Simple Summary
Cancer is the main cause of human death. Nanomedicine provides a new strategy for the treatment of diverse cancers. Among them, superparamagnetic artificial cell PLGA-Fe3O4 micro/nanocapsules demand great attention due to their excellent properties, such as targeted delivery, magnetic response, and biocompatibility. The aim of this review is to summarize the application of superparamagnetic artificial cells (PLGA-Fe3O4) micro/nanocapsules in cancer drug targeted delivery and their preparation methods, providing insights into the future development of superparamagnetic artificial cells (PLGA nano/microcapsules) for magnetic targeting in cancer treatment.
Abstract
Artificial cells have been extensively used in many fields, such as nanomedicine, biotherapy, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, and the COVID-19 vaccine. The unique properties of superparamagnetic Fe3O4 nanoparticles have contributed to increased interest in using superparamagnetic artificial cells (PLGA-Fe3O4 micro/nanocapsules) for targeted therapy. In this review, the preparation methods of Fe3O4 NPs and superparamagnetic artificial cell PLGA-drug-Fe3O4 micro/nanocapsules are discussed. This review also focuses on the recent progress of superparamagnetic PLGA-drug-Fe3O4 micro/nanocapsules as targeted therapeutics. We shall concentrate on the use of superparamagnetic artificial cells in the form of PLGA-drug-Fe3O4 nanocapsules for magnetic hyperthermia/photothermal therapy and cancer therapies, including lung breast cancer and glioblastoma.
1. Introduction
Cancer poses a severe threat to human health, and the rate of cancer incidence has been steadily increasing. In statistics compiled for 2020, an estimated 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10 million cancer deaths occurred around the world—close to one in three people during their lifetimes will be diagnosed with some type of tumor disease [1]. Nowadays, cancer treatment methods include surgical resection, radiation therapy, chemotherapy, and immunotherapy [2]. However, there are still three disadvantages for these methods: (1) it is difficult to completely remove the tumor cells by surgery [3], (2) the normal cells around the irradiated site are damaged to a certain extent while tumor cells are killed under radiotherapy [4], (3) traditional chemotherapeutic agents cannot achieve specific distribution in vivo, and they can simultaneously damage both tumor cells and normal cells [5]. Therefore, researchers are interested in the development of an improved targeting and efficient strategy for cancer treatment with minimal side effects.
In 1964, Chang published the first paper on artificial cells (semipermeable microcapsules) [6] that can be used for a number of medical applications [7,8], including drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, regenerative medicine, hemoperfusion, blood substitutes, encapsulated microbes [7,8,9,10,11,12], and others. Recent developments in artificial micro/nanomotors and COVID-19 vaccines can be found in recent publications [9,10,11,13,14]. In 1966, he reported the preparation of artificial cells containing magnetic material for targeting the movements of these artificial cells [15]. Later in 1976, he reported the preparation of biodegradable artificial cell membranes using polylactide [16]. This has since been extended by others using polylactic-co-glycolic acid.
Superparamagnetic Fe3O4 nanoparticles with core dimensions of about 10 to 20 nm exhibit superparamagnetic behavior [17,18]. They have attracted great attention in many fields, such as the development of rare earth elements such as magnetite nanoparticles [19], the use of UV-stable perovskite solar cells [20], and the application of natural polymer magnetic nanocarriers for drug delivery [21]. Due to the excellent biocompatibility and biodegradability of superparamagnetic Fe3O4 nanoparticles, Fe3O4-based nanostructured systems could be a potential platform for successfully controlled and targeted drug delivery applications using magnetic hyperthermia. Among magnetic metal nanoparticles, iron oxide nanoparticles (Fe3O4 NPs) are the only MNPs approved for clinical use by the US Food and Drug Administration [22,23]. Fe3O4 NPs are non-toxic in vivo because they can be metabolized into oxygen and elemental iron by hydrolytic enzymes, where the iron accumulates in the natural body [24]. The Fe3O4 nanoparticles as drug carriers can control the drug release pattern, improve the drug bioavailability, and reduce the systemic side effects on healthy tissues [22,25]. In addition, the Fe3O4 nanoparticles were widely used for thermotherapy in cancer treatment based on the thermal effect of the superparamagnetic Fe3O4 nanoparticles exposed to irradiation. Superparamagnetic Fe3O4 nanoparticles have been widely applied to drug delivery for chemotherapeutics and magnetic resonance imaging (MRI) [24]. However, the bare SPIONs (Superparamagnetic Iron Oxide Nanoparticles) are very easy to attract and agglomerate as they are very small in size (nano-dimension), along with the presence of van der Waals force and interparticle magnetic interactions [26]. The surface of Fe3O4 nanoparticles is prone to modification by conjugating with biochemically active functional groups [27]. The functionalized Fe3O4 NPs could extend their application to targeted drugs, molecular imaging, enzyme delivery, and cell immobilization [28].
Moreover, the biodegradable and biocompatible artificial cell invented by Chang in 1976 is an alternative solution to solve the above issue [16]. The polymeric shell of artificial cell micro/nanoparticles is a good barrier, not only protecting against the premature release and degradation of drugs, maintaining the sustained release of the drug, but also preventing aggregation from the Fe3O4 NPs [29,30]. Poly-lactic-co-glycolic acid (PLGA), an FDA and EMA (European Medicines Agency)-approved biodegradable copolymer, is one of the most exploited polymers in the design and formulation of artificial cell nano/micro-drug delivery systems for biomedical applications owing to its biodegradability, biosafety, biocompatibility, and versatility [30,31,32,33]. PLGA can be processed into any shape and size, has excellent water solubility, and allows for a tunable drug release according to its molecular weight and composition ratio [32]. In addition, PLGA can be decomposed into nontoxic compounds (H2O and CO2) through the Krebs cycle in the human body [34]. At present, more than 20 kinds of PLGA-based biodegradable microspheres and nanoparticles have already been approved by the FDA, many of which are in the stage of R&D or clinical trials [35].
