Feasibility of Synchrotron-Based Ultra-High Dose Rate (UHDR) Proton Irradiation with Pencil Beam Scanning for FLASH Research
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Modification to Existing Beam Delivery and Control System
2.2. Pulse Structure Measurement of the Synchrotron Spill in UHDR Mode
2.3. Measurement of Beam Characteristics in UHDR Mode
2.4. Measurement of Dose and Dose Profile in UHDR Mode
3. Results
3.1. Pulse Structure and Beam Fluence
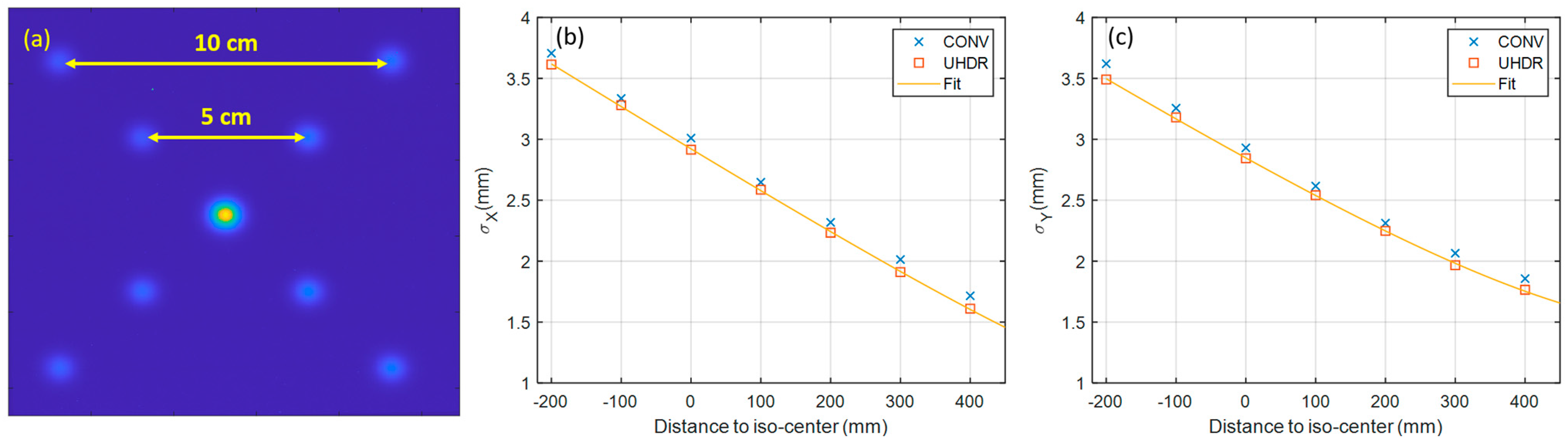
3.2. Beam Characteristics
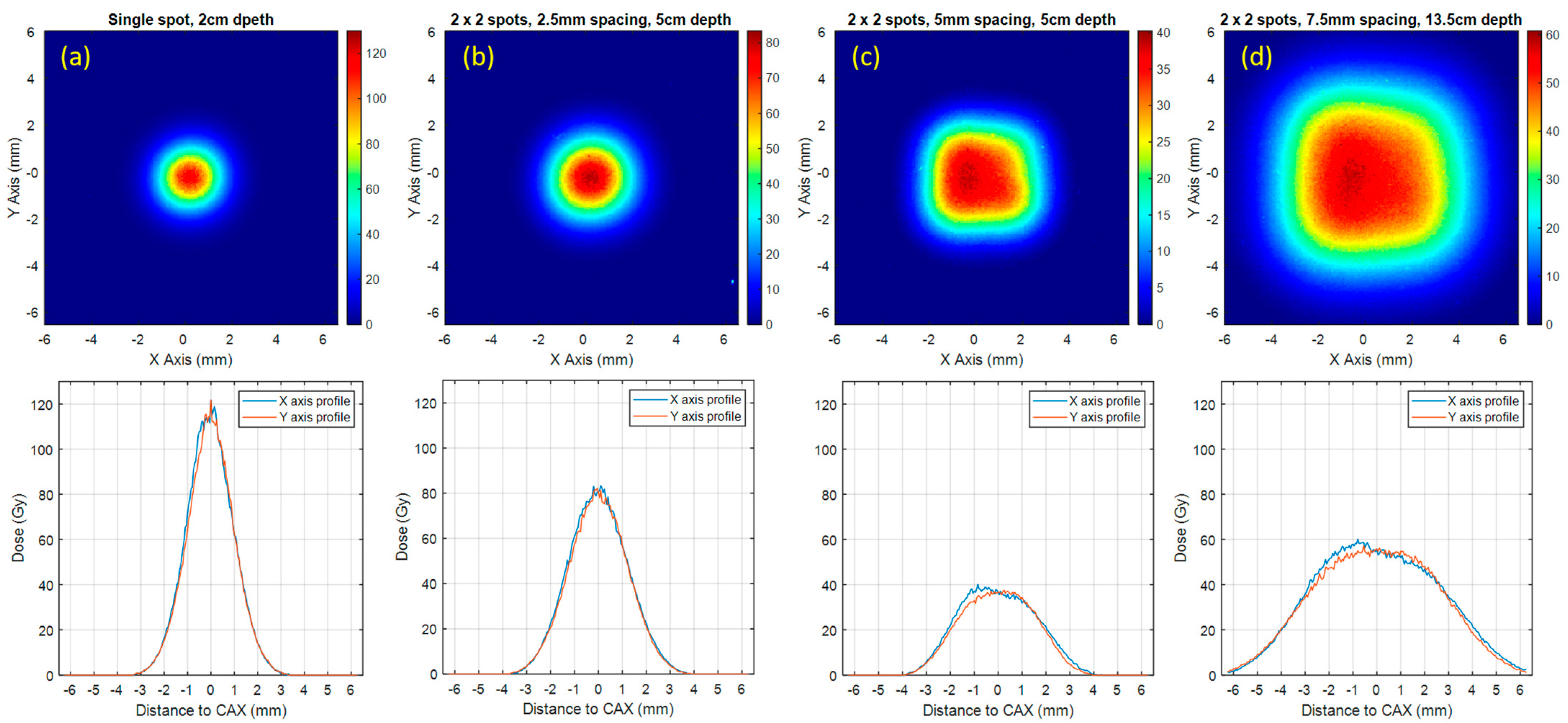
3.3. Film Dosimetry Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Sforza, D.; Wong, J.W.; Gabrielson, K.; Aziz, K.; Mahesh, M.; Coulter, J.B.; Siddiqui, I.; Tran, P.T.; Viswanathan, A.N.; et al. FLASH Effects Induced by Orthovoltage X-rays. Int. J. Radiat. Oncol. 2023, 117, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother. Oncol. 2022, 166, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xie, D.; Yang, Y.; Huang, S.; Gao, X.; Peng, Y.; Wang, B.; Wang, J.; Xiao, D.; Wu, D.; et al. Radioprotective effect of X-ray abdominal FLASH irradiation: Adaptation to oxidative damage and inflammatory response may be benefiting factors. Med. Phys. 2022, 49, 4812–4822. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin. Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef]
