Simple Summary
Malignant pleural mesothelioma is an invasive and drug-resistant tumor related to asbestos exposure, with limited therapy options. It is associated with an unfavorable prognosis and a 5-year survival rate of only 12%. Current standard-of-care treatment based on platinum-pemetrexed chemotherapy has been in place for the past two decades, though survival is increased by just a few months. In this article, we aim to review the current chemotherapy and immunotherapy options for this malignancy and highlight recent developments with regard to chemoimmunotherapy, targeted agents and cellular therapy.
Abstract
The incidence of malignant pleural mesothelioma is expected to increase globally. New treatment options for this malignancy are eagerly awaited to improve the survival and quality of life of patients. The present article highlights the results of recent advances in this field, analyzing data from several relevant trials. The heterogeneous tumor microenvironment and biology, together with the low mutational burden, pose a challenge for treating such tumors. So far, no single biomarker has been soundly correlated with targeted therapy development; thus, combination strategies are often required to improve outcomes. Locally applied vaccines, the expansion of genetically engineered immune cell populations such as T cells, the blockage of immune checkpoints that inhibit anti-tumorigenic responses and chemoimmunotherapy are among the most promising options expected to change the mesothelioma treatment landscape.
Keywords:
mesothelioma; pleura; asbestos; chemotherapy; immunotherapy; targeted agents; cellular therapy 1. Introduction
Malignant pleural mesothelioma (MPM) is a rare malignancy of the pleural lining highly associated with asbestos exposure (over 80% of the cases) [1]. In spite of its well-established relation with this mineral fiber, the risk of developing the malignancy is only 5% in high-risk populations [2]. The prevalence is higher in men due to occupational exposure, and the median age of diagnosis is 71 years [1]. This aggressive type of tumor undergoes a long latency period (10–40 years), is mostly diagnosed at late stages and has a poor prognosis and a median survival time of one year [3].
The implementation of rigorous regulations in developed countries during the 1990s led to a decrease of over 75% in asbestos usage across various industries. This regulatory approach stands as the foremost strategy for reducing the incidence of MPM within these developed nations. On the other hand, major developing economies have embraced less stringent regulations concerning asbestos, allowing for its ongoing widespread use. This disparity raises concerns about the potential for a global increase in MPM cases [4]. Even within developed nations, the potential for a sustained or increased incidence of MPM is present, primarily stemming from a demographic shift towards an aging population. While asbestos exposure continues to be the predominant risk factor, there are a few other etiologies that may be associated with mesothelioma: exposure to other mineral fibers (erionite, fluoroedenite and balangeroite), radiation, chronic pleural inflammation and germline mutations [5].
A better understanding of MPM’s molecular biology may provide a benchmark for the development of more efficient treatments. The best outcomes so far for patients with mesothelioma have been reported in those who have received multimodal therapy, which usually includes a combination of chemotherapy, surgery and radiation treatment. However, few patients are candidates for this type of approach, and systemic therapy alone is what is feasible to most. Expanding our knowledge with the use of next-generation sequencing and computational technology may further elucidate important pathologic and genomic aspects of the disease, which add to the histologic subtype, and other biomarkers are fundamental to personalizing and determining the optimal treatment [2,6].
MPM’s increasing incidence, aligned with the population aging, high lethality and only modest treatment advances in the past decade, prove to be an unmet medical need. This review highlights the recent advances in the treatment of this aggressive tumor, such as chemoimmunotherapy, and brings to light other promising strategies with the use of targeted agents and cellular therapies.
2. Molecular Biology, Genomics and Immunology
MPM can be histologically classified as an epithelioid, which accounts for the majority of cases (around 50%) and has a better prognosis when compared to the sarcomatoid (10% of cases), a more rare, invasive and resistant tumor subtype. The remaining 40% correspond to the biphasic subtype, a mosaic of the previous two [7,8]. However, intra-tumor biology is heterogeneous, and a thorough description of the pathologic specimen may have important prognostic implications. A recent multi-omic study, integrating epigenetic and transcriptomic data, proposed a new method of classification that takes into account not only the histological aspects of MPM but also its microenvironment and inter- and intra-tumor variability. The resulting classification ranks the tumor in a scale from epithelioid to sarcomatoid, creating a continuum of these two populations [9]. A further understanding of tumor biology may also provide novel predictive biomarkers to better inform therapeutic options and clinical trial design, especially in the field of targeted therapies and immunotherapies [9].
Genomic alterations in MPM are primarily related to a loss of function of tumor suppressor genes. BAP1 is the most frequently reported, and others include NF2, CDKN2A, TP53, LATS2 and SETD2 [6,10]. Mesothelioma has a lower tumor mutational burden than most solid tumors, and hence, other predictive immunotherapy biomarkers are warranted [6].
The tumor’s microenvironment, constituting endothelial, stroma and immune cells, has drawn attention as a possible driver of disease, influencing tumor progression, and, therefore, has been under extensive scrutiny for possible therapeutic targets [11]. Some of the immune infiltrating tumor cells are known to be anti-tumorigenic, while others favor tumor growth by dampening the immune response [12]. As an example, lymphocytes (cytotoxic and T helper cells), dendritic cells and natural killer cells are anti-tumorigenic, while myeloid-derived suppressor cells and regulatory T cells are pro-tumorigenic; macrophages and neutrophils have a variable role and can be related to both pro- and anti-tumor activity [13,14]. The mesothelioma microenvironment is subject to the influence of asbestos fiber exposure, which has been linked to the development of an immunosuppressive profile [11]. New therapies should employ a combination of strategies, including immunotherapies that could both inhibit and stimulate specific immune cells of the MPM microenvironment [15].