Artificial cell PLGA micro/nanocapsules encapsulating magnetically-responsive Fe3O4 nanoparticles have been established as multifunctional systems for specific, selective, and performance-enhanced anti-infective [17] and anti-cancer applications [36]. The development of superparamagnetic artificial cells would pave a promising avenue for personalized therapy and precision medicine in the future. To our knowledge, superparamagnetic artificial cell PLGA-drug-Fe3O4 micro/nanocapsules for targeted cancer therapy have been published discretely in several reviews [34,36,37]. In this review, we discuss the preparation methods of Fe3O4 NPs and artificial cell PLGA-drug-Fe3O4 micro/nanocapsules. We shall discuss in detail our approach using emulsion or microfluidizer to prepare suparamagnetic artificial cells of micro and nano dimensions. Then, the medical applications of the superparamagnetic artificial cell PLGA-drug-Fe3O4 nano/microcapsules for targeted cancer therapy in the last 5 years (2017–2022) were summarized, followed by our insights into the future development of superparamagnetic artificial cell PLGA nano/microcapsules for magnetic targeting cancer treatment.
2. Preparation of Fe3O4 NPs
Fe3O4 NPs can be synthesized via various methods, including physical, chemical, and biological methods [38]. The method produced can influence particle morphology, size, surface structure, stability, and magnetic properties. And those parameters of Fe3O4 NPs play an important role in medical applications [39]. Therefore, the selection of the desired method to form the Fe3O4 NPs shall consider their future biological applications and the differences from the preparation methods of Fe3O4 NPs. This review will briefly describe the methods of chemical co-precipitation, thermal decomposition, microemulsions, and hydrothermal synthesis, as these preparation methods are simple and convenient and can obtain relatively uniform particles.
2.1. Co-Precipitation
Co-precipitation is one of the most popular standard methods for aqueous-phase Fe3O4 NP synthesis. In simple terms, the generation of SPIONs used aqueous ferrous and ferric salt solutions in a 2:1 proportion in the basic condition, such as NaOH, NH4OH, TMAOH, etc. In the reaction process, the reaction mixture was maintained in a pH range of 9 to 11 and at room temperature or higher in an inert environment, for example, Nitrogen or Argon [40,41]. The co-precipitation method has several advantages, including mild reaction conditions, narrow size distribution, facile preparation, and convenient modification of the Fe3O4 NP surface with other functional groups. Specifically, the particle size can be tuned by adjusting the reaction temperature, the molar ratio of iron salts, solution pH, and ionic strength [42,43]. The production of Fe3O4 NPs is conducted in the presence of the oxidation/reduction potential of iron salts, which affect the physicochemical properties of the prepared nanoparticles.
2.2. Thermal Decomposition
Thermal decomposition can prepare small nanocrystals applied to non-magnetic organometallic precursors in the presence of organic solvents and surfactants at high temperatures ranging from 100 to 350 °C. High-quality SPIONs with sizes of approximately 15 nm can be obtained via this method. The size and morphology of particles mainly depend on factors like ratios and volumes of different precursor molecules and the reaction conditions. Generally, the large Fe3O4 particle and high saturation magnetization will be obtained over a long reaction time and at a high temperature. For example, Bateer et al. reported that the size of the obtained Fe3O4 particles increased from 12 nm to 20 nm produced by the thermal decomposition of iron oleate at 350 °C when increasing the reaction time from 40 min to 120 min. Moreover, the saturation magnetization of 12 and 20 nm Fe3O4 exists at 65.51 and 74.48 emu/g, respectively [44].
2.3. Microemulsions
Microemulsions consist of the oil phase, the aqueous phase, and surfactants as stabilizers to form a thermodynamically stable isotropic mixture. There are three main kinds of microemulsions: oil in water, water in oil, and double emulsion. The formation of the particles is confined inside the microemulsion droplets. Thus, the growth of particles is controlled by the size of the microcavity of the droplets. The resulting nanoparticles show small sizes and uniform morphology, good dispersity, and good crystallinity [45,46].
2.4. Hydrothermal Synthesis
A simple process for producing Fe3O4 nanoparticles is hydrothermal synthesis. The iron metal salts, or their oxides, were separated through dehydration and hydrolysis in a solvent under high pressure and temperature conditions. This process required a reactor to maintain a temperature of 150 °C and a pressure of more than 2000 psi [47]. The various physical parameters (temperature, pressure, and reaction time) that affect the distribution and nucleation of the nanoparticles are also taken into account to maintain their good properties [48].
3. Methods of Generating Superparamagnetic Artificial Cell PLGA-Drug-Fe3O4 Micro/Nanocapsules
Various techniques can be used to prepare superparamagnetic artificial cells based on the physicochemical properties of the drug and Fe3O4NPs micro/nancapsules, such as water-in-oil (W/O) single emulsion [49] and water-in-oil-in-water (W/O/W) double emulsion [50], spray drying [51,52], electrospraying [53,54,55], co-flowing devices [56], and new techniques for microfluidic devices [57,58,59,60]. The processing conditions for producing superparamagnetic artificial cell PLGA micro/nanocapsules play an important role in their final features, such as shape, size distribution, and stability [61]. Here, we describe the most common methods, and they are summarized in Table 1.

Table 1.
The summarizations of the different techniques for supramagnetic artificial cells production.
3.1. Emulsification Method
In 1957, Chang first developed the emulsion method to produce the first artificial cells (Figure 1) [62]. Most nanocapsules and microcapsules are made using the emulsification method because of its simplicity. This method includes the single emulsion method and the double emulsion method [15,16,63,64]. Factors affecting particle size, morphology, encapsulation rate, and drug release include the components of biodegradable polymers, the emulsified concentration of biodegradable polymers, solvent evaporation methods, and surfactants [8,70,71,72]. Different preparation methods can be selected based on the different physical and chemical properties of the encapsulated drug molecules. Most hydrophobic drugs and hydrophobic Fe3O4 NPs (such as OA-Fe3O4 NPs) were encapsulated in the PLGA micro/nanocapsules via the single emulsion method of oil in water (O/W) due to the fact that the water-insoluble drug can be well dissolved in the organic solvent to form a homogeneous solution as an oil phase during the emulsified process [49]. In this process, the polymeric solution consisted of a polymer and the desired Fe3O4 NP in DCM or THF. The resulting mixture solution is then emulsified in a continuous aqueous phase (PVA%) to produce microdroplets/nanocapsules under mechanical stirring or sonication. The organic solvent was removed by evaporating the organic solvent to achieve solidification and the formation of the rude microspheres. After that, the pure microspheres or nanoparticles were obtained by purification with suitable filtration and drying [73]. For hydrophilic drugs, the encapsulation efficiency is lower in a single emulsion, attributed to the fact that the water-soluble compounds could rapidly dissociate into the continuous aqueous phase, leading to much drug loss. To enhance the loading level of hydrophilic drug molecules, the hydrophilic drugs can be encapsulated into the PLGA micro/nanoparticles by the double emulsion method of oil in water in oil (O/W/O) or water in oil in water (W/O/W) [16,74,75]. Generally, the alternative for hydrophilic drugs is to transform the drug to a hydrophobic state or use a mixture in the presence of a stabilizer [75]. Moreover, water-soluble bioactive substances like peptides, proteins, sugar, and vaccines were also encapsulated into the PLGA microcapsules, usually using the water-in-oil-in-water (W/O/W) emulsion method [16].

