Simple Summary
Rare diseases represent a major health problem, since patients face difficulties in obtaining a rapid diagnosis and appropriate treatments. Vulvar dermatofibrosarcoma protuberans is one of these rare entities, in which reaching a correct pathological diagnosis is intricate and surgical techniques are not standardised. The aim of our paper is to review the available literature on vulvar dermatofibrosarcoma protuberans to summarise previous experiences and main issues, in an attempt to improve the management of this rare disease. Dermatofibrosarcoma protuberans of the vulva needs to be diagnosed early and managed by a referral centre, where the patient can receive appropriate management: surgical treatment should aim to obtain free margins, lowering the probability of recurrence. Long-term follow up is needed, since recurrences are documented even after several years.
Abstract
Background: Vulvar dermatofibrosarcoma protuberans is an extremely rare disease. Its rarity can hamper the quality of treatment; deeper knowledge is necessary to plan appropriate management. The purpose of this review is to analyse the data reported in the literature to obtain evidence regarding appropriate disease management. Methods: We made a systematic search of the literature, including the terms “dermatofibrosarcoma protuberans”, “vulva”, and “vulvar”, alone or in combination. We selected articles published in English from two electronic databases, PubMed and MEDLINE, and we analysed their reference lists to include other potentially relevant studies. Results: We selected 39 articles, with a total of 68 cases reported; they were retrospective case reports and case series. Dermatofibrosarcoma protuberans of the vulva tends towards local recurrence; an early and timely pathological diagnosis, together with an appropriate surgical approach, are of utmost importance to ensure free margins and maximise the curative potential. Conclusions: Even if this is an indolent disease and it generally shows a good prognosis, appropriate management may help in reducing the rate of local recurrences that may hamper patients’ quality of life. Management by a multidisciplinary team is highly recommended.
1. Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing, well-differentiated mesenchymal tumour arising in the dermis and usually with extension into subcutaneous tissue [1].
DFSP can occur anywhere, but the preferred sites are the trunk and extremities; a vulvar location is extremely rare, with fewer than 70 cases reported in the literature.
First described by Darier and Ferrand in 1924 [2], vulvar DFSP is characterised by slow growth, rarely leading to distant spread (less than 5% of cases); local recurrence is common, ranging between 20 and 50% of cases. Due to its indolent course, diagnosis is often made when the disease is locally advanced. Moreover, reaching a correct histopathological diagnosis is challenging, as vulvar DFSP is often misdiagnosed with other more common tumours.
The gold standard for both first diagnosis and recurrence is represented by radical surgery, aiming at complete excision with free surgical margins.
Given its rarity and the lack of available clinical guidelines for its management, treating these patients poses several challenges, from diagnosis to follow up.
The aim of the present review is to systematically collect and analyse all of the available literature, and then summarise and discuss the evidence on vulvar DFSP.
2. Materials and Methods
A systematic search of the literature, until February 2023, was performed in two electronic databases (PubMed, MEDLINE and Embase) in order to identify articles relevant to the purpose of this systematic review. The article research was carried out according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework [3], as presented in Figure 1. The search included the following keywords and medical subject heading terms, alone or in combination: “dermatofibrosarcoma protuberans”, “vulva”, and “vulvar”. All identified articles were examined and their reference lists were reviewed in order to include other potentially relevant studies. Two independent authors reviewed the studies (RM, AB) for inclusion. Discordant cases were discussed with a third author (FM). Eligibility for inclusion was initially assessed on the basis of titles and abstracts. The decision for final inclusion was made after the detailed examination of the full manuscripts.
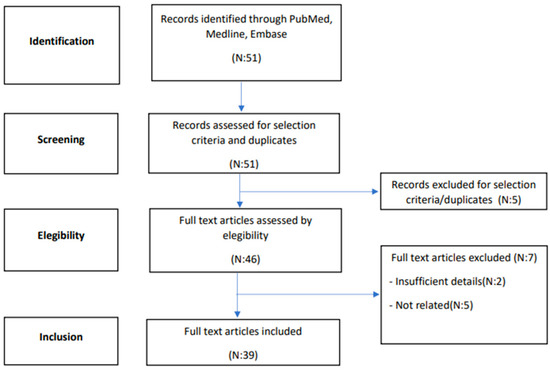
Figure 1.
