Simple Summary
Malignant Brenner tumors are rare ovarian neoplasms. Our aim is to provide insights concerning this rare entity. We reviewed 115 cases reported in the English literature until 15 September 2023, and analyzed the available demographic, clinical, and pathologic data. We also described the treatment modalities. A comparison of the available data showed that patients treated with lymph node dissection had a better disease-related survival rate. Disease recurrence was associated with tumor stage with marginal statistical significance and was more frequent in patients with ascites and those with abnormal CA-125 levels. Larger series with treatment details and long term follow-up data are needed to define the optimal management for this uncommon entity.
Abstract
Background: Malignant Brenner tumors are rare ovarian tumors, accounting for less than 1% of malignant ovarian neoplasms. The aim of this manuscript is to systematically review the current literature concerning malignant Brenner tumors. Methods: We searched three medical databases (PubMed, Scopus, and Web of Science) for relevant articles published until 15 September 2023. Results: After applying inclusion and exclusion criteria, 48 manuscripts describing 115 cases were included in this study from the English literature. Conclusions: We analyzed the demographic, clinical, pathological, and oncological characteristics of 115 patients with malignant Brenner tumors. The statistical analysis showed that recurrence was marginally statistically significantly related to tumor stage and was more common in patients with ascites and in women with abnormal CA-125 levels; patients that were treated with lymphadenectomy had better disease-specific survival.
1. Introduction
Brenner tumors are an uncommon subtype of epithelial neoplasms, accounting for less than 5% of ovarian tumors [1]. They are usually unilateral and have a propensity for postmenopausal women; they are commonly asymptomatic and incidental due to their small size, but patients sometimes experience symptoms such as pain or a palpable mass [2].
The origin of these tumors is unknown. A number of them may derive from fallopian tube epithelium or Walthard nests [3], while when rarely associated with teratomas, they may originate from germ cells [1]. MacNoughton-Jones first described Brenner tumors in 1898, whereas in 1907, Fritz Brenner published the article “Das oophoroma folliculare” [4], considering them a variant of the granulosa cell tumor [5]. This neoplasm was first called a Brenner tumor by Meyer in 1932 [6]. Von Numers was the first to describe a malignant Brenner tumor (MBT) in 1945 [7].
Brenner tumors are classified into benign, borderline, and malignant variants, with benign being the most common. Borderline variants are infrequent (less than 5% of all cases), and MBTs are extremely rare, with less than 150 cases reported in the English literature. Histologically, MBTs are composed of atypical transitional/urothelial-type cells that occasionally display focal squamous differentiation. By definition, they show stromal invasion, usually with a desmoplastic stromal response, and are associated with a benign and/or borderline element [8].
This study aims to review MBTs’ clinical, pathological, diagnostic, molecular, and treatment features, focusing on differential diagnosis.
2. Materials and Methods
2.1. Systematic Review
The systematic review of the literature was performed according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (http://www.prismastatement.org/; accessed on 15 September 2023) (Figure 1) to identify published manuscripts of malignant ovarian Brenner tumors.
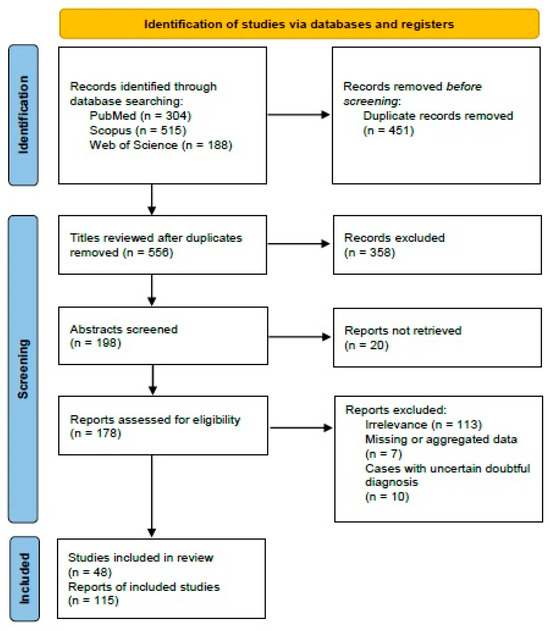
Figure 1.
PRISMA 2020 flowchart showing the search strategy, excluded studies, and finally included reports.
Our retrospective observational study search was conducted through the PICO process:
- Population: Women with a diagnosis of MBT;
- Intervention: Surgical treatment of the primary ovarian tumor;
- Comparison: None;
- Outcome: Patient treatment, follow-up.
We searched for (“malignant”) AND (“Brenner”) AND (“tumor”) AND (“ovary” OR “ovarian”) in three different databases. The search yielded results on PubMed (all fields; 304 results; https://pubmed.ncbi.nlm.nih.gov, accessed on 15 September 2023), Scopus (Title/Abstract/Keywords; 515 results; https://www.scopus.com/, accessed on 15 September 2023), and Web of Science (all fields, 188 results; https://login.webofknowledge.com, accessed on 15 September 2023). We did not set any additional limitations while performing the search.
We applied the following criteria:
- Eligibility/inclusion criteria:
- (1)
- Study design: We only included original studies and case reports describing cases of MBT.
- (2)
- Population: Studies involving adult patients diagnosed with MBT that provided adequate surgical and/or oncological information were included.
- (3)
- Intervention or exposure: We included studies that examined any treatment or intervention for MBT, including surgery, chemotherapy, radiation therapy, or targeted therapies.
- (4)
- Outcome: We included studies that reported on the presence or absence of disease relapse as an outcome measure.
- (5)
- Language: The included studies were written in the English language.
- Exclusion criteria:
- (1)
- Review articles and editorials: We excluded narrative or systematic reviews, meta-analyses, opinion pieces, and other articles that did not present original research findings.
- (2)
- Insufficient information: Cases with insufficient or too much aggregated data were excluded.
- (3)
- Uncertain diagnosis: Cases with an uncertain/doubtful diagnosis were excluded.
- (4)
- Histologic criteria: Cases lacking a benign or borderline Brenner component were excluded.
- (5)
- Language: Manuscripts in languages other than English were excluded.