Figure 1.
Emulsion method for preparing micro-dimension artificial cells. This image is from the literature. This figure has been taken from ref. [8] with written copyright permission from the publisher, Taylor and Francis.
The emulsion method can conveniently obtain large-scale magnetic PLGA nano/microcapsules in one batch. However, much larger undesired particles are present in the sample, and this method usually obtains polydisperse magnetic PLGA nano/microcapsules with an uncontrollable size distribution [76]. In addition, this approach has other drawbacks, such as residual organic solvents in the prepared nano/microcapsules and surfactant residues on the surface of the prepared nano/microcapsules. Moreover, the drug loading efficiency and particle size of microcapsules could not be precisely controlled by manipulating the stirring rate in secondary emulsion preparation, the viscosity of the organic phase, the concentration of polymer used, and osmotic pressure [77].
3.2. Spray Drying
The spray drying technique is ideal for encapsulating heat-sensitive substances into the PLGA nano/microcapsules. Schliehe et al. used the spray-drying method to prepare the magnetic microcapsules [52]. The removal of organic solvents by spraying the emulsion into a hot atmosphere can be used to encapsulate biomolecules such as proteins or peptides, plasmid DNA, and small chemically active molecules, including hydrophilic and hydrophobic drugs [65]. However, this method still has several drawbacks. Some heat-sensitive proteins will lose their activity and native structure in the hot, dry air during the spray-drying process. The laboratory setups usually obtain a low yield of production because products stick to the walls of the drying chamber [78].
3.3. Electrospray
Electrospray is particularly suited to generating a narrow-sized distribution of particles from tens of nanometers to hundreds of micrometers [79]. Faramarzi et al. produce PLGA particles containing high magnetite nanoparticles using the electrospray method [55]. In the industry, electrospray is employed to produce biodegradable polymeric encapsulations due to its advantages, including versatility, simplicity, low cost of production, high yield of microspheres, and large-scale production [80]. In addition, sensitive substances such as proteins and cells can be loaded into the PLGA nano/microcapsules [66]. The particle size and morphology can be acquired by tuning the experimental voltage and velocity [81]. For this technology, the main problem is low throughput, which limits its large-scale production [82].
3.4. Microfluidic Technology
Microfluidic technology is a relatedly new strategy for the generation of highly controlled and uniform microspheres [83]. The better formation of micro/nanoparticles can be achieved in a microchannel compared to the conventional method. The microfluidic device consists of a microchip, flow controller, and collector (Figure 2). Generally, the microfluidic chip for preparing the microdroplets (microparticles) is classified into three types: co-flow capillary, flow-focusing capillary, and a combination of co-flow and flow-focusing capillary. The herringbone chip is efficient for the preparation of nanoparticles [84]. In a microfluidic system, the flow phases usually consist of an aqueous phase and an organic phase. The size and polydispersity of the resulting particles were affected by several parameters, such as flow rate, the concentration of both disperse phase and continuous phase, and the surfactant type [85,86]. The low flow rate produces large particles compared to the high flow rate under identical working conditions. The high concentration of the dispersed phase fabricates small particles as opposed to those produced by a low concentration under identical operating parameters. The microfluidic method for preparing PLGA micro/nanoparticles has several advantages: a narrow size distribution, low polydispersity, and reproducibility by controlling flow rate and concentration from bulk preparations. However, the yield of microspheres prepared by this method is relatively low for further biological investigation. However, a series of parallel designs on the microfluidic platform may be possible to achieve the large-scale microspheres and reproductivity of superparamagnetic artificial cell PLGA-drug-Fe3O4NPs micro/nanocapsules for their further bioassays and other studies [87,88,89].
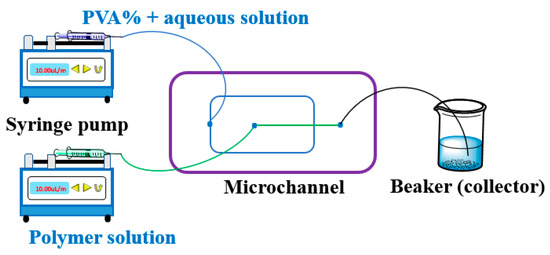
Figure 2.
Microfluidics device setup. Two syringe pumps can precisely control the flow rate of both phases, and the flow rate passes through the microchannel of the chip. The resulting microdroplets are collected in the outlet by a beaker as the collector. More information on the microchannel can be obtained from the reference [90,91,92].
3.5. Nanoprecipitation Method
The nanoprecipitation method is a commonly used technique for preparing magnetic poly(lactic-co-glycolic acid) (PLGA) nanocapsules [67,68,69]. The magnetic nanoparticles are usually coated with a surfactant to prevent agglomeration and ensure good dispersion in the polymer solution. A solution containing PLGA and magnetic nanoparticles is rapidly injected dropwise into a non-solvent solution, such as water or ethanol, under magnetic stirring. This causes the polymer to rapidly precipitate out of solution and form nanoparticles. The disadvantage of this technique is that it cannot precisely control the particle size and size distribution [93], and it is difficult to incorporate hydrophilic drugs using simple nanoprecipitation techniques [94].