Flowchart summarising the systematic literature review process.
The inclusion criteria were articles (also considering case series and case reports on vulvar DFSP) published in English in peer-reviewed journals between 1976 and 2023. Reviews or articles including tumours with mixed/other histologies or DFSP outside of the vulva were excluded.
3. Results
3.1. Study and Patient Characteristics
Figure 1 illustrates the flow of the systematic literature review. In total, 39 articles were selected for inclusion in this review, with a total of 68 cases reported (listed in Table 1). None of these were prospective series, while n = 32 were retrospective case reports and n = 7 case series. The number of patients included for each report ranged from 1 to 13.

Table 1.
Summary of the clinicopathological characteristics of cases of vulvar DFSP reported in the literature until March 2023.
This review also includes one representative case from a MITO centre that has not been previously published.
Sixty-nine cases of vulvar DFSP meeting our inclusion criteria were reported, with a median age of 46 (range: 19–83). The most common site of presentation was the labia majora (52.2%), followed by the mons pubis (11.6%). The mean size of the lesion at the time of surgery was 5.32 cm (data available for 61 cases, range 1.0–20.0 cm). Vulvar DFSP is usually described as an asymptomatic vulvar subcutaneous and firm mass, and less commonly as a plaque-like lesion. In rare cases (7.2%), the presence of a vulvar mass has been associated with pain, itching, malaise, bleeding, and dyspareunia.
The median time between the detection of the vulvar lesion and treatment was 24 months (range 1–252, data available for 29 patients, Table 1). In the included articles, the initial diagnosis was inconsistent with the final review in 22% of cases.
3.2. Pathology
Macroscopically, DFSP presented as a plaque-like cutaneous lesion, flat or elevated, firm, with irregular borders and of variable size. At the cut surface, it appeared as a single or multinodular lesion, with a translucent and gelatinous appearance, involving dermis and spreading into subcutaneous tissue.
Microscopically, the majority of our cases had the typical aspect of DFSP, presenting as low to intermediate differentiated tumours composed of spindle cells embedded in a collagenous stroma; in 3/69 cases (4.3%), the stroma was described as myxoid. In DFSP, tumour cells are typically arranged in a storiform pattern and show the entrapment of subcutaneous adipose tissue with a sparing of adnexal structures (“honeycomb” pattern). The cytoplasm is scant, eosinophilic, and fibrillary; the nuclei have low-grade atypia and low mitotic activity (Figure 2 and Figure 3). The presence of higher nuclear pleomorphism and increased mitotic count indicates the presence of fibrosarcomatous transformation (DFSP-FS) and was reported in 9/69 cases (13%) [11].
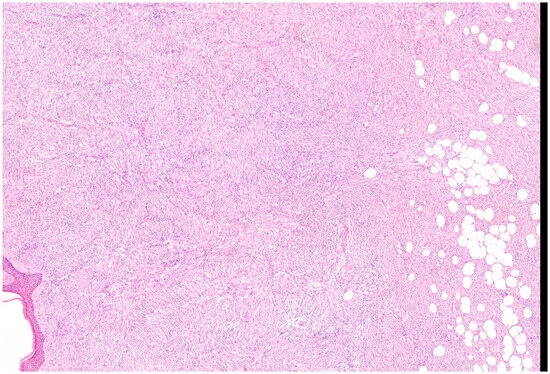
Figure 2.
Representative case of vulvar DFSP. Spindle cell proliferation infiltrates the dermis at full thickness and permeates the subcutis, saving some lobules of adipocytes (Haematoxylin–Eosin; 50×).