- Levy, K.; Natarajan, S.; Wang, J.; Chow, S.; Eggold, J.T.; Loo, P.E.; Manjappa, R.; Melemenidis, S.; Lartey, F.M.; Schüler, E.; et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci. Rep. 2020, 10, 21600. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Rohrer Bley, C.; Wolf, F.; Gonçalves Jorge, P.; Grilj, V.; Petridis, I.; Petit, B.; Böhlen, T.T.; Moeckli, R.; Limoli, C.; Bourhis, J.; et al. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clin. Cancer Res. 2022, 28, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cascio, E.; Li, C.; Yang, Q.; Gerweck, L.E.; Huang, P.; Gottschalk, B.; Flanz, J.; Schuemann, J. FLASH Investigations Using Protons: Design of Delivery System, Preclinical Setup and Confirmation of FLASH Effect with Protons in Animal Systems. Radiat. Res. 2020, 194, 656–664. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef] [PubMed]
- Singers Sørensen, B.; Krzysztof Sitarz, M.; Ankjærgaard, C.; Johansen, J.; Andersen, C.E.; Kanouta, E.; Overgaard, C.; Grau, C.; Poulsen, P. In vivo validation and tissue sparing factor for acute damage of pencil beam scanning proton FLASH. Radiother. Oncol. 2022, 167, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Sitarz, M.K.; Ankjærgaard, C.; Johansen, J.G.; Andersen, C.E.; Kanouta, E.; Grau, C.; Poulsen, P. Pencil beam scanning proton FLASH maintains tumor control while normal tissue damage is reduced in a mouse model. Radiother. Oncol. 2022, 175, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Penn Vet|Clinical Trial Detail-Evaluation of FLASH Proton RT in Naturally Occurring Canine Head and Neck Cancer. Available online: https://www.vet.upenn.edu/research/clinical-trials-vcic/all-clinical-trials/clinical-trial/evaluation-of-flash-proton-rt-in-naturally-occurring-canine-head-and-neck-cancer (accessed on 13 September 2023).
- FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases in the Thorax. Clinical Trial Registration NCT05524064. March 2023. Available online: https://clinicaltrials.gov/study/NCT05524064 (accessed on 12 September 2023).
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Patriarca, A.; Fouillade, C.; Auger, M.; Martin, F.; Pouzoulet, F.; Nauraye, C.; Heinrich, S.; Favaudon, V.; Meyroneinc, S.; Dendale, R.; et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. Int. J. Radiat. Oncol. 2018, 102, 619–626. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, M.; Chen, C.-C.; Hu, L.; Yu, F.; Tsai, P.; Huang, S.; Liu, J.; Turner, R.; Shen, B.; et al. Commissioning a 250 MeV research beamline for proton FLASH radiotherapy preclinical experiments. Med. Phys. 2023, 50, 4623–4636. [Google Scholar] [CrossRef]
- Charyyev, S.; Chang, C.-W.; Zhu, M.; Lin, L.; Langen, K.; Dhabaan, A. Characterization of 250 MeV Protons from the Varian ProBeam PBS System for FLASH Radiation Therapy. Int. J. Part. Ther. 2023, 9, 279–289. [Google Scholar] [CrossRef]
- Darafsheh, A.; Hao, Y.; Zwart, T.; Wagner, M.; Catanzano, D.; Williamson, J.F.; Knutson, N.; Sun, B.; Mutic, S.; Zhao, T. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med. Phys. 2020, 47, 4348–4355. [Google Scholar] [CrossRef]
- Darafsheh, A.; Hao, Y.; Zhao, X.; Zwart, T.; Wagner, M.; Evans, T.; Reynoso, F.; Zhao, T. Spread-out Bragg peak proton FLASH irradiation using a clinical synchrocyclotron: Proof of concept and ion chamber characterization. Med. Phys. 2021, 48, 4472–4484. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, X.; Guan, F.; Titt, U.; Iga, K.; Jiang, D.; Takaoka, T.; Tootake, S.; Katayose, T.; Umezawa, M.; et al. Adaptation and dosimetric commissioning of a synchrotron-based proton beamline for FLASH experiments. Phys. Med. Biol. 2022, 67, 165002. [Google Scholar] [CrossRef] [PubMed]
- Titt, U.; Yang, M.; Wang, X.; Iga, K.; Fredette, N.; Schueler, E.; Lin, S.H.; Zhu, X.R.; Sahoo, N.; Koong, A.C.; et al. Design and validation of a synchrotron proton beam line for FLASH radiotherapy preclinical research experiments. Med. Phys. 2022, 49, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Toshito, T.; Omachi, C.; Umezawa, M.; Shinozawa, Y.; Yamada, M.; Nakajima, K.; Nomura, K.; Ogino, H.; Shibamoto, Y. Scanning Proton FLASH Irradiation Using a Synchrotron Accelerator: Effects on Cultured Cells and Differences by Irradiation Positions. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e522. [Google Scholar] [CrossRef]