4. Superparamagnetic Artificial Cell PLGA-Drug-Fe3O4 Nanocapsules for Cancer Therapeutics
Nanoparticles (NPs) are frequently applied to drug delivery systems (DDS) as they allow delivery of the appropriate drug amount to the targeted site at the desired time [95]. Successful drug delivery systems require high encapsulation efficiency, reduced systemic clearance, and prolonged half-life circulation of the drug in vivo [96]. Biodegradable polymeric artificial cells have gained more and more attention as drug delivery systems to fight malignant tumors and other diseases [8]. Magnetic drug carriers not only achieve high local drug concentrations and avoid the toxicities on other organs and healthy cells, but they can also avoid the influences on the reticuloendothelial system [97,98]. Superparamagnetic artificial cell PLGA-drug-Fe3O4 NPs micro/nanocapsules could be a promising strategy as magnetic-targeted drug carriers for cancer therapeutics to realize fixed-point, timing, and quantitative drug release [99,100,101]. In this section, we will summarize the applications of superparamagnetic artificial cell PLGA-drug-Fe3O4 capsules as anticancer drug carriers in cancer therapy, magnetic hyperthermia, and photothermal therapy.
4.1. Superparamagnetic Artificial Cell PLGA-Fe3O4 Nanocapsules as Cancer-Targeted Therapeutics
4.1.1. Lung Cancer Therapy
Lung cancer is a malignant tumor caused by uncontrolled cell growth in the lung tissue [96]. Nanoparticles can directly deliver drugs to the lung to treat lung diseases, improving the drug treatment effect, reducing systemic toxicity, achieving sustained drug release, and improving patient compliance [102]. Numerous studies have shown that biodegradable polymeric nanocapsules can easily penetrate cell membranes, decreasing the untargeted effects and lysosomal escape following endocytosis, which significantly improves the co-delivery efficiency of chemotherapeutic agents [103,104,105]. Tetrandrine (Tet) [106,107] and doxorubicin (DOX) [108] were encapsulated into magnetic PLGA nanoparticles as nanocarriers to treat lung cancer. Tetrandrine (Tet) can inhibit lung cancer cell proliferation, induce apoptosis [109], and suppress lung cancer growth by destructing the VEGF/HIF-1α/ICAM-1 signaling pathway [110] (Table 2). The magnetic nanocapsule Tet-Fe3O4-PLGA NPs had a higher anti-proliferation effect than the free drug Tet. This is because Tet-Fe3O4-PLGA nanocapsules could effectively penetrate the A549 cell membrane and multicellular spheroids. These prepared magnetic nano-vehicles can highly injure lysosomes, leading to apoptosis [107]. Similarly, DOX was loaded onto the magnetic PLGA to fabricate nanocarriers (F/A-PLGA@DOX/SPIO NPs) as a dual probe. Folic acid (FA) and activatable cell-penetrating peptide (ACPP) were used to improve the cancer cells’ targeting abilities. In vivo studies had shown that the F/A-PLGA@DOX/SPIO NPs had better inhibitory effects on tumor growth and fewer organ toxicities compared to free DOX. In vivo MR R2* imaging studies showed that the R2* value of tumors treated with the F/A-PLGA@SPIO NPs and F/A-PLGA@DOX/SPIO NPs could increase significantly at 1 h and 4 h compared to the SPIONPs [108].

Table 2.
The summarizations of the superparamagnetic artificial cell PLGA-drug-Fe3O4 nanocapsules for cancer therapeutics.
Poly(ethylene glycol) (PEG) can escape immune recognition, prevent macrophage attachment, enhance retention time for drug delivery [139,140], and minimize the non-specific uptake in normal tissues [141]. Thus, the magnetic PLGA-PEG nanoparticle was used to fabricate a magnetic nanocarrier to deliver silibinin (Sil) [111], curcumin (Cur) [112], and co-deliver silibinin (Sil) with metformin (Met) [113]. In the nanosystem Sil/Met-PLGA-PEG-Fe3O4NPs, although the saturation magnetization of this magnetic nanocarrier is low (9 emu/g), they still exhibited superparamagnetic properties. In the cytotoxicity assay, Met/Sil-PLGA-PEG-Fe3O4 NPs at a concentration of 10.51 μM showed a significant inhibitory effect on the lung cancer cell A549 compared to the free drugs (Met and Sil). With increasing concentrations, the Sil/Met-PLGA-PEG-Fe3O4 NPs group also exhibited higher toxicity than the free Met and Sil groups. This co-delivery carrier could achieve a higher synergistic therapeutic effect compared to the individual agent and could reduce the toxicity of the single agent. That magnetic nano-vehicle followed dose-dependent and time-dependent manners in the reaction with the A549 cells. Experiments revealed that these magnetic PLGA nanoparticles exhibit an outstanding anti-cancer effect and targeting function [113].
4.1.2. Breast Cancer Therapy
Breast cancer is one of the most common forms of malignant tumors among women worldwide and poses a severe threat to their well-being [142]. Several drugs for breast cancer and Fe3O4 NPs were co-encapsulated into PLGA or PLGA-PEG shells to form the magnetic nanocapsules to carry the drugs to the breast cancer cells, such as Methotrexate (MTX) [114,115], Doxorubicin (DOX) [116,117], Salvigenin (Sal) [118], Gemcitabine (Gem) [119,120], Paclitaxel (PTX) and Transferrin (Tf) [121], Docetaxel (DTX) [122], Vitamin C (Vc) [123], Olaparib (Olb) [124], and Chlorin E6 (Ce6) [125].
To improve the bioavailability and reduce the toxicity of MTX, the MTX was efficiently encapsulated into the magnetic polymer PLGA to obtain a magnetically guided system for breast cancer. The magnetic MPNP-35 with rough surfaces followed the sustained release mode and showed an apparent anti-proliferation effect on breast cancer cells. The optimized experimental condition achieved a high drug encapsulation efficiency of 84.31% in this study [114].