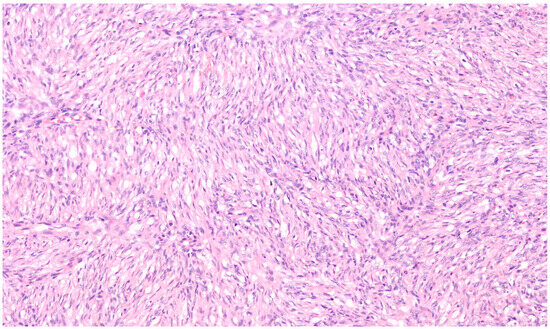
Figure 3.
Spindle cells have elongated and mildly hyperchromatic nuclei and are arranged in a storiform pattern, generally around small vessels (Haematoxylin–Eosin; 200×).
Immunohistochemical staining for CD34 was performed in 71% of patients (49/69), showing diffuse and strong expression in all cases of DFSP, except for those with fibrosarcomatous transformation, where staining was low or negative [11,15,28]. Vimentin was always positive, while staining for Desmin was negative in all available cases: S-100 was negative in 90% of cases.
Molecular studies have described that DFSP may often harbour a common chromosomal translocation t (17;22) (q22;q13) with the COL1A1-PDGFB fusion gene between the collagen type Iα1 gene (COL1A1) and the platelet-derived growth factor β-chain gene (PDGFB). The analysis of this rearrangement has only been recently performed; for this reason, this information is available in our records for 39/69 patients with vulvar DFSP (42%), with a positivity of 75.8%.
3.3. Treatment and Clinical Course
The details regarding treatment and clinical course are summarised in Table 2. Surgical excision with tumour-free margins is the gold standard of treatment for this disease. For limited volume lesions, wide local excision (WLE) was the most commonly applied surgical technique at primary surgery (61/68 = 89.7%); in cases of positive margins (26/65 = 40%), repeated surgery with WLE or vulvectomy has usually been proposed.

Table 2.
Summary of the treatment approaches and disease course of vulvar DFSP cases reported in the literature until March 2023.
Mohs microsurgery (MMS) was successfully applied in two cases following the positive experience of DFSP affecting other disease sites. These previous experiences have reported a lower rate of recurrence with this technique compared to wide excision (1.6% vs. 20%) [42].
Lymph node involvement has never been detected, confirming that lymphadenectomy is not recommended.
Adjuvant therapy is generally not recommended when radical excision is feasible. According to the present literature review, medical treatment with Imatinib was only offered in three cases, as neoadjuvant treatment for a large unresectable lesion or as adjuvant treatment in case of incomplete resection [11,15,17]. Adjuvant RT was administered in four cases: in two of them for positive margins after excision, in one case for local recurrence after WLE, and in one case for local recurrence without local excision [11,26,28,30].
Relapses of DFSP were frequent (20/68 = 29.4%). Most of the recurrences occurred locally (19/20 = 95.0%), particularly in cases of positive margins at local excision. In this analysis, the local recurrence rate was 42% in the case of positive margins (11/26) vs. 10.8% in the case of negative margins (4/37) (p = 0.003).
Distant spread was rare (3/20 cases = 15%), with the most commonly involved site being the lung; one of these cases was a DFSP-FS, and in the remaining two cases, classic DFSP was diagnosed. Notably, one of the patients experiencing relapse did not attend the recommended follow-up schedule [13,22,30]. Lung metastases were treated with Imatinib in one case, achieving partial response [22], or with conventional chemotherapy [30].
Deaths from disease have been rarely reported (2/68 = 2.9%), and in both cases, they were related to the presence of distant metastases.
4. Discussion
The present analysis confirms that vulvar DFSP is an indolent disease, with slow growth and a tendency towards local recurrence. Despite its general good prognosis, this disease requires proper management, given the high incidence of local and repeated recurrences that may negatively impact quality of life.
Given the rarity of this disease, many challenges in diagnosis and management need to be faced, from the correct and timely diagnosis to radical surgery. For this reason, management by an expert multidisciplinary team is highly recommended.