Pre-clinical models have increasingly played an important role for better understanding MPM’s development and for the investigation of newer interventions and drug testing. However, cell lines, either 2D or 3D spheroids, suffer from similar limitations in which the replication of the true tumor microenvironment and tumor heterogeneity are hard to accomplish. Animal models are helpful in understanding tumorigenesis with specific gene knockouts or asbestos-infused murine pleura, but limitations include the preferential development of aggressive sarcomatoid models in the former and one more similar to human histology in the latter, although unviable for drug testing because of its long latency period [16]. The most promising innovation for the acceleration of drug development in cancer precision medicine may rely on organoids, which have been shown to be applicable in the prediction of cisplatin sensitivity in mesothelioma models [17].
3. Current Treatments
The current treatment landscape for MPM emphasizes its palliative intent. The 5-year overall survival estimates are 5–12% at best [18]. Supportive care strategies must encompass pleural effusion and pain management. Surgery is indicated for a small fraction of patients with mesothelioma due to its complexity and high morbidity rate, even in those with a favorable performance status and tumor characteristics [19]. Prior to surgery, pleuroscopy may be needed to elucidate the necessity and feasibility of surgery. Even for patients who are candidates for surgery, the randomized phase 3 trial MARS2 did not show an advantage for patients who were operated on versus those who underwent chemotherapy alone, though some patients can still be individually benefited [20]. Most patients are candidates for systemic treatment, which can improve the survival and quality of life. Palliative radiation can be judiciously used at the physician’s discretion.
3.1. Chemotherapy and the Vascular Endothelial Growth Factor Receptor (VEGFR) Pathway
The established treatment for MPM is platinum-antifolate chemotherapy, with a combination of pemetrexed and cisplatin, or carboplatin for patients who cannot tolerate cisplatin. This treatment strategy was established in 2003 in the EMPHACIS trial, which showed an increased median overall survival by 2–3 months when compared to that of cisplatin alone [21,22,23]. Since then, a long gap of treatment approvals was initiated until more recent advances with immunotherapy. An exception occurred with the possible addition of bevacizumab, an anti-VEGF monoclonal antibody, to the treatment regimen. The Mesothelioma Avastin plus Pemetrexed-Cisplatin Study (MAPS) tested a combination of bevacizumab to the present standard of care (pemetrexed and cisplatin) compared to chemotherapy alone, showing an improved median overall survival by 2.8 months and a possible benefit in pain control [24]. Despite the improvement in the quality of life and survival, this treatment regimen has not been filed for an FDA-license [7]. As a second-line regimen, several agents have been tested, with limited activity [25,26,27]. Notably, gemcitabine combined with ramucirumab, an anti-VEGFR-2 monoclonal antibody, improved overall survival compared to gemcitabine alone in a randomized phase 2 trial (RAMES), emphasizing the role of VEGF pathway blockage [28].
3.2. Immunotherapy
Several immune checkpoint inhibitors (ICI) are under investigation as potential treatments for mesothelioma. Nivolumab, a programmed cell death 1 (PD-1) inhibitor, was evaluated in the CONFIRM phase III trial, in which patients with refractory disease following platinum-based doublet chemotherapy were given nivolumab or placebo [29]. Patients in the experimental arm showed longer progression-free survival and overall survival with the use of nivolumab. The benefit seemed to be driven by those with tumor-expressing PD-L1, which was more common in non-epithelioid tumors.
Another strategy is to target two immune checkpoints with the association of an anti- PD-1 antibody and anti-cytotoxic T-lymphocyte protein 4 (CTLA-4) antibody. MAPS2 was a non-comparative, randomized phase 2 trial testing the efficacy of nivolumab alone and nivolumab-ipilimumab (anti CTLA-4 antibody) regimens in patients with relapsed disease. The trial showed numerically similar disease control rates, with 44% with nivolumab and 50% with nivolumab-ipilimumab progression-free at 12 weeks [30]. The phase III Checkmate 743 study randomized 600 patients to either cisplatin-pemetrexed or nivolumab-ipilimumab as a first-line treatment [31]. The nivolumab plus ipilimumab regimen had a median OS of 18.1 months compared to 14.1 months of the chemotherapy regimen (HR: 0.73; 95% CI: 0.61–0.87). At three years, the progression-free survival rates were 14% versus 1%. The duration of response at the three-year mark also favored nivolumab plus ipilimumab versus nivolumab alone, 28% versus 0%, respectively. At four years, the OS rates were 17% versus 11% [32]. The results indicated that nivolumab plus ipilimumab should be the standard-of-care treatment for unresectable MPM based on the evidence of a longer survival benefit over chemotherapy regardless of tumor histology, though the benefit was particularly more pronounced in the sarcomatoid subtype. The outcomes of this trial for unresectable MPM led the FDA to approve the use of nivolumab/ipilimumab as a first-line treatment.
Another potential treatment uses pembrolizumab, an anti-PD-1 antibody [33], which has been tested in the KEYNOTE-028 phase I trial in patients with MPM. As an early phase trial, it presented promising results; however, the PROMISE-meso phase 3 trial was negative in the second-line setting [33,34].
Table 1 summarizes the trials with relevant results related to the current practice.

Table 1.
Relevant clinical trials on current MPM treatment.
4. Novel Treatments for MPM
4.1. Chemoimmunotherapy
A phase II, multicenter, single-arm trial (DREAM), conducted with 54 previously untreated patients, evaluated the combination of durvalumab (anti-PD-L1 antibody) and chemotherapy with cisplatin and pemetrexed for advanced MPM [35]. The results were promising, with 57% of patients alive and progression-free at 6 months.