Three authors (I.B., D.D., and K.S.) worked independently to remove duplicate papers. They also reviewed the titles and abstracts of all the search results (n = 1007). Any disagreement was resolved by consensus. After applying eligibility and exclusion criteria, 48 manuscripts describing 115 cases of MBT were included in this review (Table 1 and Supplementary Table S1).

Table 1.
Clinic-pathologic and treatment features of the cases of malignant Brenner tumors.
2.2. Statistical Analysis
Statistical analysis was performed via the SAS for Windows 9.4 software platform (SAS Institute Inc., Cary, NC, USA). Descriptive values were expressed as the mean ± standard deviation (SD) and, when no normality was confirmed (via the Shapiro–Wilk test), as median value, 1st (Q1) and 3rd (Q3) quartile values, respectively. For categorical data we reported the appearance frequency and the relevant percentages.
Comparisons between groups for the qualitative parameters were made using the chi-square test. For the numerical data (such as a woman’s age), normality was not possible to ensure, therefore, non-parametric tests were applied, specifically the Kruskal–Wallis test.
Furthermore, we estimated survival time using the Kaplan–Meier method; we considered that the follow-up time reported in the studies was equal to the survival time for those women that died from the disease, while in all other cases, the follow-up time was considered as the time point for censored cases. Additional tests for factors that could affect survival time were performed using the log-rank method.
The significance level (α) was set to 0.05 for all statistical tests; thus, a statistically significant difference between compared groups was when p < 0.05 and all tests were two sided.
3. Results
3.1. Demographic and Clinical Data
The publication years ranged from 1956 to 2023. The age in 108/115 (93.9%) cases [2,8,9,10,11,12,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] was reported. Specifically, the mean age at presentation was 59 ± 13 years, ranging from 22 to 87 years. Presenting symptoms were reported in 113/115 (98.3%) patients [2,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. The most common presenting symptom was abdominal pain, which was present in 42/113 patients (37.1%) [2,13,19,22,26,27,30,36,37,38,39,40,41,45,47,48,49,51], followed by adnexal mass (15/113, 13.3%) [13,24,51], abdominal/pelvic mass (15/113, 13.3%) [24,31,35,36,40,42,43,44,48,53], abdominal distention (16/113, 14.1%) [13,14,34,36,40,43,48,52], vaginal bleeding (15/113, 13.3%) [10,13,21,24,25,27,38,40,43,48], weight loss (8/113, 7.1%) [20,25,26,31,43,44,53], abnormal uterine bleeding (6/113, 5.3%) [10,11,14,39,45], and nausea and/or vomiting (6/113, 5.3%) [10,13,47]. Other symptoms included diarrhea (2/113, 1.8%) [15,18], constipation (2/113, 1.8%) [44,47], hematuresis (1/113, 0.9%) [40] and acute urinary retention (1/113, 0.9%) [17]. Ascites was present in 33/113 (29.2%) cases [9,13,20,24,25,26,27,29,31,34,36,38,39,40,42,43,44,46,48,53]. The patient presented by Baizabal-Carvallo et al., had a bifrontal headache, tinnitus, blurred vision, and dizziness due to dural metastasis [33]. Each of these symptoms occurred alone or in combination with other symptoms. In 7/113 (6.2%) [12,13,36,38,45,50] cases, patients were asymptomatic. Details concerning symptoms can be seen in Supplementary Table S2.
Data concerning laterality were provided in 97/115 (84.3%) cases [2,8,9,10,11,12,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,42,43,44,46,47,48,49,50,51,52,53]; 45/97 (46.4%) cases involved the right ovary [2,9,16,17,18,21,24,25,26,29,30,31,32,35,36,37,38,39,40,42,43,46,47,48,49,51,53], 36/97 (37.1%) cases arose from the left ovary [8,10,11,12,14,19,24,27,28,30,32,33,34,36,38,40,44,48,50,51], and 16/97 (16.5%) cases showed bilateral ovarian involvement [2,20,22,23,27,36,38,40,51,52]. Tumor size was reported in 105/115 (91.3%) cases, ranging from 2 to 30 cm, with a mean value of 12.2 cm [2,8,9,10,11,13,14,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50,51,52,53]. Two manuscripts, Miles and Norris [13] and Zhang et al. [45], reported the mean value and SD; these values were used for each individual patient. There was no information regarding tumor size in 9/115 (7.8%) cases [19,32,33,40,41,51]. In a single case, the tumor size was mentioned as >10 cm [42].
CA-125 serum levels were reported in 65/115 (56.5%) cases [2,8,30,32,34,36,37,38,39,40,42,43,44,45,46,47,48,51,52]. Five reports mentioned the CA-125 level as normal without providing an exact value [3,31,50]. The mean value was 202.69 U/mL, ranging from 4 to 4073.3 U/mL). Details showing patients’ demographic, treatment, and outcome characteristics are presented in Table 2.

Table 2.
Detailed results of the MBT patients’ characteristics.
Staging was performed in 100/115 (86.9%) cases [2,8,10,13,17,18,20,21,22,23,24,26,27,28,29,30,31,32,33,34,36,38,39,40,42,44,45,47,48,49,50,51,52,53]. Stage I disease was assigned to 50/100 (50%) patients [8,10,13,21,23,24,28,30,32,34,36,38,40,45,48,49,50,51], stage II to 7/100 (7%) patients [38,42,45,48,51], stage III to 32/100 (32%) patients [2,13,18,22,26,27,29,30,36,38,39,40,45,47,48,51,52,53], and stage IV to 11/100 (11%) patients [17,20,31,33,36,38,40,44,45]. One patient was not staged due to her poor medical status [35]. Staging was not mentioned in 14/115 (12.1%) cases [9,11,12,14,15,16,19,25,32,37,41,43,46]. The details of the staging are presented in Supplementary Table S3.
3.2. Diagnosis
The diagnosis of MBT, according to the latest edition of the WHO diagnostic criteria (5th edition, 2020) [1], requires the presence of invasive urothelial-like carcinoma and the presence of a benign and/or borderline Brenner tumor component. The cases included in this review satisfied these diagnostic criteria. Immunohistochemically, MBTs were positive for PAX-8 (1/3, 33%) [42,49,52], CK7 (6/6, 100%) [42,43,44,49,52], Uroplakin III (1/2, 50%) [42,52], GATA-3 (4/4, 100%) [42,43,49,50], p63 (6/6, 100%) [42,43,44,49,50,52], and negative for WT-1 (0/2, 0%) [43,52]. Some authors have described some morphologic variants of MBT. St. Pierre-Robson et al., published three cases with an unusual pattern of invasion without a desmoplastic response [8]. McGinn et al., reported two cases of a possibly novel variant of the Brenner tumor; these neoplasms consisted of a benign Brenner component associated with a low-grade basaloid carcinoma [50].