Early diagnosis is of utmost importance to allow appropriate and conservative surgery; since DFSP is usually paucisymptomatic at first presentation, diagnosis is often made several months after tumour appearance. Diagnostic delay is also conditioned by DFSP being an extremely rare tumour that uncommonly presents in the vulva; thus, achieving a correct final diagnosis is challenging.
The spindled cells are usually arranged in a storiform pattern and are typically associated with minimal cytologic atypia. Immunohistochemistry for CD34 is mostly positive. The presence of DFSP-FS is associated with a high risk of metastatic disease. For unclear lesions, fluorescence in situ hybridisation (FISH), polymerase chain reaction (PCR), or conventional cytogenetics can be useful to detect t(17;22) (q22;q13), which is a distinctive feature of DFSP.
Several tumours may resemble DFSP. The most common differential diagnoses include neurofibroma, schwannoma, malignant peripheral nerve sheath tumour (MPNST), solitary fibrous tumour (SFT), leiomyosarcoma, myxoid liposarcoma, and desmoplastic melanoma. Notably, in this review, 22% of the final diagnoses were inconsistent with initial pathological diagnosis. In particular, the most common misleading diagnosis was that of dermatofibroma—the benign counterpart of DFSP—generally composed of a mixture of spindle cells and inflammatory cells, with a minor subcutaneous involvement, that could be differentiated from DFSP by negative staining for CD34. The other reported misdiagnoses were histiocytoma, fibrosarcoma, leiomyosarcoma, and neurofibroma.
The misdiagnosis is often due to inadequate tissue sampling or superficial biopsy; NCCN guidelines recommend a punch or incisional biopsy, including the deeper subcutaneous layer [42].
The interval between the clinical presentation of the lesion and first surgery can be considered prognostically relevant. In the present review, the longest was the interval between first presentation and surgery, and the largest was the tumour volume, with wider resection necessary to reach surgical free margins.
After preliminary workup, with haematoxylin and eosin (H&E) and immunopanel (i.e., for CD34 positivity), patients should be submitted for an accurate clinical exam, followed by multidisciplinary consultation and MRI with contrast, to plan appropriate treatment [42].
Wide surgical excision without lymphadenectomy is the gold standard for the treatment of this disease for both primary and recurrent lesions. To minimise the consequences of tissue defect, optimise the aesthetic result, and reduce the risk of relapse, surgery should be proposed at first appearance of the disease and performed by a surgeon with extensive expertise in vulvar surgery. Mohs micrographic surgery helped two patients in obtaining free margins and ensuring the complete resection of DFSP [43]. Excision with Mohs or other forms of margin assessment should be used; for unresectable disease, neoadjuvant Imatinib could be considered, following the execution of tumour mutation analysis.
Adjuvant treatment in cases of surgical free margins is not recommended. Radiation therapy can be advised in cases of positive surgical margins, when further resection is not feasible.
Limited long-term follow up information was reported. The prognosis in terms of disease-free survival is negatively affected by lesion size and positive surgical margins. Interestingly, recurrences were documented even after several years, suggesting a recommendation for long-term follow up. Patients should be informed about the peculiarity of the disease and educated to conduct regular self-examinations. Clinical follow-up should be integrated with MRI surveillance.
In the setting of recurrent disease, patients should be evaluated for repeated surgery or radiotherapy if resection is not feasible. When the disease is not resectable, or in the metastatic setting, treatment with Imatinib can be considered [42].
5. Conclusions
DFSP of the vulva is a slow-growing entity and surgery is the mainstay of treatment in this disease. Patients should be encouraged to seek medical attention when a new lesion—even apparently benign—persists or grows. A timely correct pathological diagnosis is essential to ensure proper management and limit the morbidities associated with surgical excision. Given the rarity of this disease, patients should be referred to high-volume centres to discuss diagnostic and therapeutic issues. Multicentre collaboration is essential for polling data and increasing the knowledge on this rare disease.