The IND227 phase 3 trial tested the combination of pembrolizumab with pemetrexed-platinum chemotherapy to chemotherapy alone, showing a statistically significant OS benefit with the combination (HR: 0.79; 95% CI: 0.64–0.98; p = 0.0324), with a median OS of 17.3 months compared to 16.1 months in the control arm. The three-year survival rates were also higher in the experimental arm (25% against 17%). The study showed that the addition of pembrolizumab to platinum-pemetrexed improves the overall response rate from 38% to 62%, with no new safety concerns. Exploratory analysis indicates again that non-epithelioid histologies may benefit the most from the addition of immunotherapy to the treatment regimen [36,37].
The Bevacizumab and Atezolizumab in Malignant Pleural Mesothelioma (BEAT-meso), a randomized phase III trial, is assessing the efficacy of atezolizumab (a PD-L1 blocker) combined with bevacizumab in addition to standard chemotherapy compared to the administration of bevacizumab and chemotherapy [38]. The study has recruited 400 patients across Europe, and its results are expected to be released in 2024. Similarly, the DREAM3R, a phase III randomized trial, is evaluating the use of anti PD-L1 and durvalumab, in combination with cisplatin and pemetrexed, for the first-line treatment of advanced MPM [39].
4.2. Novel Immunotherapy Approaches
4.2.1. Immune Checkpoints
New emerging immune checkpoints, such as lymphocyte activation gene-3 (LAG-3), are being evaluated in MPM. LAG-3 is expressed on the surface of T cells, whose negative regulatory role hampers T-cell activation and proliferation against tumor antigens [40]. It has been shown that LAG-3 is expressed on immune cell infiltrates isolated from patients with MPM. Pre-clinical models have shown delayed tumor growth and a survival benefit in mice with the administration of an anti-PD-1 plus anti-LAG-3 antibody [41,42]. A phase I trial designed to assess the safety and tolerability of tebotelimab, a bispecific antibody designed to bind PD-1 and LAG-3 and restore the function of exhausted T cells in advanced solid tumors, showed encouraging preliminary results [43]. VISTA is another relevant immune checkpoint, expressed mostly by epithelioid MPM tumors, and is being investigated as a potential target for MPM treatment in several studies that combine the anti-VISTA antibody with vaccines and other ICIs [44,45].
4.2.2. Oncoviral Therapy
Oncoviral therapy also represents a potential line of treatment for mesothelioma. Modified viruses, such as adenovirus or measles virus containing human genes, are injected into patients to induce polyclonal anti-tumor activity by their own immune system [46]. A phase II trial with 40 patients demonstrated the safe and feasible results of administering an intrapleural injection of a non-replicating adenoviral vector (Ad) expressing the immune-activating cytokine interferon-alpha (IFN) in patients with MPM, followed by celecoxib and chemotherapy. Celecoxib is an inhibitor of the immunosuppressive molecule PGE2 used to further manipulate the tumor microenvironment. The regimen causes a large production of interferon in the pleura, translated into an intense stimulus to the patient’s immune system, with a promising disease control rate of 88% [46]. A larger, randomized phase III trial (INFINITE) is currently underway, testing the administration of adenovirus-delivered Interferon Alpha-2b (rAd-IFN) in combination with celecoxib and gemcitabine in 53 patients with MPM [47]. The results are expected by the end of 2024.
4.2.3. Cellular Therapy
CAR-T
Cellular therapy involving Chimeric Antigen Receptor (CAR) T cells has been proven to be a successful treatment for hematological tumors but still has not presented compelling evidence in the treatment of solid tumors due to its heterogeneous nature [48]. For T-cells to be able to exert anti-tumoral activity, they need to fulfill several steps. They must infiltrate the tumor tissue and be activated against tumor antigens. T-cell therapies are challenged with some of the following barriers: (i) an immunosuppressive microenvironment of solid tumors imposes resistance to T-cell therapy, (ii) an expression of PD-L1 in tumor cells inactivates T-cells and (iii) genomic instability leads to tumor cell heterogeneity, with different clonal populations expressing different antigens [49]. To overcome such barriers, researchers have: (i) applied CAR-T cells regionally on the pleura to increase tumor infiltration, a strategy that granted better success rates compared to intravenous infusion, (ii) carried out the blockage of inhibitory signals by tumor cells and the tumor microenvironment and (iii) focused on using targets for CAR-T cell therapy that are less expressed in healthy tissues but overexpressed in MPM cells, which is the case for the antigen mesothelin (MSLN)—overexpressed in 80–90% of MPM [49]. So far, phase I/II clinical trials using anti-MSLN CAR T cells to treat MPM have been promising, showing anti-tumor activity and good safety outcomes.
CAR-T cell therapy is likely the most promising treatment strategy compared to other targeted therapies. Once infused, CAR-T cells multiply and persist in the patient’s body, which may overcome the tumor’s immune tolerance and promote long-term immune surveillance, preventing recurrence through immune-reactivation once re-encountering the tumor’s antigens [50]. CAR-T cell therapy may be enhanced with the combination of ICI therapies, such as PD-1 or PD-L1 blockade, further preventing tumors from immune evasion. A phase I trial demonstrated that the intrapleural administration of MSLN CAR T-cell followed by a PD-1 blockade (pembrolizumab) in pretreated patients with MPM was feasible and well tolerated [51]. Furthermore, patients who received the combined treatment had a median overall survival of almost two years compared to 17.7 months in patients who received only CAR-T cells. Several groups are currently conducting trials evaluating different CAR-T cell products in mesothelin-expressing tumors [52,53,54].
Dendritic Cell Therapy
Although checkpoints inhibitors have been shown to improve outcomes for MPM patients, only a few derive significant benefits from immunotherapy. The use of PD-1 or PD-L1 inhibitors is expected to activate the T-cell killing capacity [55]. In this sense, a low density of CD8+ T-cells may limit its single-agent activity [56]. CD8+ T-cell infiltration positively correlates with a better overall survival in MPM patients.
