, V.; Landmesser, U.; Schellenberger, E. Macrophage uptake switches on OCT contrast of superparamagnetic nanoparticles for imaging of atherosclerotic plaques. Int. J. Nanomed. 2018, 13, 7905–7913. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Serkova, N.J.; Groman, E.V.; Scheinman, R.I.; Simberg, D. Feraheme (Ferumoxytol) Is Recognized by Proinflammatory and Anti-inflammatory Macrophages via Scavenger Receptor Type AI/II. Mol. Pharm. 2019, 16, 4274–4281. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, M.; Nangaku, M. Ferumoxytol: An emerging therapeutic for iron deficiency anemia. Expert Opin. Pharmacother. 2023, 24, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, X.; Li, X.; Zhang, H.; Wu, M.; Liu, J.; Palmen, M.; Roubert, B.; Li, C. Pharmacokinetic, Pharmacodynamic, and Safety Profiles of Ferric Carboxymaltose in Chinese Patients with Iron-deficiency Anemia. Clin. Ther. 2020, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.R.; Stirrat, C.; Richards, J.; Mirsadraee, S.; Semple, S.I.; Tse, G.; Henriksen, P.; Newby, D.E. Vascular and plaque imaging with ultrasmall superparamagnetic particles of iron oxide. J. Cardiovasc. Magn. Reson. 2015, 17, 83. [Google Scholar] [CrossRef]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef]
- Mikhalkevich, N.; O’Carroll, I.P.; Tkavc, R.; Lund, K.; Sukumar, G.; Dalgard, C.L.; Johnson, K.R.; Li, W.; Wang, T.; Nath, A.; et al. Response of human macrophages to gamma radiation is mediated via expression of endogenous retroviruses. PLoS Pathog. 2021, 17, e1009305. [Google Scholar] [CrossRef]
- Carney, B.W.; Gholami, S.; Fananapazir, G.; Sekhon, S.; Lamba, R.; Loehfelm, T.W.; Wilson, M.D.; Corwin, M.T. Utility of combined gadoxetic acid and ferumoxytol-enhanced liver MRI for preoperative detection of colorectal cancer liver metastases: A pilot study. Acta Radiol. 2023, 64, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Tonan, T.; Fujimoto, K.; Qayyum, A.; Morita, Y.; Nakashima, O.; Ono, N.; Kawahara, A.; Kage, M.; Hayabuchi, N.; Ueno, T. CD14 expression and Kupffer cell dysfunction in non-alcoholic steatohepatitis: Superparamagnetic iron oxide-magnetic resonance image and pathologic correlation. J. Gastroenterol. Hepatol. 2012, 27, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Maurea, S.; Mainenti, P.P.; Tambasco, A.; Imbriaco, M.; Mollica, C.; Laccetti, E.; Camera, L.; Liuzzi, R.; Salvatore, M. Diagnostic accuracy of MR imaging to identify and characterize focal liver lesions: Comparison between gadolinium and superparamagnetic iron oxide contrast media. Quant. Imaging Med. Surg. 2014, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.J. Nanoparticle-Based Magnetic Resonance Imaging on Tumor-Associated Macrophages and Inflammation. Front. Immunol. 2017, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.H.; Kandala, S.K.; Yang, C.T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci. Adv. 2020, 6, eaay1601. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, Y.; Liu, X.; Sun, Z.; Pan, Y.; Lu, X.; Liang, H.; Dou, H.; Hou, Y. Ferumoxytol Attenuates the Function of MDSCs to Ameliorate LPS-Induced Immunosuppression in Sepsis. Nanoscale Res. Lett. 2019, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Beach, C.; MacLean, D.; Majorova, D.; Arnold, J.N.; Olcina, M.M. The effects of radiation therapy on the macrophage response in cancer. Front. Oncol. 2022, 12, 1020606. [Google Scholar] [CrossRef]
- Wu, C.; Shen, Z.; Lu, Y.; Sun, F.; Shi, H. p53 Promotes Ferroptosis in Macrophages Treated with Fe(3)O(4) Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 42791–42803. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.; Decker, C.; Sansbury, B.E.; Marinello, M.; Seyfried, A.; Howard, J.; Mori, M.; Hosseini, Z.; Arunachalam, T.; Finn, A.V.; et al. Radiation-Induced Macrophage Senescence Impairs Resolution Programs and Drives Cardiovascular Inflammation. J. Immunol. 2021, 207, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanisms and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, B.; Auger, J.P.; Dutz, S.; Cicha, I.; Schreiber, E.; Band, J.; Boccacccini, A.R.; Krönke, G.; Alexiou, C.; Tietze, R. Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release. Int. J. Mol. Sci. 2021, 22, 4143. [Google Scholar] [CrossRef]
- Su, L.; Dong, Y.; Wang, Y.; Wang, Y.; Guan, B.; Lu, Y.; Wu, J.; Wang, X.; Li, D.; Meng, A.; et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 2014, 1846, 121–129. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Tang, Y.; Liu, S.J.; Zeng, P.H.; Qu, L.; Jing, Q.C.; Yin, W.J. Radiation-induced liver disease: Beyond DNA damage. Cell Cycle 2022, 22, 506–526. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurin, M.R.; Kirichenko, V.A.; Shurin, G.V.; Lee, D.; Crane, C.; Kirichenko, A.V. Radiomodulating Properties of Superparamagnetic Iron Oxide Nanoparticle (SPION) Agent Ferumoxytol on Human Monocytes: Implications for MRI-Guided Liver Radiotherapy. Cancers 2024, 16, 1318. https://doi.org/10.3390/cancers16071318
Shurin MR, Kirichenko VA, Shurin GV, Lee D, Crane C, Kirichenko AV. Radiomodulating Properties of Superparamagnetic Iron Oxide Nanoparticle (SPION) Agent Ferumoxytol on Human Monocytes: Implications for MRI-Guided Liver Radiotherapy. Cancers. 2024; 16(7):1318. https://doi.org/10.3390/cancers16071318
Chicago/Turabian StyleShurin, Michael R., Vladimir A. Kirichenko, Galina V. Shurin, Danny Lee, Christopher Crane, and Alexander V. Kirichenko. 2024. "Radiomodulating Properties of Superparamagnetic Iron Oxide Nanoparticle (SPION) Agent Ferumoxytol on Human Monocytes: Implications for MRI-Guided Liver Radiotherapy" Cancers 16, no. 7: 1318. https://doi.org/10.3390/cancers16071318
APA StyleShurin, M. R., Kirichenko, V. A., Shurin, G. V., Lee, D., Crane, C., & Kirichenko, A. V. (2024). Radiomodulating Properties of Superparamagnetic Iron Oxide Nanoparticle (SPION) Agent Ferumoxytol on Human Monocytes: Implications for MRI-Guided Liver Radiotherapy. Cancers, 16(7), 1318. https://doi.org/10.3390/cancers16071318