3.3. Surgical Management
Information regarding surgical treatment was mentioned in 110/115 (95.6%) cases [2,5,8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,49,50,51,52,53]. A woman with stage IV disease did not receive surgical treatment [33]. The majority of patients (88/109, 80.7%) underwent hysterectomy and bilateral salpingo-oophorectomy (HBSO) [2,8,10,12,13,15,17,18,19,20,21,23,24,25,26,27,28,29,30,32,34,36,37,38,39,40,41,43,44,45,46,47,49,51,52,53]. The rest of the patients were treated with other procedures, such as hysterectomy and right salpingo-oophorectomy (1/109, 0.9%) [38], bilateral salpingo-oophorectomy (BSO) (10/109, 9.1%) [13,16,22,27,31,43,45,50], left salpingo-oophorectomy (5/109, 4.5%) [8,11,14,38,50], right salpingo-oophorectomy (2/109, 1.8%) [30,42], or right oophorectomy (2/109, 1.8%) [35,40]. In 2/109 (1.8%) cases [13], the procedure was salpingo-oophorectomy without mentioning the side. Omentectomy was performed in addition to HBSO or BSO in 64/109 (58.7%) patients [2,8,22,25,26,27,30,34,36,37,38,39,40,41,43,44,45,51,52,53]. Other procedures included omental biopsy/sampling (4/109, 3.6%) [8,20,27,49], excision of mesenteric nodules (1/109, 0.9%) [17], resection of bladder-involved focus (1/109, 0.9%) [40], splenectomy (1/109, 0,9%) [2], right hemicolectomy (1/109, 0.9%) [47], and appendectomy (23/109, 21.1%) [2,34,38,39,40,51]. Lymph node dissection was performed in 41/109 (37.6%) [2,36,38,39,42,43,45,51,52,53] and lymph node biopsy in 1/109 (0.9%) [37] of the cases. The applied surgical approach is detailed in Supplementary Table S4.
3.4. Adjuvant Therapy
Information concerning adjuvant treatment was reported in 96/115 (83.5%) of cases [2,5,10,13,15,16,17,18,19,21,22,23,27,30,31,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Adjuvant therapy was not administered to 27/96 (28.1%) patients [10,13,15,17,18,19,23,31,33,38,40,43,45,47,49,50,51]. Most of them had stage I disease. In one case, the patient refused adjuvant therapy [19]. In two cases with stage IV disease, the reasons were the patient’s poor status in the first [31] and that the patient died a few hours after surgery in the second [33]. Radiotherapy was offered alone in 3/69 (4.3%) [10,13,16] or in combination with chemotherapy in 4/69 (5.8%) patents [21,38,51,52]. Chemotherapy was administered in 63/93 (67.7%) patients [2,21,22,27,30,32,35,36,37,38,39,40,41,42,44,45,46,48,51,52,53]. The most commonly used regimen was paclitaxel-carboplatin (TC) in 41/63 (65%) of patients [2,3,38,39,42,45,46,48,51,52,53], followed by Melphalan (Alkeran) (5/63, 7.9%) [21,22,27], paclitaxel-cisplatin (3/63, 4.7%) [40], and various other drug combinations [27,30,32,37,38,40,45]. Neoadjuvant chemotherapy was administered in two cases with stage IIIb and stage IV disease, consisting of six cycles of TC and five cycles of paclitaxel-cisplatin, respectively [38,45].
In 33/46 (71.7%) cases with disease relapse, information concerning treatment was available [10,15,19,22,23,27,30,32,36,38,40,41,42,45,48,51], including tumor debulking surgery (6/33, 18.1%) [32,41,42,45,48,51], radiotherapy (6/33, 18.1%) alone [15,27] or in combination with surgery and/or chemotherapy [42,45,51]. In 27/33 (81.8%) patients, chemotherapy was administered [22,23,30,32,36,38,40,41,42,45,48,51]. The most common therapeutic regimen was TC used in 12/27 (48%) of cases [36,38,42,48,51] with various other combinations [22,23,30,38,40,41,42,45,48,51]. Details of adjuvant treatment for each patient are presented in Supplementary Table S1.
3.5. Molecular Findings
Two cases were tested for BRCA1/2 mutations [41,49]. A BRCA-2 pathogenic mutation was present in the case reported by Toboni et al. [41]. No other information was provided.
3.6. Follow-up and Survival
Follow-up data were available in 106/115 (92.1%) cases [2,8,9,10,11,13,15,16,17,18,19,21,22,23,24,25,27,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53]; 53/106 (50%) patients were alive without evidence of the disease [2,8,10,11,13,21,24,25,32,34,36,37,38,39,40,42,43,44,45,49,50,51,52,53], 10/106 (9.4%) were alive with the disease [27,31,38,41,45,48], 30/106 (28.3%) succumbed to the disease [9,10,13,15,16,17,18,19,22,23,27,29,30,32,33,35,36,38,40,45,47,51], 6/106 (5.7%) died of other causes [13,24,38,40], and 5/106 (4.7%) were lost at follow-up [38,45,48].
Follow-up time was specified in 102/115 (88.7%) cases [2,8,9,10,11,13,15,16,17,18,19,21,22,27,29,30,31,32,33,34,35,36,37,38,39,40,42,43,45,48,49,50,51,52,53], ranging from 1 to 173 months (mean: 40.1 months). For all except one woman, information on the outcome was available, thus survival curves were possible to construct; the mean survival time for all patients was estimated with the Kaplan–Meier approach at 80.9 months (standard error: 5.5 months) (Figure 2).