Dendritic cell (DC) therapy aims to induce the proliferation of T cells and promote the activation of CD4+ and CD8+ T-cells by presenting them with tumor antigens, allowing CD8+ T-cells to infiltrate the tumor microenvironment [57]. DC can be derived from the patient’s bone marrow or peripheral blood through an ex vivo maturation process stimulated by cytokines. Matured DCs are loaded with tumor antigens (peptides, lysate and others) that are processed by the cell and transferred to its surface (through MHC I and II molecules). These processed DC cells are then transferred back to the patient to stimulate the immune response against the tumor [58].
Phase I trials have demonstrated that autologous tumor lysate-pulsed DC immunotherapy increased the T-cell response against MPM; however, using autologous tumor material imposes many challenges to conducting larger clinical trials [59]. Therefore, efforts have been made to verify the plausibility of using allogeneic tumor lysate as an antigen source. The DENIM randomized phase II/III trial is assessing the efficacy of autologous DCs loaded with allogeneic tumor lysate as a potential maintenance treatment for MPM following first-line treatment with chemotherapy in patients who had not shown disease progression [57].
4.3. Targeted Agents
4.3.1. EZH2
The hyperexpression of the enhancer of zeste homolog 2 (EZH2) is related to cancer progression. EZH2 is a subunit of the oncogenic polycomb repressive complex 2, frequently present in association with BAP1 loss [36]. Mesothelioma cells with inactivated BAP1 are sensitive to EZH2 pharmacologic inhibition, a fact that led investigators to launch a phase II trial to assess the effect of tazemetostat, an EZH2 inhibitor, in relapsed MPM patients with inactivated BAP1 [60,61]. The disease control rate was 54% at week 12 (primary outcome of the trial) and 28% at 24 weeks. Tazemetostat also presented a favorable toxicity profile [61].
4.3.2. ASS1
Arginine is an amino acid synthesized by cells and is essential for their growth. Notwithstanding, some tumors lack an important enzyme in the process of synthesizing arginine, called argininosuccinate synthetase 1 (ASS1), depending on the exogenous supply [62]. Lower ASS1 expression has been associated with more aggressive tumors and worse prognoses in different malignancy types, including mesotheliomas [62]. Arginine deprivation, therefore, is currently being evaluated by several studies as a potential therapy. Arginine-depleting agent (ADI-PEG 20) has already presented promising outcomes in treating patients with MPM in a phase I trial combined with cisplatin and pemetrexed chemotherapy, paving the way for a randomized phase II/III trial to scale this potential therapy (ATOMIC-Meso Phase 2/3 Study) [63]. ATOMIC recruited 249 MPM patients and is currently investigating the safety and efficacy of the same treatment regimen tested in the phase I trial mentioned before.
4.3.3. Molecular-Stratified Therapy
The Mesothelioma Stratified Therapy (MiST) is a multicenter ongoing clinical trial being conducted in the United Kingdom (UK), trying to identify predictive biomarkers and evaluate new personalized therapy for mesothelioma [64]. It seeks to stratify patients based on the molecular characteristics of their disease to better individualize treatment strategies. MiST has been designed with three different arms.
MiST1: Patients in this arm were selected based on mutations in the BRCA-1 or BAP1, known to be found in MPM tumors. BAP1, similar to the BRCA1 gene, is involved in DNA repair and can potentially be targeted with the use of poly-ADP ribose polymerase inhibitors (PARPi). The Mesothelioma Stratified Therapy 1 (MiST1) is a phase II trial studying the use of rucaparib, a PARPi, in 26 patients diagnosed with relapsed mesothelioma with BAP1 or BRCA-1 deficiency. The results showed some activity, with manageable toxicity, with a 58% disease control rate at 12 weeks and one of 23% at 24 weeks [65].
MiST2: MiST2 is a phase II trial focused on p16ink4A-negative mesothelioma previously treated with chemotherapy. The loss of the gene CDKN2A, frequently found in mesotheliomas, is associated with poorer prognosis due to the loss of the tumor suppressor p16ink4A, an endogenous suppressor of cyclin-dependent kinase (CDK)4 and CDK6. The trial is investigating the use of abemaciclib, an inhibitor of CDK4/6, in 26 patients; the results have also shown a 54% disease control rate at 12 weeks [66].
MiST3: The third arm trial, MiST3, will test the inhibition of AXL, a member of the TAM (Tyro3, AXL, Mer) family of receptor tyrosine kinases. AXL is a key regulator of tumor plasticity and immune evasion, contributing to tumor-intrinsic and microenvironmental immune suppression [67]. The overexpression of AXL in 74% of mesothelioma tumors examined by an analysis led to an ongoing trial investigating the potential of bemcentinib, an AXL inhibitor, combined with pembrolizumab in patients with relapsed mesothelioma.
4.4. Tumor-Treating Fields
In 2019, The Food and Drug Administration (FDA) approved the NovoTTF system, a humanitarian use device, to be used in combination with first-line standard chemotherapy (platinum-pemetrexed) for the treatment of MPM. NovoTT is a device based on the application of specific electric frequencies (tumor treatment fields, TTF) to diminish cancer growth [6,68]. This approval occurred sooner than expected, since the results from a randomized phase III trial have not yet confirmed the results of the phase II STELLAR trial, a single-arm study with 80 patients conducted in Europe, which demonstrated that TTF combined with chemotherapy had an overall survival of 18.2 months [69].
Table 2 summarizes the most relevant trials related to novel treatment pathways.

Table 2.
Relevant clinical trials on novel MPM treatment.