Author Contributions
Conceptualisation, R.M. and A.B.; methodology, R.M., A.B. and L.A.; resources, A.B., R.M., G.M., M.S. and G.T.; data curation, R.M., F.M. and A.B.; writing—original draft preparation, R.M., A.B. and R.C.; writing—review and editing, all authors; visualisation, all authors; supervision, A.B.; project administration, G.M., S.P. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, A.H.; Detty, S.Q.; Gonzaga, M.I.; Huerter, C. Clinical Features and Treatment of Dermatofibrosarcoma Protuberans Affecting the Vulva: A Literature Review. Dermatol. Surg. 2017, 43, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Darier, J.; Ferrand, M. Dermatofibromes progressifs et récidivants ou fibrosarcomes de la peau. Ann. Dermatol. Syphil. 1924, 5, 545–562. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Aartsen, E.J.; Albus-Lutter, C.E. Vulvar sarcoma: Clinical implications. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 56, 181–189. [Google Scholar] [CrossRef]
- Agress, R.; Figge, D.C.; Tamimi, H.; Greer, B. Dermatofibrosarcoma protuberans of the vulva. Gynecol. Oncol. 1983, 16, 288–291. [Google Scholar] [CrossRef]
- Alverez-Cañas, M.C.; Mayorga, M.; Fernandez, F.; Val-Bernal, J.F.; Moral, E.; Leon, C.; Erasun, F.; Lerma, D. Dermatofibrosarcoma protuberans of the vulva: Clinico-pathological, immunohistochemical and flow cytometric study of a case. Acta Obstet. Gynecol. Scand. 1996, 75, 82–85. [Google Scholar] [CrossRef]
- Barnhill, D.R.; Boling, R.; Nobles, W.; Crooks, L.; Burke, T. Vulvar dermatofibrosarcoma protuberans. Gynecol. Oncol. 1988, 30, 149–152. [Google Scholar] [CrossRef]
- Barrios Barreto, R.; Mendoza Suarez, L.; Del Valle, A.; Silvera Redondo, C.; De La Hoz Pabola, A. Dermatofibrosarcoma protuberans with unusual presentation in vulva. Medicina 2022, 82, 441–444. (In English) [Google Scholar]
- Bernárdez, C.; Machan, S.; Molina-Ruiz, A.M.; Pérez de la Fuente, T.; Pavón, M.; Carrillo, I.; Fortes, J.; Requena, L. Dermatofibrosarcoma Protuberans of the Vulva with Myxoid Differentiation. Am. J. Dermatopathol. 2015, 37, e107-11. [Google Scholar] [CrossRef]
- Bertolli, E.; Bretchbuhl, E.R.; Camarço, W.R.; Campagnari, M.; Molina, A.S.; Baiocchi, G.; Macedo, M.P.; Pinto, C.A.; Cunha, I.W.; Neto, J.P. Dermatofibrosarcoma protuberans of the vulva: Margins assessment and reconstructive options—A report of two cases. World J. Surg. Oncol. 2014, 12, 399. [Google Scholar] [CrossRef][Green Version]
- Bock, J.E.; Andreasson, B.; Thorn, A.; Holck, S. Dermatofibrosarcoma protuberans of the vulva. Gynecol. Oncol. 1985, 20, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Cromi, A.; Uccella, S.; Serati, M.; Casarin, J.; Cimetti, L.; Donadello, N.; Ghezzi, F. Dermatofibrosarcoma protuberans of the vulva. J. Obstet. Gynaecol. 2015, 35, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Davos, I.; Abell, M.R. Soft tissue sarcomas of vulva. Gynecol. Oncol. 1976, 4, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Doufekas, K.; Duncan, T.J.; Williamson, K.M.; Varma, S.; Nunns, D. Mohs micrographic surgery for dermatofibrosarcoma protuberans of the vulva. Obstet. Gynecol. Int. 2009, 2009, 547672. [Google Scholar] [CrossRef]