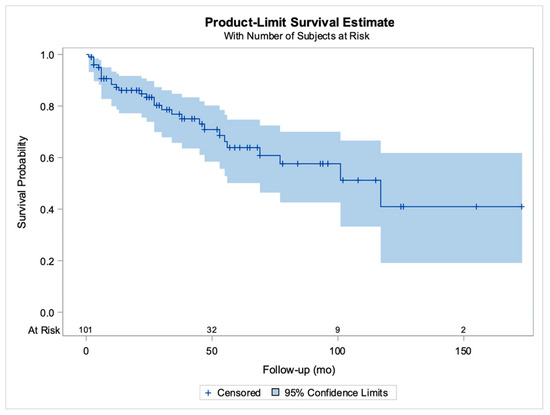
Figure 2.
Kaplan–Meier curves for patient survival. The horizontal axis shows the follow-up period in months and the number of patients at risk for various time points, vertical lines correspond to censored cases (previously unpublished original photo).
Relapse information was available in 104/115 (90.4%) cases [2,10,11,13,15,16,17,18,19,21,22,23,24,25,27,29,30,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]; 46/104 (43.2%) patients had one or more relapses [10,13,15,19,22,23,27,29,30,32,35,36,38,40,41,42,45,48,50,51], while there was no disease relapse in 59/104 (56.8%) cases [2,10,11,13,16,17,18,21,24,25,32,33,34,36,37,38,39,40,43,44,45,46,47,49,50,51,52,53]. The median time to relapse was 13 months (Q1–Q3: 9–36 months), and the mean time was 25.5 months (range 3–116 months). Regarding the relapse site, there was available information for 27/46 (60%) patients [10,15,17,19,20,23,27,29,30,32,38,42,48,50,51]. The most common sites were the liver in 11/27 (40.7%) [10,20,38,40,48,51], lymph nodes in 6/27 (22.2%) [22,30,38,42,51], bone in 5/27 (18.5%) [15,27,32,38,50], lung in 4/27 (14.8%) [38,40,50,51], peritoneum in 5/27 (18.5%) [10,19,27,30,48], and the omentum in 4/27 (14.8%) of the cases [20,27,30].
3.7. Results of Inferential Statistical Analysis
The available data allowed for the performance of inferential statistics and the extraction of possible relations. A possible role of the tumor side (left or right) and the development of ascites was not possible to confirm (p = 0.1165). We furthermore studied all collected data for their role in recurrence, with the results being summarized in Table 3.

Table 3.
Comparison of results between women with recurrence and no recurrence.
Age, tumor size, tumor location (left or right), and the administration of adjuvant therapy did not have any statistically significant impact on subsequent recurrence. CA-125 was higher in women with recurrence (median: 91.7 Q1–Q3: 43–273.4, vs. median: 27 Q1:Q3: 13–184.2, p = 0.1164). When considering CA-125 levels as normal/abnormal (using 35 U/mL as a cut-off the value), the percentage of women who had normal CA-125 levels and still recurred was only 29.63%, while it was 70.37% for women without recurrence. The correlation of CA125 to disease recurrence was marginally significant (p = 0.0522) without enough statistical power to make a definitive statement about it. Moreover, it was observed that in women with recurrence, ascites was more common (38.1% vs. 22.5%, p = 0.1033). Clearly, stage was a decisive factor for recurrence (see Table 3), since 24.4% of the women with stage I had a recurrence, while the percentage was more than 60% for disease at stage II–IV (p = 0.0018).
The tumor side (left, right, or bilateral) had no role in patient survival time (log-rank p = 0.9378; Figure 3 highlights relevant survival curves and the number of women at risk).
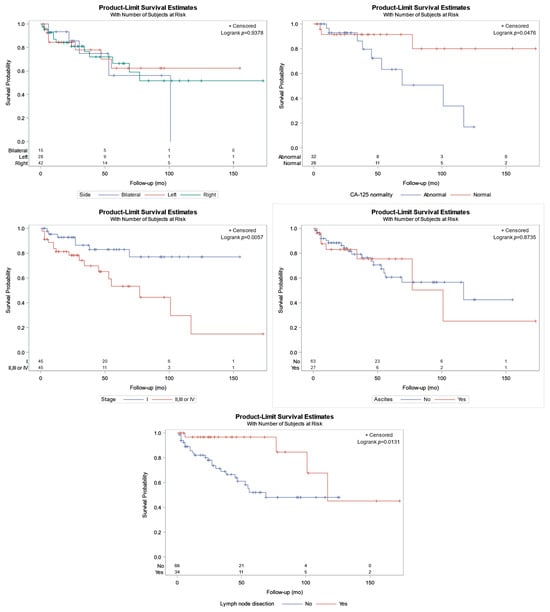
Figure 3.
Kaplan–Meier curves for patient survival in relation to the upper left: bilaterality; upper right: CA-125 characterization (normal/abnormal); middle left: stage (I vs. II, II, or IV); middle right: ascites; bottom: lymph node dissection (previously unpublished original photo).
In contrast, an abnormal CA-125 level was linked to lower survival (Figure 3, p = 0.0476), with a mean survival of 29 months (Q1–Q3: 20–64 months) and 47 months (Q1–Q3: 24–96 months) for abnormal and normal CA-125 status, respectively. Similarly, women with tumors at stage I experienced better survival than women at stages higher than I (Figure 3, p = 0.0057); specifically, the median survival was 53 months (Q1–Q3: 24–94 months) for stage I cases and 39 months (Q1–Q3: 20–78 months) for tumors at stage higher than I, respectively. Furthermore, ascites was not an important factor for lower survival (p = 0.8735). Finally, patients with lymph node dissection (LND), had better survival than patients without LND (p = 0.0131); specifically, the median survival for the 34 women in whom LND was performed was 117 months, and for the women without LND, it was 69 months.
4. Discussion
Ovarian cancer is the fifth most common cause of cancer-related death from gynecological carcinomas [54,55]. Due to their rarity, MBTs comprise only a small fraction of these tumors. To our knowledge, this study is the first to review the literature systematically. In 1988, Austin and Morris first recognized that a subgroup of MBTs lacking a benign Brenner component represented, in fact, high-grade ovarian serous carcinomas with a transitional architectural pattern [56]. To ensure that we did not include such cases, we included, for cases reported before 1988, only invasive tumors associated with a benign and/or borderline Brenner component.