5. Discussion
The global incidence of MPM has been suffering upward pressure due to the widespread use of asbestos by industries in large developing economies and the aging population shift in developed countries. The aggressiveness of the disease also drives researchers to look for more favorable treatments that could improve survival and reduce morbidity [4]. Given the proven efficacy of chemotherapy and immunotherapy, the possibility of achieving better outcomes from combining both strategies led researchers to investigate novel therapies. However, the low mutational burden coupled with a diverse tumor microenvironment and biology pose challenges to defining a predictive biomarker for more suitable therapeutic options [9].
Mesothelioma has an immunosuppressive profile; hence, focus may be on boosting immune cells to increase the anti-tumorigenic response and on inhibiting pro-tumorigenic cell functions. In this direction, several immunotherapy strategies are being evaluated, but still with small practical breakthroughs (Figure 1). Nivolumab/ipilimumab is the only one approved by the FDA as a first-line treatment for unresectable MPM. The FDA’s decision was supported by the results of the Checkmate 743 trial, which showed a 4-month increase in overall survival compared to chemotherapy regardless of the tumor histology type [31].
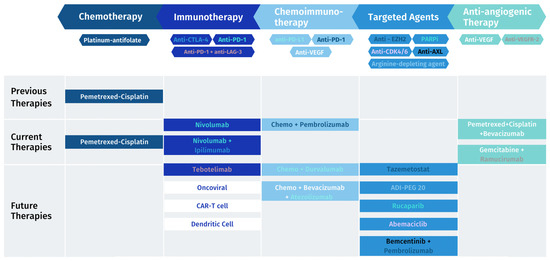
Figure 1.
MPM treatment landscape: previous, current and future therapies.
Clinical trials have been assessing the potential of chemoimmunotherapy, combining standard-of-care chemotherapy (platinum-pemetrexed) with different immunotherapeutic strategies. The addition of pembrolizumab (anti-PD-1 antibody) to chemotherapy seems to improve overall survival by only a month, with a more pronounced effect in non-epithelioid histologies, as reported by the IND227 trial [37]. Several ongoing phase III trials may reveal interesting options of combining available ICI and anti-VEGF agents with chemotherapy.
The evolution of immune oncology has led to the discovery of several other checkpoints currently being evaluated in most solid tumors, including MPM. LAG-3 and VISTA are among promising proteins to be targeted. The use of vaccines to stimulate the immune system coupled with chemotherapy is another avenue that could lead to positive outcomes. Delivering such therapies locally in the pleura may enhance their potential while minimizing systemic toxicities [15]. Even though preliminary data on oncoviral therapy regionally applied are favorable, more sound results are still necessary. The phase III trial INFINITE is testing adenovirus-delivered Interferon Alpha-2b efficacy and should indicate whether oncoviral treatment may be used as MPM therapy [47]. Similarly, CAR-T cells directed to mesothelin, locally administered, are expected to improve outcomes. Dendritic cell therapies may also increase CD8+ T-cell density and enhance anti-PD-1 activity.
Even though the search for biomarkers has been under a lot of focus, there does not seem to be a single driver alteration that is amenable to targeted therapy. The loss of tumor suppressor genes, mainly BAP1 and CDKN2A, is the most predominant genomic alteration in MPM, and it could represent an important biomarker. In this sense, agents targeting PARP enzymes, CDK4/6 and AXL are being evaluated.
6. Conclusions
The recent advances in understanding the immune landscape and molecular profile of MPM allowed for several agents used in different scenarios to be investigated for this disease. Moreover, novel biomarker-directed therapies are being developed to target specific mechanisms of mesotheliomas. The complexity and heterogeneity of this deadly disease may have an increased chance of success with combined approaches.
Author Contributions
Conceptualization, G.S., B.B. and M.Z.; methodology, B.B.; writing—original draft preparation, B.B.; writing—review and editing, B.B., G.S. and M.Z.; visualization, B.B. and G.S.; supervision, G.S.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
G.S. declares receiving consulting fees from AstraZeneca, Bristol-Myers Squibb, Merck, Sharp and Dome, Daiichi Sankyo, Sanofi, Roche and Takeda.
References
- Broeckx, G.; Pauwels, P. Malignant Peritoneal Mesothelioma: A Review. Transl. Lung Cancer Res. 2018, 7, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, B.; De Rienzo, A.; Bueno, R. The Molecular Basis of Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020, 30, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Vaynblat, A.; Pass, H.I. Diagnosis and Prognosis-Review of Biomarkers for Mesothelioma. Ann. Transl. Med. 2017, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific Clues for Prevention, Diagnosis, and Therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed]
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Pass, H.I.; Rimner, A.; Mansfield, A.S. New Era for Malignant Pleural Mesothelioma: Updates on Therapeutic Options. J. Clin. Oncol. 2022, 40, 681–692. [Google Scholar] [CrossRef]
- Janes, S.M.; Alrifai, D.; Fennell, D.A. Perspectives on the Treatment of Malignant Pleural Mesothelioma. N. Engl. J. Med. 2021, 385, 1207–1218. [Google Scholar] [CrossRef]
- Courtiol, P.; Maussion, C.; Moarii, M.; Pronier, E.; Pilcer, S.; Sefta, M.; Manceron, P.; Toldo, S.; Zaslavskiy, M.; Le Stang, N.; et al. Deep Learning-Based Classification of Mesothelioma Improves Prediction of Patient Outcome. Nat. Med. 2019, 25, 1519–1525. [Google Scholar] [CrossRef]
- Blum, Y.; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting Heterogeneity in Malignant Pleural Mesothelioma through Histo-Molecular Gradients for Clinical Applications. Nat. Commun. 2019, 10, 1333. [Google Scholar] [CrossRef]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.I.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1549–1565. [Google Scholar] [CrossRef]
- Minnema-Luiting, J.; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, R.; Lievense, L.A.; Maat, A.P.; Hendriks, R.W.; Hoogsteden, H.C.; Bogers, A.J.; Hegmans, J.P.; Aerts, J.G. Ratio of Intratumoral Macrophage Phenotypes Is a Prognostic Factor in Epithelioid Malignant Pleural Mesothelioma. PLoS ONE 2014, 9, e106742. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.J.J.; Aerts, J.G.J.V. Immunomodulation in Cancer. Curr. Opin. Pharmacol. 2014, 17, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Kadota, K.; Nitadori, J.I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The Tumoral and Stromal Immune Microenvironment in Malignant Pleural Mesothelioma: A Comprehensive Analysis Reveals Prognostic Immune Markers. Oncoimmunology 2015, 4, e1009285. [Google Scholar] [CrossRef] [PubMed]