Similarly, to enhance the therapeutic efficacy of the chemotherapeutic agent paclitaxel (PTX) and its magnetic targeting ability, PTX was co-encapsulated with Tf into a magnetic PLGA nanoparticle to obtain enhanced magnetic nanocomposite (Tf-PLGA-PTX-Fe3O4NPs). Tf is an iron-binding plasma glycoprotein mediated by cell surface Tf receptors overexpressed in proliferating cancer cells. In vitro results and confocal images showed that, compared to the PLGA-PTX-Fe3O4NPs, the Tf-PLGA-PTX-Fe3O4NPs exhibited the highest cytotoxicity on the MCF-7 and U-87 cells due to the fact that Tf could promote cellular uptake and drug accumulation in the tumor cells. Moreover, the cellular uptake efficiency of those magnetic nanoparticles was higher when exposed to an external magnetic field [121].
To improve the activation and release of Vitamin C (Vc) and superparamagnetic iron oxide by specific stimuli in the TME, a magnetic nanoreactor system was designed to incorporate Vc and Fe3O4 NPs [123]. The magnetic system (PLGA-SPIO&Vc) has excellent colloidal stability in the physiological environment within 7 days. However, PLGA-SPIO&Vc would swell well under acidic conditions (pH 6.5) due to the acidic degradation of the PLGA shell. Vc could be significantly released on exposure to LIFU irradiation, especially in acidic conditions, which can promote the chemodynamic therapy efficacy associated with the Fenton reaction. In the flow cytometry, the highest apoptosis of MDA-MB-231 cells (breast cancer cells) was observed in the Ma/PLGA-SPIO&Vc + LIFU group since LIFU irradiation can enhance the permeability of PLGA-SPIO&Vc into the cell membrane, thus facilitating cell phagocytosis. The PLGA-SPIO&Vc were injected into the tumor mice to investigate their pharmacokinetics and biodistribution. The result was that sufficient accumulation of PLGA-SPIO&Vc could be achieved in tumor tissues.
In the case concerning the synergistic platform, both agents of indocyanine green (ICG) and zoledronic acid (ZOL) were co-encapsulated into the magnetic PLGA nanoparticles to form nanocarriers (ICG/Fe3O4@PLGA-ZOL). With the ability of ICG and Fe3O4 to convert light into heat, in vivo experiments showed that ICG/Fe3O4@PLGA-ZOL NPs had remarkable antitumor effects on breast cancer tibial metastasis and could be accurately located in the medullary cavity of the tibia [126].
Likewise, the natural glucose oxidase (GOx) and superparamagnetic Fe3O4 nanoparticles were co-loaded into magnetic PLGA nanocapsules to fabricate a sequential nanocatalyst (GOx@PLGA-Fe3O4). The GOx@PLGA-Fe3O4 NPs group showed higher cytotoxicity than the GOx or Fe3O4 NPs groups. This is because the GOx@PLGA-Fe3O4 NPs can produce highly toxic hydroxyl radicals. Moreover, the GOx@PLGA-Fe3O4 NPs group generated more cellular ROS compared to other groups. The GOx@PLGA-Fe3O4 nanoparticles were injected into mice to evaluate their tumor-catalytic therapeutics. The results showed that the 4T1 mammary cancer cell growth in the GOx@PLGA-Fe3O4+magnetic targeting group was significantly inhibited with a suppression rate of about 87.44%, while the non-magnetic targeting group was approximately 46.73% [127].
Polymeric NPs modified with targeting ligands can increase their intracellular delivery ability and maintain sustained drug release [143]. For example, to improve the targeting ability of magnetic PLGA nanoparticles to the human epidermal growth factor receptor 2 (Her2/neu) overexpressed in breast cancers, Herceptin was grafted onto the PLGA-Fe3O4NPs surface by chemical reaction. In the FACS and MR molecular imaging experiments, a strong contrast enhancement was observed on the Her2/neu overexpressing cell line compared to the Her2/neu non-expressing cell lines, and the signal intensity of in vivo MR imaging was inversely proportional to the concentration of Herceptin [144]. Similarly, L-arginine-based magnetic nanoparticles were constructed for multiple synergistic tumor therapies. The produced Fe3O4@PLGA/LA NPs could significantly enhance the therapeutic effect since drugs accumulate more and have prolonged retention at the tumor site under an external magnet. This nanoplatform exhibited synergistic therapeutic effects by combining HIFU-based tumor ablation and nitric oxide (NO) to assist antitumor gas therapy. Experiments showed that this novel strategy had excellent synergistic effects for tumor suppression compared to individual HIFU or gas therapy [145]. The surface of PEI-PLGA nanoparticles also allowed further modification with hyaluronic acid (HA) to actively target the CD44 receptor [124].
Immunotherapy has gradually become a new approach to cancer treatment in recent years. However, the massive immunosuppressive cells and tumor-associated macrophages are unfavorable to the therapeutic effects. To solve the challenge of an immunosuppressive tumor microenvironment (TME), a magnetic nanocarrier (PLGA-ION-R837@M (PIR@M)) was developed to selectively target and polarize the macrophages to boost and pave the way for immunotherapy in breast cancer [130].
4.1.3. Glioblastoma Therapy
Glioblastoma (GBM) is one of the fatal malignant tumors occurring in the central nervous system of the human body [146]. It possesses several adverse features, including rapid proliferation, high invasiveness, extensive infiltration, and hyper-neoangiogenesis, complicating treatment with the current therapies [146]. In comparison to conventional chemical drugs, nanomedicine possesses several advantages, such as crossing biological barriers, high accumulation at the targeted brain tumor site, and low systemic toxicity [147].