- Zou, W.; Diffenderfer, E.S.; Ota, K.; Boisseau, P.; Kim, M.M.; Cai, Y.; Avery, S.M.; Carlson, D.J.; Wiersma, R.D.; Lin, A.; et al. Characterization of a high-resolution 2D transmission ion chamber for independent validation of proton pencil beam scanning of conventional and FLASH dose delivery. Med. Phys. 2021, 48, 3948–3957. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, M.; Petersson, K.; Buchillier, T.; Germond, J.-F.; Durán, M.T.; Vozenin, M.-C.; Bourhis, J.; Bochud, F.O.; Bailat, C. High dose-per-pulse electron beam dosimetry: Usability and dose-rate independence of EBT3 Gafchromic films. Med. Phys. 2017, 44, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wang, X.; Yang, M.; Draeger, E.; Han, D.; Iga, K.; Guo, F.; Perles, L.; Li, Y.; Sahoo, N.; et al. Dosimetric response of GafchromicTM EBT-XD film to therapeutic protons. Precis. Radiat. Oncol. 2023, 7, 15–26. [Google Scholar] [CrossRef]
- Villoing, D.; Koumeir, C.; Bongrand, A.; Guertin, A.; Haddad, F.; Métivier, V.; Poirier, F.; Potiron, V.; Servagent, N.; Supiot, S.; et al. Technical note: Proton beam dosimetry at ultra-high dose rates (FLASH): Evaluation of GAFchromicTM (EBT3, EBT-XD) and OrthoChromic (OC-1) film performances. Med. Phys. 2022, 49, 2732–2745. [Google Scholar] [CrossRef]
- Lewis, D.; Micke, A.; Yu, X.; Chan, M.F. An efficient protocol for radiochromic film dosimetry combining calibration and measurement in a single scan. Med. Phys. 2012, 39, 6339–6350. [Google Scholar] [CrossRef]
- Folkerts, M.M.; Abel, E.; Busold, S.; Perez, J.R.; Krishnamurthi, V.; Ling, C.C. A framework for defining FLASH dose rate for pencil beam scanning. Med. Phys. 2020, 47, 6396–6404. [Google Scholar] [CrossRef]
- Yin, L.; Zou, W.; Kim, M.M.; Avery, S.M.; Wiersma, R.D.; Teo, B.-K.K.; Dong, L.; Diffenderfer, E.S. Evaluation of Two-Voltage and Three-Voltage Linear Methods for Deriving Ion Recombination Correction Factors in Proton FLASH Irradiation. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 263–270. [Google Scholar] [CrossRef]