- Bograd, A.J.; Suzuki, K.; Vertes, E.; Colovos, C.; Morales, E.A.; Sadelain, M.; Adusumilli, P.S. Immune Responses and Immunotherapeutic Interventions in Malignant Pleural Mesothelioma. Cancer Immunol. Immunother. 2011, 60, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Shamseddin, M.; Obacz, J.; Garnett, M.J.; Rintoul, R.C.; Francies, H.E.; Marciniak, S.J. Use of Preclinical Models for Malignant Pleural Mesothelioma. Thorax 2021, 76, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Kato, K.; Yanatori, I.; Maeda, Y.; Murohara, T.; Toyokuni, S. Matrigel-Based Organoid Culture of Malignant Mesothelioma Reproduces Cisplatin Sensitivity through CTR1. BMC Cancer 2023, 23, 487. [Google Scholar] [CrossRef] [PubMed]
- Malignant Mesothelioma Early Detection, Diagnosis, and Staging. Available online: https://www.cancer.org/cancer/types/malignant-mesothelioma/detection-diagnosis-staging.html (accessed on 27 November 2023).
- Pemetrexed for the Treatment of Malignant Pleural Mesothelioma. Available online: www.nice.org.uk/guidance/ta135 (accessed on 1 August 2023).
- Lim, E.; Darlison, L.; Edwards, J.; Elliott, D.; Fennell, D.A.; Popat, S.; Rintoul, R.C.; Waller, D.; Ali, C.; Bille, A.; et al. Mesothelioma and Radical Surgery 2 (MARS 2): Protocol for a Multicentre Randomised Trial Comparing (Extended) Pleurectomy Decortication versus No (Extended) Pleurectomy Decortication for Patients with Malignant Pleural Mesothelioma. BMJ Open 2020, 10, e038892. [Google Scholar] [CrossRef]
- Nadal, E.; Bosch-Barrera, J.; Cedrés, S.; Coves, J.; García-Campelo, R.; Guirado, M.; López-Castro, R.; Ortega, A.L.; Vicente, D.; de Castro-Carpeño, J. SEOM Clinical Guidelines for the Treatment of Malignant Pleural Mesothelioma (2020). Clin. Transl. Oncol. 2021, 23, 980–987. [Google Scholar] [CrossRef]
- Brims, F.; Gunatilake, S.; Lawrie, I.; Marshall, L.; Fogg, C.; Qi, C.; Creech, L.; Holtom, N.; Killick, S.; Yung, B.; et al. Early Specialist Palliative Care on Quality of Life for Malignant Pleural Mesothelioma: A Randomised Controlled Trial. Thorax 2019, 74, 354–361. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for Newly Diagnosed Pleural Mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.P.C.; Shamash, J.; Evans, M.T.; Gower, N.H.; Tischkowitz, M.D.; Rudd, R.M. Phase II Study of Vinorelbine in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2000, 18, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; O’brien, M.E.R.; Byrne, M.J.; Bard, M.; Kaukel, E.; Smit, B. Phase II Trial of ZD0473 as Second-Line Therapy in Mesothelioma. Eur. J. Cancer 2002, 38, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; Ceresoli, G.L.; Garassino, I.; De Vincenzo, F.; Cavina, R.; Campagnoli, E.; Cappuzzo, F.; Salamina, S.; Soto Parra, H.J.; Santoro, A. Gemcitabine and Vinorelbine in Pemetrexed-Pretreated Patients with Malignant Pleural Mesothelioma. Cancer 2008, 112, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Zucali, P.A.; Pagano, M.; Grosso, F.; Pasello, G.; Garassino, M.C.; Tiseo, M.; Soto Parra, H.; Grossi, F.; Cappuzzo, F.; et al. Gemcitabine with or without Ramucirumab as Second-Line Treatment for Malignant Pleural Mesothelioma (RAMES): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol. 2021, 22, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus Placebo in Patients with Relapsed Malignant Mesothelioma (CONFIRM): A Multicentre, Double-Blind, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Dô, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or Nivolumab plus Ipilimumab in Patients with Relapsed Malignant Pleural Mesothelioma (IFCT-1501 MAPS2): A Multicentre, Open-Label, Randomised, Non-Comparative, Phase 2 Trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-Line Nivolumab plus Ipilimumab versus Chemotherapy in Patients with Unresectable Malignant Pleural Mesothelioma: 3-Year Outcomes from CheckMate 743. Ann. Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef]
- Zalcman, G.; Oulkhouir, Y.; Cornelissen, R.; Greillier, L.; Cid, J.R.R.; Mazieres, J.; Briggs, P.; Nowak, A.K.; Tsao, A.; Fujimoto, N.; et al. LBA71 First-Line Nivolumab (NIVO) plus Ipilimumab (IPI) vs Chemotherapy (Chemo) in Patients (Pts) with Unresectable Malignant Pleural Mesothelioma (UMPM): 4-Year Update from CheckMate 743. Ann. Oncol. 2022, 33, S1438–S1439. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical Safety and Activity of Pembrolizumab in Patients with Malignant Pleural Mesothelioma (KEYNOTE-028): Preliminary Results from a Non-Randomised, Open-Label, Phase 1b Trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A Multicentre Randomised Phase III Trial Comparing Pembrolizumab versus Single-Agent Chemotherapy for Advanced Pre-Treated Malignant Pleural Mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-Meso Trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.-S.; Brown, C.; Hughes, B.G.; Karikios, D.J.; John, T.; Kao, S.C.-H.; Leslie, C.; Cook, A.M.; et al. Durvalumab with First-Line Chemotherapy in Previously Untreated Malignant Pleural Mesothelioma (DREAM): A Multicentre, Single-Arm, Phase 2 Trial with a Safety Run-In. Lancet Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Deiana, C.; Fabbri, F.; Tavolari, S.; Palloni, A.; Brandi, G. Improvements in Systemic Therapies for Advanced Malignant Mesothelioma. Int. J. Mol. Sci. 2023, 24, 10415. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, M.C.; Chu, Q.; Bradbury, P.; Tu, W.; Coschi, C.H.; Grosso, F.; Florescu, M.; Mencoboni, M.; Goffin, J.R.; Pagano, M.; et al. Brief Report: Canadian Cancer Trials Group IND.227: A Phase 2 Randomized Study of Pembrolizumab in Patients With Advanced Malignant Pleural Mesothelioma (NCT02784171). J. Thorac. Oncol. 2023, 18, 813–819. [Google Scholar] [CrossRef]
- Study Details|BEAT-Meso: Bevacizumab and Atezolizumab in Malignant Pleural Mesothelioma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03762018 (accessed on 27 November 2023).