Research demonstrated that the oleic-based magnetic PEG-PLGA nanoparticles (OAMNP) could serve as a potential magnetic targeting nanocarrier for glioblastoma treatment [148]. Magnetic RGD-PLGA-PEG NPs wrapped paclitaxel (PTX) to obtain a magnetic drug delivery system (PTX/SPIO-RGD-PLGA-PEG NPs), where RGD could enhance the cellular uptake through specific binding to the αvβ3 receptors expressed on U87MG and HUVEC cells. The cellular internalization of PTX/SPIO-RGD-PLGA-PEG was nearly twofold greater than that of PTX/SPIO-PLGA-PEG, with no significant cytotoxic effect on the U87MG cells at a concentration of about 0.01 ng/mL. Their anti-tumor efficacy was evaluated by injecting the PTX-SPIO-PLGA NPs into the orthotopic glioma-bearing mice. In the PTX-SPIO-PLGA NPs group, the tumor growth and tumor area in mice were significantly smaller than in other groups. In comparison to the magnetic therapy, the accumulation of the prepared NPs in the active + magnetic group and the magnetic group was all higher than that for the other group. More importantly, the 6 doses of PTX/SPIO-NPs (5 mg/kg PTX) had no toxic effects on the liver, kidney, or heart compared to Taxol [128]. Similarly, Doxorubicin (DOX) was loaded into the magnetic PLGA nanoparticles to generate PLGA-DOX-Fe3O4 NPs for fighting glioma cells. The EC50 was around 20 µg/mL for all the synthesized SPION-DOX HPNPs in glioma cell lines U87 and 9L after treatment for 48 h. This nanocarrier could also enhance the T2 contrast in MRI [129].
Lanthanide doping with iron oxide nanoparticles could enhance the T1 contrast in MRI [149]. Gadolinium (Gd) was doped into the iron oxide magnetic core to obtain a complex superparamagnetic core (Gd-Fe3O4 nanoparticles) to produce Gd-Fe3O4-Lenalidomide-PEI-PLGA NPs. These synthesized nanocarriers still exhibited the superparamagnetic property in the presence of Gd. As a result, the Gd-Fe3O4-Lenalidomide-PEI-PLGA NPs can significantly inhibit the proliferation of glioblastoma cells, effectively accumulate drugs in the tumor site, and target mitochondria [150].
4.1.4. Other Cancer Therapies
Besides lung, breast, and glioblastoma cancer, the drug-loaded magnetic PLGA nanocapsules were also used to treat other cancers. For example, silibinin (SLB) was loaded into magnetic PLGA nanoparticles to treat renal cell cancer [131]. Paclitaxel (PTX) was encapsulated into magnetic PLGA nanoparticles to obtain magnetic nano-vehicles (PTX-Fe3O4-PLGA NPs) for fighting endometrial cancer [132]. Curcumin and verapamil were co-encapsulated into magnetic PLGA nanoparticles for the HepG2 cancer treatment [134]. Mohammed Sedki et al. prepared a hybrid nanocarrier system (SMV- Fe3O4-PLGA-COOH) for enhancing simvastatin cytotoxic activity against prostate cancer [133]. Curcumin (Cur) was loaded in the magnetic PLGA-PEG-FA nanoparticles to obtain multifunctional nano-niosomes (Cur-Fe3O4-PLGA-PEG-FA) for the treatment of cervical cancer (the HeLa229 cells) [135].
A synergistic nanosystem with magnetically targeted and temperature-responsive functions was designed to target hepatoma cells (Figure 3). This system (PFH/DOX@PLGA/Fe3O4–FA) was constructed with biodegradable PLGA, targeting material folate (FA), liquid-gas phase-changeable PFH, and superparamagnetic Fe3O4 NPs. Both pH and HIFU could control the release of loaded anticancer drugs from the nanocomposites. The animal experiments showed that the PFH/DOX@PLGA/Fe3O4–FA group had a higher accumulation of magnetic nanoparticles in the tumor site compared to other groups. The PFH/DOX@PLGA/Fe3O4–FA nanoparticles could enhance the T2 signal contrast. Both contrast-enhanced ultrasound imaging and HIFU ablation efficacy could be improved when PFH/DOX@PLGA/Fe3O4–FA nanoparticles were treated by high-intensity focused ultrasound. Thus, those prepared nanoparticles could efficiently suppress tumor growth through synergistic chemotherapy and HIFU ablation. Additionally, no toxic effects on normal organs had been observed [136].
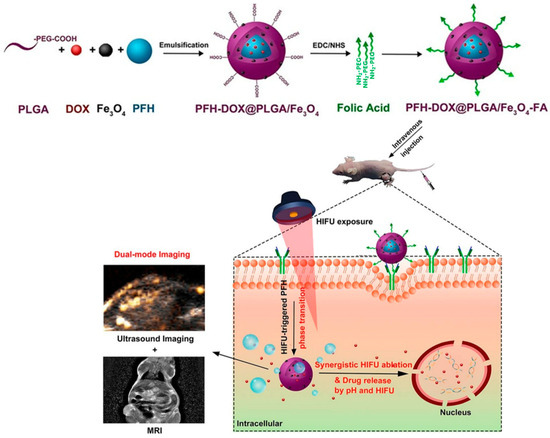
Figure 3.
Schematic illustration of the synthesis of PFH/DOX@PLGA/Fe3O4-FA nanocomposites and corresponding synergistic cancer therapy modals for targeted US/MRI-guided HIFU/chemo. This figure has been taken from ref. [136] with permission from the American Chemical Society, Copyright © 2018.
The anti-CD133 Ab is the cell surface marker in various cancers, such as brain, prostate, and colorectal cancer. The anti-CD133 Ab was conjugated to superparamagnetic PLGA nanoparticles as a targeting nanocarrier to load Oxaliplatin against CaCo-2 colorectal carcinoma cells [137]. The cRGDa can enhance the targeting ability of the magnetic PLGA nanoparticles to the ovarian cancer cells and also improve ultrasound imaging and magnetic resonance imaging (MRI) in vivo. Thus, the multifunctional tumor-targeted PLGA nanoparticles (PFH/siBIRC5/Fe3O4@Pt(IV) NPs-cRGD) were prepared to deliver Pt(IV)/siBIRC5 to ovarian cancer cells [138]. Shortly, combining antibodies on the particle surface can enhance the specific affinity of magnetic nanocarriers to tumor cells [140].