In our study, the mean age of patients presenting with MBT is 59 years. In comparison, a previous study reported the mean age of patients to be 65 years [57]. For other histotypes, the age of presentation ranges from 55 years for mucinous and endometrioid carcinoma, 56 years for clear-cell carcinoma, and 65 years for serous carcinoma [1]. MBTs tend to present at a lower stage compared to serous carcinoma [1]. The symptoms of MBT are similar to those of other epithelial ovarian carcinomas. The most common symptoms reported were abdominal pain, adnexal, abdominal or pelvic mass, abdominal distention, and vaginal bleeding. According to the literature, ascites is present in <10% of MBT cases. Our study reveals a much higher (28.9%) percentage. MBTs have no specific ultrasound or MRI findings [58,59].
The inferential statistical analysis performed in our study showed that disease stage I is associated with a statistically significant lower percentage of disease recurrence compared to stages II-IV. Also, disease recurrence is more commonly related to the presence of ascites and elevated CA-125 levels. Furthermore, the analysis showed a relation between higher CA-125 levels and a stage higher than I with decreased survival. In contrast to the study by Nasioudis et al., our analysis showed that patients treated with LND had a better survival rate [57].
The first step in correctly managing every malignancy is a precise diagnosis. The differential diagnosis of MBT includes high-grade ovarian serous carcinoma with a transitional architectural pattern, primary squamous cell carcinoma (SqCC), SqCC arising in a mature cystic teratoma, endometrioid borderline tumor, endometrioid carcinoma, metastatic SqCC, and metastatic urothelial carcinoma.
High-grade ovarian serous carcinoma with a transitional architectural pattern shows areas of conventional high-grade serous carcinoma with high-grade nuclear atypia, prominent nucleoli, and significant pleomorphism. It lacks a benign Brenner component, and, immunohistochemically, it is positive for WT-1 and estrogen receptors [60].
Primary ovarian SqCC usually shows keratinization and high-grade nuclear features, lacking a benign Brenner component; it may arise from a mature teratoma [61,62]. Endometrioid borderline tumors and endometrioid carcinoma show at least partially endometrioid-type glands and are immunohistochemically positive for ER; they are frequently related to endometriosis.
In the differential diagnosis of metastatic tumors (either SqCC or urothelial carcinoma), knowledge of the previous clinical history is of great importance. Furthermore, metastatic tumors tend to be bilateral, displaying a multinodular growth pattern and lacking a benign Brenner component.
For instance, metastatic SqCC also does not show a papillary architecture. The summary of essential clinical, histologic, and immunohistochemical features for the distinction of the entities mentioned above is shown in Table 4 and Table 5.

Table 4.
Clinical and histologic features of malignant Brenner tumors and their differential diagnoses.

Table 5.
Immunohistochemical features of malignant Brenner tumors and their differential diagnoses.
Concerning the molecular findings in MBTs, the most common are inactivating mutations in the CDKN2A and CDKN2B loci encoding the cyclin-dependent kinase inhibitors p16INK4a and p15INK4b, respectively, followed by activating mutations in FGFR3 and PIK3CA [63]. Notably, the p53 signaling was frequently disrupted in MBTs. The amplification of murine double minute 2 (MDM2)—encoding an E3 ubiquitin ligase that counteracts p53 suppressor activity—was a frequent event [63]. Only a few cases harbored TP53 truncating and missense mutations, which were shown in a mutually exclusive pattern with MDM2 amplification [64]. Interestingly, MDM2 amplification or TP53 mutations were mainly present in FGFR3 wild-type cases [63]. Wang et al., reported amplification of MDM2 and CCND1 (encoding Cyclin D1), and loss of CDKN2A and CDKN2B in one case of MBT [48]. Also, MBTs lack TERT promoter mutations, commonly found in urothelial carcinoma [65,66]. Genomic alterations in genes involved in the homologous recombination deficiency (HRD) pathway were rare; Lin et al., revealed homozygous inactivating mutations only in BAP1 in rare cases [63]. A pathogenic BRCA2 mutation was found in the case presented by Toboni et al. [41]. Overall, it seems that MBT has unique molecular features among gynecological malignancies. In addition, previous data revealed that the FGFR3 and MDM2/P53 pathways, along with CDKN2A/B loss, play a key role in the pathogenesis of MBT. However, as MBT is rare, additional studies are required to shed light on the molecular events driving this entity. A summary of the molecular alterations is presented in Supplementary Table S5.
Surgery is the basis of MBT treatment. The majority of patients in our review were treated with HBSO, with or without omentectomy, appendectomy, and lymph node dissection.
The role of adjuvant chemotherapy has yet to be defined. In early stage disease, the benefit of chemotherapy is not clear. For instance, Gezginc et al., reported that patients of stages IA and IB could be followed up, and Han et al., spared patients of stage IA disease from chemotherapy [45]. It is reasonable, therefore, to discuss with the patient the pros and cons and potentially offer adjuvant chemotherapy to those with stage IC and higher disease due to increased recurrence risk.
Literature shows that most clinicians have been using alkylating agents (such as cisplatin, cyclophosphamide, and melphalan), tumor antibiotics (mitomycin C and doxorubicin), and, importantly, taxanes (mainly docetaxel and paclitaxel) in treating MBT, either in the adjuvant or metastatic setting [2,27,30,36,37,38,39,40,41,42,48,51,52,53]. Since 2012, the combination of platinum with taxane has been gaining rising acceptance among clinicians, and carboplatin with paclitaxel is currently the most used regimen [2,36,38,39,40,41,42,44,45,48,51,52,53,67]. This is in line with the international guidelines, which suggest that patients with high grade histology should be treated with six cycles of carboplatin and paclitaxel chemotherapy.
Importantly, antiangiogenic factors increase the progression free survival of patients with locally advanced and metastatic, high-grade epithelial ovarian cancer; however, patients with MBT were not included in these clinical trials [68,69]. Lang et al., reported clinical benefit with the addition of Bevacizumab in a patient with recurrent MBT [42].
Due to the rarity of the disease, patients with recurrent or metastatic disease should be encouraged to undergo a genetic next-generation sequencing analysis of the tumor. This may shed light on the pathogenesis of this malignancy and allow for a treatment approach tailored to the patient.