- Kok, P.S.; Forde, P.M.; Hughes, B.; Sun, Z.; Brown, C.; Ramalingam, S.; Cook, A.; Lesterhuis, W.J.; Yip, S.; O’Byrne, K.; et al. Protocol of DREAM3R: DuRvalumab with ChEmotherapy as First-Line TreAtment in Advanced Pleural Mesothelioma-a Phase 3 Randomised Trial. BMJ Open 2022, 12, e057663. [Google Scholar] [CrossRef]
- Marcq, E.; De Waele, J.; Van Audenaerde, J.; Lion, E.; Santermans, E.; Hens, N.; Pauwels, P.; Van Meerbeeck, J.P.; Smits, E.L.J. Abundant Expression of TIM-3, LAG-3, PD-1 and PD-L1 as Immunotherapy Checkpoint Targets in Effusions of Mesothelioma Patients. Oncotarget 2017, 8, 89722–89735. [Google Scholar] [CrossRef]
- Gray, S.G. Emerging Avenues in Immunotherapy for the Management of Malignant Pleural Mesothelioma. BMC Pulm. Med. 2021, 21, 148. [Google Scholar] [CrossRef]
- Marcq, E.; Van Audenaerde, J.R.M.; De Waele, J.; Merlin, C.; Pauwels, P.; Van Meerbeeck, J.P.; Fisher, S.A.; Smits, E.L.J. The Search for an Interesting Partner to Combine with PD-L1 Blockade in Mesothelioma: Focus on TIM-3 and LAG-3. Cancers 2021, 13, 282. [Google Scholar] [CrossRef]
- Luke, J.J.; Patel, M.R.; Hamilton, E.P.; Chmielowski, B.; Ulahannan, S.V.; Kindler, H.L.; Bahadur, S.W.; Clingan, P.R.; Mallesara, G.; Weickhardt, A.J.; et al. A Phase I, First-in-Human, Open-Label, Dose-Escalation Study of MGD013, a Bispecific DART Molecule Binding PD-1 and LAG-3, in Patients with Unresectable or Metastatic Neoplasms. J. Clin. Oncol. 2020, 38, 3004. [Google Scholar] [CrossRef]
- Lines, J.L.; Sempere, L.F.; Broughton, T.; Wang, L.; Noelle, R. VISTA Is a Novel Broad-Spectrum Negative Checkpoint Regulator for Cancer Immunotherapy. Cancer Immunol. Res. 2014, 2, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, M.; Robinson, B.W.; Campbell, P.J. The TCGA Malignant Pleural Mesothelioma (MPM) Project: VISTA Expression and Delineation of a Novel Clinical-Molecular Subtype of MPM. J. Clin. Oncol. 2018, 36, 8516. [Google Scholar] [CrossRef]
- Sterman, D.H.; Alley, E.; Stevenson, J.P.; Friedberg, J.; Metzger, S.; Recio, A.; Moon, E.K.; Haas, A.R.; Vachani, A.; Katz, S.I.; et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin. Cancer Res. 2016, 22, 3791–3800. [Google Scholar] [CrossRef]
- Study Details|A Phase 3, Open-Label, Randomized, Parallel Group Study to Evaluate the Efficacy and Safety of Intrapleural Administration of Adenovirus-Delivered Interferon Alpha-2b (RAd-IFN) in Combination with Celecoxib and Gemcitabine in Patients with Malignant Pleural Mesothelioma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03710876 (accessed on 27 November 2023).
- Castelletti, L.; Yeo, D.; van Zandwijk, N.; Rasko, J.E.J. Anti-Mesothelin CAR T Cell Therapy for Malignant Mesothelioma. Biomark. Res. 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kiesgen, S.; Chicaybam, L.; Chintala, N.K.; Adusumilli, P.S. Chimeric Antigen Receptor (CAR) T-Cell Therapy for Thoracic Malignancies. J. Thorac. Oncol. 2018, 13, 16–26. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-Cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti–PD-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef]
- Study Details|A Phase 1 Study of SynKIR-110, Autologous T Cells Transduced with Mesothelin KIR-CAR, in Subjects with Mesothelin-Expressing Advanced Ovarian Cancer, Cholangiocarcinoma, or Mesothelioma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT05568680 (accessed on 5 September 2023).