4.2. Superparamagnetic Artificial Cell PLGA-Fe3O4 Nanocapsule for Magnetic Hyperthermia and Photothermal Therapy
4.2.1. Superparamagnetic Artificial Cell PLGA-Fe3O4 Nanocapsules for Magnetic Hyperthermia
Hyperthermia is a promising adjuvant therapy for the treatment of many tumors. Cancer cells have been proven to be more sensitive than normal cells to temperature and pH [151]. Cancer cells would be destroyed at temperatures 41–45 °C by destroying the cell membrane and proteins, which would disturb the nucleic acid synthesis, while healthy cells can still survive [152,153]. Magnetite nanoparticles (MNPs) can generate localized heat under an external magnet and reduce adverse side effects for the normal system. The drug release from superparamagnetic PLGA nanoparticles can be effectively controlled by magnetic induction heating during the treatment of cancer. The glass transition temperature (Tg) of PLGA is around 40 °C [154]. When the temperature is higher than the glass transition temperature of PLGA itself, the polymeric structure will change and accelerate its degradation. Thus, the diffusion and release of the encapsulated drug will be accelerated. Superparamagnetic PLGA nanoparticles can be inductively heated under an external alternating magnetic field (AMF) [155]. These characteristics benefit magnetic hyperthermia ( Table 3) [153,156].

Table 3.
The summarizations of the superparamagnetic artificial cell PLGA-Fe3O4 nanocapsules for magnetic hyperthermia and photothermal therapy.
Approximately 5-fluorouracil is a common anticancer drug with undesired side effects on healthy organs and low bioavailability. When 5-fluorouracil was loaded into the magnetic PLGA nanoparticles, these synthesized PLGA-Fe3O4-5-FU NPs could achieve hyperthermia in the human colon cancer cell line HT-29. The release of 5-FU from PLGA-Fe3O4-5-FU NPs had no effect at 37 and 43 °C. This property is helpful for the use of cancer treatment. The combination group (PLGA-Fe3O4-5-FU nanoparticles + hyperthermia) could significantly reduce the survival of tumor cells compared to the 5-FU-PLGA non-hyperthermia nanoparticles group, indicating that the combined method had an excellent inhibitory ability for tumor colony-formation. Moreover, the group treated with 5-FU-loaded nanoparticles could significantly reduce the survival fraction compared to those treated with the free drug 5-FU [157]. These results were because hyperthermia made 5-FU transfer to active forms more rapidly [164], and the cells at 43 °C were more sensitive to hyperthermia than those at physiological temperatures [165].
Similarly, DOX and Fe3O4 nanoparticles were incorporated into temperature-responsive polymer PLGA nanoparticles to achieve hyperthermia and local heat-triggered chemotherapy for efficient dual cancer treatment [155]. In another study, a thermal-sensitive nano theragnostic agent and phase-shifted magnetic nanoparticles (PMNPs) were combined in a single nanosystem. The PMNPs, which comprise the biocompatible polymer PLGA shell and the liquid core perfluoropentane (PFP), can easily be converted into nano- and/or microbubbles upon heating to physiological temperatures. The PMNPs and MNPs groups could increase the temperature of cell media rapidly compared with the other groups, indicating that PMNPs could act as efficient NIR heat absorbers for hyperthermia therapy (HTT) [158].
Approximately 17-N-allylamino-17-demethoxygeldanamycin (17AAG) was the first Hsp90 inhibitor with broad-spectrum anticancer activities. Combining 17AAG (a Hsp90 inhibitor) and Fe3O4 magnetic nanoparticles could provide a magnetic hyperthermia strategy for pancreatic cancer treatment [159]. Glycine-arginine-glycine-aspartic acid-serine (GRGDS) can selectively recognize the vβ3/vβ5 integrins overexpressed on the glioblastoma cells. GRGDS conjugated to curcumin-Fe3O4-PLGA nanoparticles can increase cellular uptake to achieve hyperthermia treatment in glioblastoma cells. These synthesized nanoparticles could generate heat to elevate the local temperature to the hyperthermia treatment degree (~42–45 °C) when exposed to an AC magnetic field for 15 min. GRGDS-Cur-m-PNPs could damage the intact cell membrane when exposed to an RF field [160]. This is because the magnetic field could increase the cellular uptake and permeability of the blood-brain barrier [166].
4.2.2. Superparamagnetic Artificial Cell PLGA-Fe3O4 Nanocapsule for Photothermal Therapy
Photothermal therapy (PTT) is a less invasive and highly effective therapeutic alternative for cancer treatment that employs photo-absorbing nanoparticles to convert photon energy from near-infrared (NIR) lasers into thermal energy to “burn” the cancer cells.
A triple-modal imaging-guided magnetic nanocarrier (IR780/Fe3O4@PLGA/PFP/DOX NPs) was produced for breast cancer treatment. Specifically, this complex nano-system consists of near-infrared fluorescence, magnetic resonance, and ultrasound. The samples were irradiated with an 808 nm laser for 5 min in vitro. The temperatures of IR780/Fe3O4@PLGA/PFP/DOX NPs were 54 °C, and the photothermal conversion efficiency (η) of them (1.0 mg/mL) was 35.7%, showing excellent PTT performance. When combined with laser irradiation, the DOX release from the IR780/Fe3O4@PLGA/PFP/DOX NPs was as high as 35% within 30 min and 90% within 72 h. Both in vitro and in vivo studies revealed that the prepared magnetic nano-system possesses excellent synergistic performance in magnetic targeting, chemo-photothermal therapy of cancer [117].
Indocyanine green (ICG) is the only NIR agent approved by the U.S. Food and Drug Administration (FDA) for human clinical NIR fluorescence (NIRF) imaging and diagnosis [167]. To overcome its instability in aqueous solutions, rapid blood clearance, and light- and temperature-dependent properties, ICG and pefluoropentane (PFP) were integrated into magnetic PLGA nanoparticles to form dual NIR light-absorbing PFC-based polymeric NPs (Fe3O4/ICG@PLGA/PFP NPs). Because Fe3O4 NPs and ICG contribute to NIR light-absorbing agents efficiently converting absorbed light into heat, Fe3O4/ICG@PLGA/PFP NPs could effectively generate heat when exposed to NIR laser irradiation. When MCF-7 breast cancer cells were treated with Fe3O4/ICG@PLGA/PFP NPs and irradiated by an NIR laser, the cells were severely damaged. Fe3O4/ICG@PLGA/PFP NPs were injected in mice with MCF-7 breast tumor cells and could ablate tumor cells under NIR laser irradiation [161].