Data on the role of radiotherapy are lacking in the literature. Only a few cases are reported, receiving radiotherapy as part of their adjuvant treatment [21,51] and in the case of recurrence [27,42,45,51]. The use of radiotherapy cannot be advocated, particularly in early stage disease; it is reasonable, however, to consider targeted radiotherapy for symptom control.
Besides, the low incidence of this disease does not permit clinicians to carry out randomized clinical trials. Treatment protocols are therefore based on a case-by-case experience. It is therefore highly recommended that these cases be discussed in multidisciplinary team boards and published to accumulate clinical evidence.
5. Conclusions
In the present manuscript, we have collected data presenting a systematic review of MBTs’, presenting their demographic, clinical, pathological, molecular, and treatment characteristics, with a special focus on the differential diagnosis. To our knowledge, this is the first study to systematically review the characteristics of these tumors. More multicentric studies reporting in detail treatment modalities and long-term follow-up are needed to define the optimal management for this rare entity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16061106/s1, Table S1: Clinicopathologic and treatment features of the cases of malignant Brenner tumor; Table S2: Symptoms at presentation; Table S3: Details from tumor staging; Table S4: Details of surgery type; Table S5: Molecular alterations.
Author Contributions
Conceptualization, N.I.K. and A.P. (Andrea Palicelli); methodology, A.Z. and M.G.S.; software, D.D.; validation, I.B., D.D., K.S. and A.K.; formal analysis, M.Z. and C.K.; investigation, D.L. and A.S.; resources, A.Z.; data curation, A.P. (Andrea Palicelli); writing—original draft preparation, N.I.K., A.P. (Abraham Pouliakis), I.S.P. and J.S.; writing—review and editing, all authors; visualization, A.-I.I.; supervision, I.G.P.; project administration, I.G.P.; funding acquisition, A.P. (Andrea Palicelli). All authors have read and agreed to the published version of the manuscript.
Funding
The study was partially supported by the Italian Ministry of Health—Ricerca Corrente Annual Program 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Francesca Sabrina Vinci, Giovanni Mattia and Virginia Dolcini of Grant Office and Research Administration (Azienda USL-IRCCS di Reggio Emilia) for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lax, S.F.; Folkins, A.K.; Palacios, J.; Xue, W.C. Brenner tumor. In Female Genital Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Turgay, B.; Koyuncu, K.; Taşkın, S.; Ortaç, U.F. Features of ovarian Brenner tumors: Experience of a single tertiary center. Turk. J. Obstet. Gynecol. 2017, 14, 133. [Google Scholar] [CrossRef]
- Kuhn, E.; Ayhan, A.; Shih, I.e.M.; Seidman, J.D.; Kurman, R.J. Ovarian Brenner tumour: A morphologic and immunohistochemical analysis suggesting an origin from fallopian tube epithelium. Eur. J. Cancer 2013, 49, 3839–3849. [Google Scholar] [CrossRef]
- Brenner, F. Das oophoroma folliculare. Frankf. Z. Pathol. 1907, 1, 150. [Google Scholar]
- Speert, H. Obstetrical-gynecological eponyms: Fritz Brenner and Brenner tumors of the ovary. Cancer 1956, 9, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R. Über verschiedene Erscheinungsformen der als Typus Brenner bekannten Eierstocksgeschwulst, ihre Absonderung von den Granulosazelltumoren und Zuordnung unter andere Ovarialgeschwülste. Arch. Gynak. 1932, 148, 541–596. [Google Scholar] [CrossRef]
- Von Numers, C. A Contribution to the Case Knowledge and Histology of the Brenner Tumor: Do malignant Forms of the Brenner Tumor also occur? Acta Obstet. Gynecol. Scand. 1945, 25, 114–127. [Google Scholar] [CrossRef]
- St Pierre-Robson, K.; Dunn, P.J.; Cooper, E.; Tofazzal, N.; Hirschowitz, L.; McCluggage, W.G.; Ganesan, R. Three cases of an unusual pattern of invasion in malignant Brenner tumors. Int. J. Gynecol. Pathol. 2013, 32, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mackinlay, C.J. Brenner tumours of the ovary: A report of nine cases including one with malignant degeneration. J. Obstet. Gynaecol. Br. Emp. 1956, 63, 58–67. [Google Scholar] [CrossRef]
- Abell, M.R. Malignant brenner tumors of ovary with report of three cases. Cancer 1957, 10, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Reel, P.J. Malignant brenner tumor of the ovary: With the report of one case. Am. J. Obstet. Gynecol. 1958, 76, 872–876. [Google Scholar] [CrossRef]
- Marshall, C. Malignant ovarian brenner tumour: A case report. Pathology 1970, 2, 169–174. [Google Scholar] [CrossRef]
- Miles, P.A.; Norris, H.J. Proliferative and malignant Brenner tumors of the ovary. Cancer 1972, 30, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Toriumi, J.; Ijima, Y. Malignant Brenner tumor-a case report. Acta Pathol. Jpn. 1973, 23, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.G.; Campbell, G.R. The malignant Brenner tumor. Obstet. Gynecol. 1973, 42, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pratt-Thomas, H.R.; Kreutner, A., Jr.; Underwood, P.B.; Dowdeswell, R.H. Proliferative and malignant Brenner tumors of ovary: Report of two cases, one with Meigs’ syndrome, review of literature, and ultrastructural comparisons. Gynecol. Oncol. 1976, 4, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Raahave, D.; Boiesen, P. A malignant Brenner tumour of the ovary with subcutaneous metastases. Acta Pathol. Microbiol. Scand. A 1977, 85, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Shafeek, M.; Osman, M.; Hussein, M. Malignant Brenner tumor of the ovary. Gynecol. Oncol. 1978, 6, 282–289. [Google Scholar] [CrossRef]