- Study Details|A Single-Arm, Open-Label, Phase I Trial to Assess the Safety of Genetically Engineered Autologous T Cells Targeting the Cell Surface Antigen Mesothelin with Cell-Intrinsic Checkpoint Inhibition in Patients with Mesothelioma|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04577326 (accessed on 5 September 2023).
- Study Details|Phase I Study of Human Chimeric Antigen Receptor Modified T Cells in Patients with Mesothelin Expressing Cancers|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT03054298 (accessed on 5 September 2023).
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Aerts, J.G.; Hegmans, J.P. Tumor-Specific Cytotoxic T Cells Are Crucial for Efficacy of Immunomodulatory Antibodies in Patients with Lung Cancer. Cancer Res. 2013, 73, 2381–2388. [Google Scholar] [CrossRef]
- Belderbos, R.A.; Baas, P.; Berardi, R.; Cornelissen, R.; Fennell, D.A.; van Meerbeeck, J.P.; Scherpereel, A.; Vroman, H.; Aerts, J.G.J.V. A Multicenter, Randomized, Phase II/III Study of Dendritic Cells Loaded with Allogeneic Tumor Cell Lysate (MesoPher) in Subjects with Mesothelioma as Maintenance Therapy after Chemotherapy: DENdritic Cell Immunotherapy for Mesothelioma (DENIM) Trial. Transl. Lung Cancer Res. 2019, 8, 280–285. [Google Scholar] [CrossRef]
- Buonaguro, L.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M. Translating Tumor Antigens into Cancer Vaccines. Clin. Vaccine Immunol. 2011, 18, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.G.J.V.; de Goeje, P.L.; Cornelissen, R.; Kaijen-Lambers, M.E.H.; Bezemer, K.; van der Leest, C.H.; Mahaweni, N.M.; Kunert, A.; Eskens, F.A.L.M.; Waasdorp, C.; et al. Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human. Clin. Cancer Res. 2018, 24, 766–776. [Google Scholar] [CrossRef] [PubMed]
- LaFave, L.M.; Béguelin, W.; Koche, R.; Teater, M.; Spitzer, B.; Chramiec, A.; Papalexi, E.; Keller, M.D.; Hricik, T.; Konstantinoff, K.; et al. Loss of BAP1 Function Leads to EZH2-Dependent Transformation. Nat. Med. 2015, 21, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Zauderer, M.G.; Szlosarek, P.W.; Le Moulec, S.; Popat, S.; Taylor, P.; Planchard, D.; Scherpereel, A.; Koczywas, M.; Forster, M.; Cameron, R.B.; et al. EZH2 Inhibitor Tazemetostat in Patients with Relapsed or Refractory, BAP1-Inactivated Malignant Pleural Mesothelioma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2022, 23, 758–767. [Google Scholar] [CrossRef]
- Beddowes, E.; Spicer, J.; Chan, P.Y.; Khadeir, R.; Corbacho, J.G.; Repana, D.; Steele, J.P.; Schmid, P.; Szyszko, T.; Cook, G.; et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1–Deficient Thoracic Cancers. J. Clin. Oncol. 2017, 35, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Randomized, Double-Blind, Phase 2/3 Study in Subjects with Malignant Pleural Mesotheliomato Assess ADI-PEG 20 with Pemetrexed and Cisplatin (ATOMIC-Meso Phase 2/3 Study)|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT02709512 (accessed on 23 July 2023).
- Fennell, D.; Hudka, M.; Darlison, L.; Lord, K.; Bzura, A.; Dzialo, J.; Pritchard, C.; Harber, J.; Takata, T.; Popat, S.; et al. P2.06-02 Mesothelioma Stratified Therapy (MiST): A Phase IIA Umbrella Trial for Accelerating the Development of Precision Medicines. J. Thorac. Oncol. 2019, 14, S755–S756. [Google Scholar] [CrossRef]
- Fennell, D.A.; King, A.; Mohammed, S.; Branson, A.; Brookes, C.; Darlison, L.; Dawson, A.G.; Gaba, A.; Hutka, M.; Morgan, B.; et al. Rucaparib in Patients with BAP1-Deficient or BRCA1-Deficient Mesothelioma (MiST1): An Open-Label, Single-Arm, Phase 2a Clinical Trial. Lancet Respir. Med. 2021, 9, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; King, A.; Mohammed, S.; Greystoke, A.; Anthony, S.; Poile, C.; Nusrat, N.; Scotland, M.; Bhundia, V.; Branson, A.; et al. Abemaciclib in Patients with P16ink4A-Deficient Mesothelioma (MiST2): A Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol. 2022, 23, 374–381. [Google Scholar] [CrossRef]
- Krebs, M.; Carter, L.; Villa, S.; King, A.; Massey, C.; Lorens, J.; Darlington, E.; Fennell, D. P2.06-09 MiST3: A Phase II Study of Oral Selective AXL Inhibitor Bemcentinib (BGB324) in Combination with Pembrolizumab in Pts with Malignant Mesothelioma. J. Thorac. Oncol. 2018, 13, S745. [Google Scholar] [CrossRef]
- Nicolini, F.; Bocchini, M.; Bronte, G.; Delmonte, A.; Guidoboni, M.; Crinò, L.; Mazza, M. Malignant Pleural Mesothelioma: State-of-the-Art on Current Therapies and Promises for the Future. Front. Oncol. 2020, 9, 1519. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crinò, L.; et al. Tumour Treating Fields in Combination with Pemetrexed and Cisplatin or Carboplatin as First-Line Treatment for Unresectable Malignant Pleural Mesothelioma (STELLAR): A Multicentre, Single-Arm Phase 2 Trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).