- Edelweiss, M.; Malpica, A. Dermatofibrosarcoma protuberans of the vulva: A clinicopathologic and immunohistochemical study of 13 cases. Am. J. Surg. Pathol. 2010, 34, 393–400. [Google Scholar] [CrossRef]
- Ghorbani, R.P.; Malpica, A.; Ayala, A.G. Dermatofibrosarcoma protuberans of the vulva: Clinicopathologic and immunohistochemical analysis of four cases, one with fibrosarcomatous change, and review of the literature. Int. J. Gynecol. Pathol. 1999, 18, 366–373. [Google Scholar] [CrossRef]
- Gilani, S.; Al-Khafaji, B. Dermatofibrosarcoma protuberans of the vulva: A mesenchymal tumour with a broad differential diagnosis and review of literature. Pathologica 2014, 106, 338–341. [Google Scholar]
- Goyal, L.D.; Garg, P.; Kaur, M.; Sharma, D. Recurrent Dermatofibrosarcoma Protuberans of the Vulva: A Rare Occurrence and Review of Literature. J. Fam. Reprod. Health. 2021, 15, 136–140. [Google Scholar] [CrossRef]
- Hammonds, L.M.; Hendi, A. Dermatofibrosarcoma protuberans of the vulva treated using mohs micrographic surgery. Dermatol. Surg. 2010, 36, 558–563. [Google Scholar] [CrossRef]
- Hancox, J.G.; Kelley, B.; Greenway, H.T., Jr. Treatment of dermatofibroma sarcoma protuberans using modified Mohs micrographic surgery: No recurrences and smaller defects. Dermatol. Surg. 2008, 34, 780–784. [Google Scholar] [CrossRef]
- Jahanseir, K.; Xing, D.; Greipp, P.T.; Sukov, W.R.; Keeney, G.L.; Howitt, B.E.; Schoolmeester, J.K. PDGFB Rearrangements in Dermatofibrosarcoma Protuberans of the Vulva: A Study of 11 Cases Including Myxoid and Fibrosarcomatous Variants. Int. J. Gynecol. Pathol. 2018, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.; Stefanovic, A.; Jeremic, K.; Jovic, M.; Pilic, I.; Cvetkovic, A.; Stojanovic, M. Giant dermatofibrosarcoma protuberans vulvae: Rare clinical presentation and literature review. J. BUON 2019, 24, 1289–1295. [Google Scholar] [PubMed]
- Karlen, J.R.; Johnson, K.; Kashkari, S. Dermatofibrosarcoma protuberans of the vulva. A case report. J. Reprod. Med. 1996, 41, 267–269. [Google Scholar] [PubMed]
- Leake, J.F.; Buscema, J.; Cho, K.R.; Currie, J.L. Dermatofibrosarcoma protuberans of the vulva. Gynecol. Oncol. 1991, 41, 245–249. [Google Scholar] [CrossRef]
- Merlo, G.; Cozzani, E.; Comandini, D.; Trave, I.; Centurioni, M.G.; Franchelli, S.; Zena, M.; Vellone, V.G.; Biatta, C.M.; Parodi, A. Neoadjuvant imatinib as treatment preceding surgery for vulvar dermatofibrosarcoma protuberans. Dermatol. Ther. 2021, 34, e14860. [Google Scholar] [CrossRef]
- Messalli, E.M.; D’Aponte, M.L.; Luise, R.; Rossiello, L.; Rotondi, M.; De Franciscis, P. An apparently benign vulvar mass: Possibly a rare malignancy. Eur. J. Gynaecol. Oncol. 2012, 33, 441–444. [Google Scholar]
- Moodley, M.; Moodley, J. Dermatofibrosarcoma protuberans of the vulva: A case report and review of the literature. Gynecol. Oncol. 2000, 78, 74–75. [Google Scholar] [CrossRef]
- Neff, R.; Collins, R.; Backes, F. Dermatofibrosarcoma protuberans: A rare and devastating tumor of the vulva. Gynecol. Oncol. Rep. 2019, 28, 9–11. [Google Scholar] [CrossRef]
- Nirenberg, A.; Ostör, A.G.; Slavin, J.; Riley, C.B.; Rome, R.M. Primary vulvar sarcomas. Int. J. Gynecol. Pathol. 1995, 14, 55–62. [Google Scholar] [CrossRef]