- Chiarelli, S.M.; De Marchi, A.; Piazza, M. Malignant Brenner tumor of the ovary: Case report with a review of the literature. Tumori J. 1978, 4, 597–605. [Google Scholar] [CrossRef]
- Hayden, M.T. Bilateral malignant Brenner tumor: Report of a case with ultrastructural study. Hum. Pathol. 1981, 12, 89–92. [Google Scholar] [CrossRef]
- Magrina, J.F.; Villamaria, F.J.; Masterson, B.J.; Lin, F. Malignant Brenner tumour of the ovary. A case report: Negative second look laparotomy following surgery, radiation and chemotherapy. Int. J. Gynaecol. Obstet. 1982, 20, 155–158. [Google Scholar] [CrossRef]
- Haid, M.; Victor, T.A.; Weldon-Linne, C.M.; Danforth, D.N. Malignant Brenner tumor of the ovary: Electron microscopic study of a case responsive to radiation and chemotherapy. Cancer 1983, 51, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T. Bilateral malignant Brenner tumor of the ovary. J. Surg. Oncol. 1984, 26, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Czernobilsky, B. Ovarian brenner tumors. II. Malignant. Cancer 1985, 56, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Seldenrijk, C.A.; Willig, A.P.; Baak, J.P.; Kühnel, R.; Rao, B.R.; Burger, C.W.; van der Harten, J.J.; Dijkhuizen, G.H.; Meijer, C.J. Malignant Brenner tumor: A histologic, morphometrical, immunohistochemical, and ultrastructural study. Cancer 1986, 58, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Takahashi, K.; Sonobe, H.; Ohtsuki, Y.; Yoshino, T.; Tsutsumi, A.; Taguchi, K.; Fujimori, T. Malignant Brenner tumor with peritoneal metastasis. Acta Pathol. Jpn. 1987, 37, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Hoffmann, K.D. Malignant Brenner tumor of the ovary. J. Surg. Oncol. 1988, 39, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Thirumavalavan, V.; Rufaie, A.; Biedrzycki, T. Malignant Brenner tumour. J. Obstet. Gynaecol. 1992, 12, 342–343. [Google Scholar] [CrossRef]
- Joh, K.; Aizawa, S.; Ohkawa, K.; Dohzono, H.; Aida, S.; Ohgoshi, E. Case report of a malignant Brenner tumor with hyperestrogenism. Pathol. Int. 1995, 45, 75–84. [Google Scholar] [CrossRef]
- Kataoka, A.; Nishida, T.; Imaishi, K.; Sugiyama, T.; Yakushiji, M. Malignant Brenner tumor of the ovary: Two cases of unusual histologic features of relapsed tumors. J. Obstet. Gynaecol. 1995, 21, 249–256. [Google Scholar] [CrossRef]
- Ahr, A.; Arnold, G.; Göhring, U.J.; Costa, S.; Scharl, A.; Gauwerky, J.F. Cytology of Ascitic Fluid in a Patient with Metastasizing Malignant Brenner Tumor of the Ovary. Acta Cytol. 1997, 41, 1299–1304. [Google Scholar] [CrossRef]
- Yamamoto, R.; Fujita, M.; Kuwabara, M.; Sogame, M.; Ebina, Y.; Sakuragi, N.; Kato, H.; Fujimoto, S. Malignant Brenner tumors of the ovary and tumor markers: Case reports. Jpn. J. Clin. Oncol. 1999, 29, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Barragán-Campos, H.M.; Alonso-Juárez, M.; Gamboa-Domínguez, A.; Gutierrez-Manjarréz, F.; Caro-Sánchez, C.; García-Ramos, G. Dural metastases as a presentation of a Brenner tumor. J. Clin. Neurosci. 2010, 17, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Driss, M.; Mrad, K.; Dhouib, R.; Doghri, R.; Abbes, I.; Ben Romdhane, K. Ascitic Fluid Cytology in Malignant Brenner Tumor. A Case Report. Acta Cytol. 2010, 54, 598–600. [Google Scholar] [CrossRef]
- Roth, L.M.; Goheen, M.P.; Broshears, J.R. Malignant Brenner tumor of the ovary with transformation to trabecular carcinoid: An immunocytochemical and electron microscopic study. Int. J. Gynecol. Pathol. 2012, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gezginç, K.; Karatayli, R.; Yazici, F.; Acar, A.; Celik, C.; Capar, M.; Tavli, L. Malignant Brenner tumor of the ovary: Analysis of 13 cases. Int. J. Clin. Oncol. 2012, 17, 324–329. [Google Scholar] [CrossRef]
- Verma, A.; Chander, B.; Verma, S.; Soni, A. Malignant brenner tumor of ovary. J. Obstet. Gynecol. India 2014, 64, 148–149. [Google Scholar] [CrossRef][Green Version]
- Han, J.H.; Kim, D.Y.; Lee, S.W.; Park, J.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Intensive systemic chemotherapy is effective against recurrent malignant Brenner tumor of the ovary: An analysis of 10 cases within a single center. Taiwan J. Obstet. Gynecol. 2015, 54, 178–182. [Google Scholar] [CrossRef]
- Di Donato, V.; Donfrancesco, C.; Bracchi, C.; Marchetti, C.; Schiavi, M.; Colagiovanni, V.; Monti, M.; Bogani, G.; Maturo, A.; Panici, P.B. Brain metastases from malignant Brenner tumor of the ovary: A case report and a literature review. G. Ital. Ostet. Ginecol. 2016, 38, 339–344. [Google Scholar] [CrossRef]
- Yue, Z.; Si, T.; Pan, Z.; Cao, W.; Yan, Z.; Jiang, Z.; Ouyang, H. Malignant Brenner tumor of the ovary: Clinical, pathological and demographic analyses of 10 cases. Int. J. Clin. Exp. Pathol. 2016, 9, 5642–5646. [Google Scholar]
- Toboni, M.D.; Smith, H.J.; Dilley, S.E.; Novak, L.; Leath, C.A. Malignant Brenner tumor associated with a germline BRCA2 mutation. Gynecol. Oncol. Rep. 2017, 21, 17. [Google Scholar] [CrossRef]
- Lang, S.M.; Mills, A.M.; Cantrell, L.A. Malignant Brenner tumor of the ovary: Review and case report. Gynecol. Oncol. Rep. 2017, 22, 26–31. [Google Scholar] [CrossRef]
- King, L.; Gogoi, R.P.; Hummel, C.; Smith, A. Malignant Brenner tumor: Two case reports. Case Rep. Womens Health 2018, 20, e00082. [Google Scholar] [CrossRef] [PubMed]