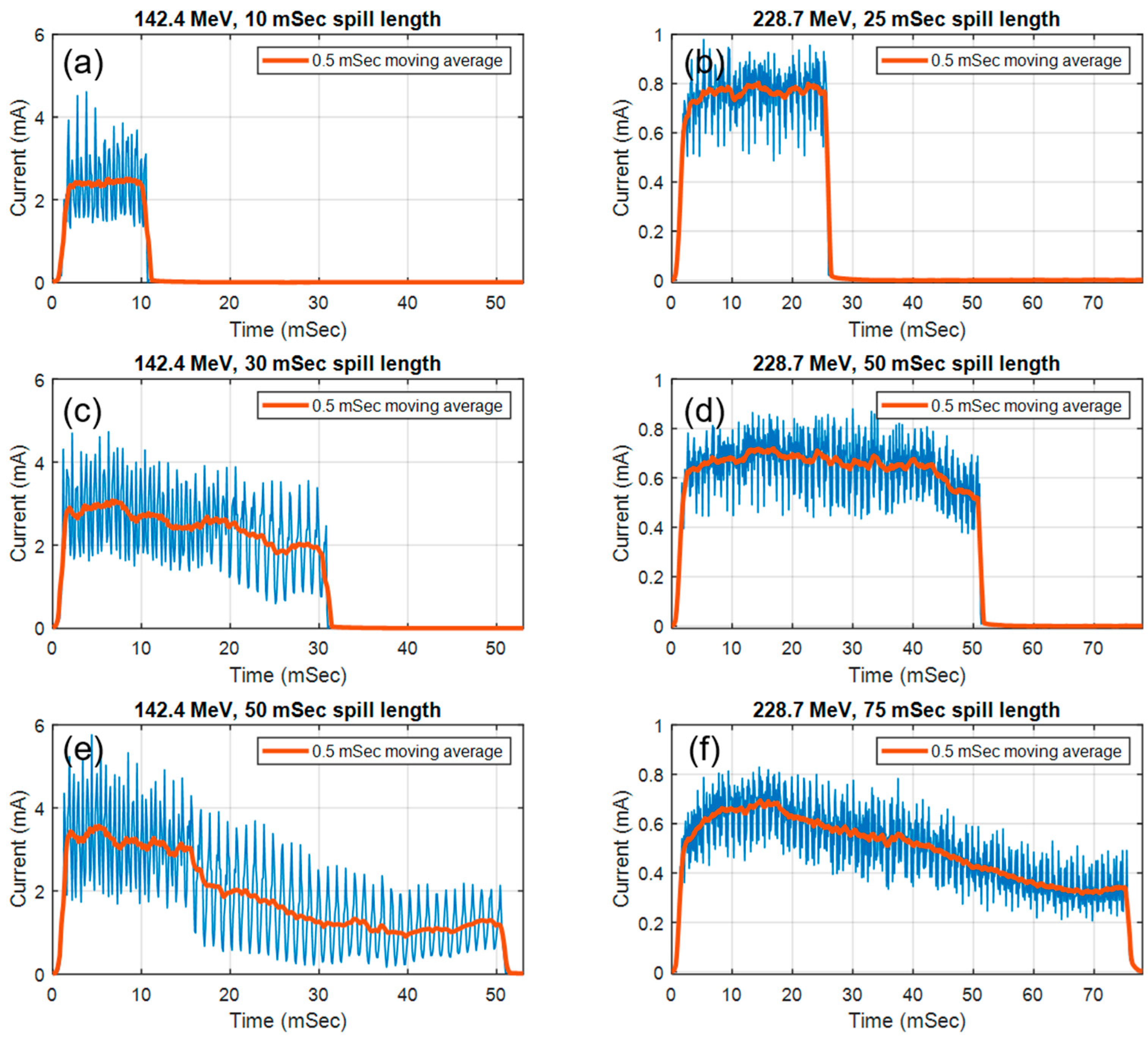


| Spill Length | 10 ms | 20 ms | 30 ms | 40 ms | 50 ms | |
|---|---|---|---|---|---|---|
| Charge per spill (nC) | Mean | 1.43 | 2.76 | 3.91 | 4.50 | 4.96 |
| Standard deviation | 0.14 | 0.27 | 0.16 | 0.10 | 0.10 | |
| Average synchrotron current (nA) | 143.20 | 138.15 | 130.33 | 112.50 | 99.20 | |
| Spill Length | 9 ms | 15 ms | 45 ms | 75 ms | |
|---|---|---|---|---|---|
| Charge per spill (nC) | Mean | 0.49 | 0.73 | 1.93 | 3.08 |
| Standard deviation | 0.07 | 0.03 | 0.17 | 0.28 | |
| Average synchrotron current (nA) | 143.20 | 54.44 | 48.67 | 41.07 | |
| Spot Pattern | Single Spot | 2 × 2 Spots with 2.5 mm Spot Spacing | 2 × 2 Spots with 5.0 mm Spot Spacing | 2 × 2 Spots with 7.5 mm Spot Spacing |
|---|---|---|---|---|
| Measurement Depth (cm) | 2 | 5 | 5 | 13.5 |
| D100% (Gy, Mean ± SD) | 121.8 ± 5.6 | 81.2 ± 3.8 | 36.3 ± 1.7 | 56.2 ± 2.6 |
| Field size at D90% (mm) | 0.6 | 1.1 | 2.3 | 3.7 |
| Field size at D50% (FWHM, mm) | 2.1 | 2.9 | 4.2 | 6.9 |
| Dose rate (Gy/s) | 2436.2 | 1624.1 | 726.3 | 1124.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Masumi, U.; Ota, K.; Sforza, D.M.; Miles, D.; Rezaee, M.; Wong, J.W.; Jia, X.; Li, H. Feasibility of Synchrotron-Based Ultra-High Dose Rate (UHDR) Proton Irradiation with Pencil Beam Scanning for FLASH Research. Cancers 2024, 16, 221. https://doi.org/10.3390/cancers16010221
Yin L, Masumi U, Ota K, Sforza DM, Miles D, Rezaee M, Wong JW, Jia X, Li H. Feasibility of Synchrotron-Based Ultra-High Dose Rate (UHDR) Proton Irradiation with Pencil Beam Scanning for FLASH Research. Cancers. 2024; 16(1):221. https://doi.org/10.3390/cancers16010221
Chicago/Turabian StyleYin, Lingshu, Umezawa Masumi, Kan Ota, Daniel M. Sforza, Devin Miles, Mohammad Rezaee, John W. Wong, Xun Jia, and Heng Li. 2024. "Feasibility of Synchrotron-Based Ultra-High Dose Rate (UHDR) Proton Irradiation with Pencil Beam Scanning for FLASH Research" Cancers 16, no. 1: 221. https://doi.org/10.3390/cancers16010221
APA StyleYin, L., Masumi, U., Ota, K., Sforza, D. M., Miles, D., Rezaee, M., Wong, J. W., Jia, X., & Li, H. (2024). Feasibility of Synchrotron-Based Ultra-High Dose Rate (UHDR) Proton Irradiation with Pencil Beam Scanning for FLASH Research. Cancers, 16(1), 221. https://doi.org/10.3390/cancers16010221