- Ohlinger, R.; Kühl, A.; Schwesinger, G.; Bock, P.; Lorenz, G.; Köhler, G. Dermatofibrosarcoma protuberans of the vulva. Acta Obstet. Gynecol. Scand. 2004, 83, 685–686. [Google Scholar] [CrossRef]
- Ozmen, E.; Güney, G.; Algin, O. Magnetic resonance imaging of vulvar dermatofibrosarcoma protuberans—Report of a case. Radiol. Oncol. 2013, 47, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Rousso, D.; Achparaki, A.; Georgiadis, H.; Vlassis, G. Recurrence of dermatofibrosarcoma protuberans of the vulva. A clinical, histological, and ultrastructural study. Eur. J. Gynaecol. Oncol. 1993, 14, 182–186. [Google Scholar] [PubMed]
- Pascual, A.; Sánchez-Martínez, C.; Moreno, C.; Burdaspal-Moratilla, A.; López-Rodriguez, M.J.; Rios, L. Dermatofibrosarcoma protuberans with areas of giant cell fibroblastoma in the vulva: A case report. Eur. J. Gynaecol. Oncol. 2010, 31, 685–689. [Google Scholar] [PubMed]
- Schwartz, B.M.; Kuo, D.Y.; Goldberg, G.L. Dermatofibrosarcoma protuberans of the vulva: A rare tumor presenting during pregnancy in a teenager. J. Low. Genit. Tract. Dis. 1999, 3, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Soergel, T.M.; Doering, D.L.; O’connor, D. Metastatic dermatofibrosarcoma protuberans of the vulva. Gynecol. Oncol. 1998, 71, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.H. Dermatofibrosarcoma protuberans of the vulva. Case Report. Br. J. Obstet. Gynaecol. 1981, 88, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Vanni, R.; Faa, G.; Dettori, T.; Melis, G.B.; Dumanski, J.P.; O’Brien, K.P. A case of dermatofibrosarcoma protuberans of the vulva with a COL1A1/PDGFB fusion identical to a case of giant cell fibroblastoma. Virchows Arch 2000, 437, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Vathiotis, I.A.; Psichogiou, E.; Syrigos, K.N.; Kotteas, E.A. Lung Metastasis from Fibrosarcomatous Dermatofibrosarcoma Protuberans of the Vulva: A Rare Case Report. J. Low. Genit. Tract. Dis. 2018, 22, 85–87. [Google Scholar] [CrossRef]
- Wiszniewska, J.; Roy, A.; Masand, R.P. Myxoid Dermatofibrosarcoma Protuberans of the Vulva: Case Report of a Rare Variant in an Unusual Location, with Unusual Morphologic and Immunohistochemical Features. Am. J. Dermatopathol. 2016, 38, 226–230. [Google Scholar] [CrossRef]
- Zemni, I.; Sassi, I.; Boujelbene, N.; Haddad, S.; Doghri, R.; Chargui, R.; Rahal, K. Vulvar Darier-Ferrand dermatofibrosarcoma: Unusual localization of a rare tumor. Pan Afr. Med. J. 2019, 33, 46. [Google Scholar] [CrossRef]
- Zlatnik, M.G.; Dinh, T.V.; Lucci, J.A., 3rd; Hannigan, E.V. Dermatofibrosarcoma protuberans of the vulva: Report of two new cases and review of the literature. J. Low. Genit. Tract. Dis. 1999, 3, 135–138. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Dermatofibrosarcoma Protuberans: NCCN Guidelines Version 1.2024; NCCN: Plymouth Meeting, PA, USA, 2023. [Google Scholar]
- Udkoff, J.; Russell, E.; Beal, B.T.; Holzer, A.M.; Brodland, D.G.; Knackstedt, T. Cost effectiveness of dermatofibrosarcoma protuberans treated with Mohs micrographic surgery compared with wide local excision. J. Am. Acad. Dermatol. 2022, 87, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).