- Agius, M.P.; Collict, M.; Custo, R.C.; Milic, M.; Dingli, M.; Scerri, A. Brenner Tumour. The Rare Malignant variant. Malta Med. J. 2018, 30, 57–59. [Google Scholar]
- Zhang, Y.; Staley, S.A.; Tucker, K.; Clark, L.H. Malignant Brenner tumor of the ovary: Case series and review of treatment strategies. Gynecol. Oncol. Rep. 2019, 28, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Toshniwal, S.M.; Inamdar, S.A.; Agarwal, H.D.; Sharma, S.R. Malignant Brenner Tumor-A rare case of ovarian carcinoma. Med. Sci. 2020, 24, 3550–3554. [Google Scholar]
- Singh, B.K.; Saha, S.; Agarwal, S.; Rathore, Y.S. Malignant Brenner tumour of the ovary manifesting as distal intestinal obstruction and perforation. BMJ Case Rep. 2020, 13, e235394. [Google Scholar] [CrossRef] [PubMed]
- Bouhani, M.; Slimane, M.; Sghaier, S.; Bouida, A.; Chargui, R.; Rahal, K. Malignant Brenner tumor of the ovary: One single institute experience and a review of the literature. Anatol. J. Fam. Med. 2020, 3, 71–75. [Google Scholar] [CrossRef]
- Wang, L.; Allison, D.; Shukla, P.S. Amplification of MDM2 and Loss of p16 expression: Do they have a role in malignant transformation of ovarian brenner tumor? A morphologic and immunohistochemical study. Am. J. Clin. Pathol. 2020, 154, 133–141. [Google Scholar] [CrossRef]
- McGinn, B.; Lemaire, A.S.; McCluggage, W.G. Ovarian Low-grade Basaloid Carcinoma Arising in Brenner Tumor: A New Variant of Malignant Brenner Tumor. Int. J. Gynecol. Pathol. 2022, 41, 276–284. [Google Scholar] [CrossRef]
- Yüksel, D.; Kılıç, Ç.; Çakır, C.; Cömert, G.K.; Turan, T.; Ünlübilgin, E.; Boran, N.; Kayıkçıoğlu, F.; Koç, S. Brenner tumors of the ovary: Clinical features and outcomes in a single-center cohort. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 22. [Google Scholar] [CrossRef]
- Zou, C.; Li, Q.; Zhao, J.; Chen, Y. Coexistence of malignant ovarian Brenner tumor and borderline mucinous cystadenoma, combined with primary uterine corpus endometrioid carcinoma: A case report and literature review. Oncol. Lett. 2022, 24, 272. [Google Scholar] [CrossRef] [PubMed]
- Kurniadi, A.; Anfasa, M.K.; Agustina, H.; Dewayani, B.M.; Kireina, J. A Rare Case of Ruptured Malignant Ovarian Brenner Tumor. Am. J. Case Rep. 2023, 24, e938680-1. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Pouliakis, A.; Boutas, I.; Samaras, M.G.; Kontogeorgi, A.; Dimas, D.; Sitara, K.; Zacharatou, A.; Zanelli, M.; Palicelli, A. Axillary Lymph Node Metastasis from Ovarian Carcinoma: A Systematic Review of the Literature. J. Pers. Med. 2023, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.M.; Norris, H.J. Malignant Brenner tumor and transitional cell carcinoma of the ovary: A comparison. Int. J. Gynecol. Pathol. 1987, 6, 29–39. [Google Scholar] [CrossRef]
- Nasioudis, D.; Sisti, G.; Holcomb, K.; Kanninen, T.; Witkin, S.S. Malignant Brenner tumors of the ovary; a population-based analysis. Gynecol. Oncol. 2016, 142, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Matsuzaki, K.; Sano, N.; Furumoto, H.; Nishitani, H. Malignant Brenner tumor with transition from benign to malignant components: Computed tomographic and magnetic resonance imaging findings with pathological correlation. J. Comput. Assist. Tomogr. 2008, 32, 553–554. [Google Scholar] [CrossRef]
- Dierickx, I.; Valentin, L.; Van Holsbeke, C.; Jacomen, G.; Lissoni, A.A.; Licameli, A.; Testa, A.; Bourne, T.; Timmerman, D. Imaging in gynecological disease (7): Clinical and ultrasound features of Brenner tumors of the ovary. Ultrasound Obstet. Gynecol. 2012, 40, 706–713. [Google Scholar] [CrossRef]
- Ali, R.H.; Seidman, J.D.; Luk, M.; Kalloger, S.; Gilks, C.B. Transitional cell carcinoma of the ovary is related to high-grade serous carcinoma and is distinct from malignant brenner tumor. Int. J. Gynecol. Pathol. 2012, 31, 499–506. [Google Scholar] [CrossRef]
- Goudeli, C.; Varytimiadi, A.; Koufopoulos, N.; Syrios, J.; Terzakis, E. An ovarian mature cystic teratoma evolving in squamous cell carcinoma: A case report and review of the literature. Gynecol. Oncol. Rep. 2017, 19, 27–30. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Nasi, D.; Goudeli, C.; Antoniadou, F.; Kokkali, S.; Pigadioti, E.; Provatas, I.; Maggo, E.; Ardavanis, A.; Terzakis, E.; et al. Primary squamous cell carcinoma of the ovary. Review of the literature. J. BUON 2019, 24, 1776–1784. [Google Scholar]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Lin, D.I.; Killian, J.K.; Venstrom, J.M.; Ramkissoon, S.H.; Ross, J.S.; Elvin, J.A. Recurrent urothelial carcinoma-like FGFR3 genomic alterations in malignant Brenner tumors of the ovary. Mod. Pathol. 2021, 34, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, N.; Darb-Esfahani, S.; Leichsenring, J.; Taube, E.; Boxberg, M.; Braicu, I.; Jesinghaus, M.; Penzel, R.; Endris, V.; Noske, A.; et al. Mutational profiles of Brenner tumors show distinctive features uncoupling urothelial carcinomas and ovarian carcinoma with transitional cell histology. Genes Chromosomes Cancer 2017, 56, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Khani, F.; Diolombi, M.L.; Khattar, P.; Huang, W.; Fallon, J.T.; Epstein, J.I.; Zhong, M. Benign and malignant Brenner tumors show an absence of TERT promoter mutations that are commonly present in urothelial carcinoma. Am. J. Surg. Pathol. 2016, 40, 1291–1295. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Ellen M Hartenbach, E.M.; Baergen, R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).