In another case, ICG and glucose oxidase (GOx) were covalently attached to the surface of the iron oxide nanoparticles to obtain a magnetic photothermal agent (ICGOx@IO). Then this generated magnetic core (ICGOx@IO NPs), DOX, and EGCG ((-)-epigallocatechin-3-gallate) were co-encapsulated into PLGA NPs against melanoma tumors. This complex nanosystem exhibited excellent photothermal efficiency when irradiated for 10 min with an external magnet. The prepared magnetic nanoparticles exhibited significantly higher cytotoxicity than the free DOX and EGCG [162]. Similarly, only ICG was covalently attached to the iron oxide (IO) nanoparticle surface to obtain a magnetic photothermal agent (PIO NPs). Then the PIO NPs, DOX, and Mcl-1-siRNA were co-encapsulated into PLGA-CS NPs to treat breast cancer. In this nanosystem, the siRNAs can overcome the chemoresistance in the breast cells. PIO-DOX-siRNA-PCSCM NPs could maintain good photothermal effects and increase solution temperature under NIR laser irradiation [163].
AS1411 is a specific aptamer to nucleolin expressed in pancreatic cancer cells. The magnetic PLGA-curcumin nanoparticles modified with AS1411 can enhance their specific targeting capability for cancer cells. This prepared nanocomposite could also enhance the photoacoustic (PA) signal contrast (around 900–950 nm). They showed good biocompatibility and low cellular cytotoxicity on the L929 cells compared to SPION alone, attributed to the presence of the AS1411 aptamer. The temperature of the cancer cells treated with the produced magnetic nanoparticles could reach 65 ± 3 °C from 27 °C when exposed to an NIR laser within 5 min, induced photothermal ablation of cancer cells [69].
5. Superparamagnetic Artificial Cell PLGA-Drug-Fe3O4 Microcapsules for Cancer Therapeutics
Along with nanoparticles, micro-dimension microspheres (MPs) are another popular drug delivery system (DDS), as they can achieve targeted treatment and control drug amounts [29,36,142]. The drug release rate from the polymer microspheres could be controlled by optimizing the polymeric coating formulation, such as the molecular weight and the composition of the polymers [168]. The superparamagnetic polymeric microspheres (MMS) usually consist of a magnetic core (Fe3O4 NPs) and polymeric shells, including PLA, PLGA, and PLGA-PEG [169]. The microsphere size usually ranges from 10 to 200 μm in the clinic. Outside of these ranges, the smaller microspheres will be engulfed by the immune cells, and the larger microspheres will cause inflammation [170]. The PLGA-embedded drug micro-carriers have exhibited outstanding sustained release characteristics and achieved local accumulations in the desired tumor sites. Several drugs were successfully encapsulated into the magnetic PLGA microcapsules to form the micro-carriers, such as 5-fluorouracil (5-FLU) [171], curcumin (CUR) [49,172], camptothecin (CPT) [173,174,175], paclitaxel (PTX) [176], doxorubicin (DOX) [177], and vincristine (Vin) [54] (Table 4 ). To achieve a targeted therapy and reduce the surrounding healthy cell damage caused by the AMF irradiation, the Fe3O4 nanoparticles and PTX were co-loaded into PLGA microspheres to achieve magnetic navigation [176].

Table 4.
The summarizations of the superparamagnetic artificial cell PLGA-drug-Fe3O4 microcapsule for cancer therapeutics.
To solve the limitations on solid tumor treatments, including the limited penetration of anti-cancer drugs in solid tumors, the side effects, the development of drug resistance, and the poor cellular uptake of common cancer therapies, the chemical drugs DOX and Vin were co-encapsulated into magnetic PLGA microspheres. The magnetic saturation of the PLGA-Fe3O4 microspheres (0.6–1.4 emu/g) is much lower than that of free Fe3O4 NPs (70.5 emu/g). This is because the content of Fe3O4 in the magnetic microspheres is about 2%. Despite this, those magnetic PLGA microspheres still exhibited superparamagnetic properties. The entrapment efficiency of the drug in the shell is greater than that inside the microsphere. This drug distribution would affect the drug release pattern and the release rate. However, those generated magnetic microspheres followed a sustained release profile. Especially, Fe3O4@(P + V)/D microspheres could significantly inhibit the growth of osteosarcoma saos-2 cells at a concentration of 100 μg/mL within 48 h with only 14.9% cell viability, indicating that they had an excellent anti-tumor effect [54].
Magnetic microspheres can generate heat by magnetothermal conversion when exposed to an alternating magnetic field [175]. The drug diffusion rate and the polymer degradation rate can be changed when the local temperature is increased by magnetothermal conversion, resulting in an influence on drug release kinetics [178]. A magnetic drug microcarrier was made of magnetic nanoparticles (MNP), camptothecin (CPT), and a polymeric matrix PLGA shell. The drug burst release occurred at 37 and 43 °C in the first hour, followed by a plateau phase. The magnetic PLGA microspheres obtained had an excellent heating performance of 161 W/gM. When applying a magnetic field, the magnetic microspheres were heated to 44 °C within one hour, and the drug from the prepared microspheres released about 50% more than that at 37 °C [175].
Reactive oxygen species (ROS) can damage biological macromolecules such as nucleic acids [179]. Hydrogen peroxide (H2O2) is one source of ROS, which plays a crucial role in cell signaling pathways. The toxic effects of excess ROS will cause a range of pathologies, such as cancer, aging, and diabetes [180]. A ROS-sensitive magnetic micro-carrier was fabricated to deliver the antitumor drug camptothecin (CPT). This magnetic micro-carrier consisted of ROS-sensitive tween 80-coated Fe3O4 NPs, CPT, and a biodegradable PLGA-PVA layer. The addition of PVA increased the swelling properties of the CPT-MMSs, which further improved a significant drug encapsulation efficiency of ∼92% and had a higher drug content of ∼15%. CPT-MMSs showed a significantly higher releasing efficiency (90%) in the presence of ROS (H2O2) than in the absence of H2O2 (40%) at pH 5.4. CPT-MMS-4 exhibited the highest cytotoxic effect on HeLa cells (IC50 = 0.9 ± 0.38 μg/mL) compared to free CPT (5.2 ± 0.23 μg/mL) and Tween 80-MIONs (19.5 ± 0.98 μg/mL) [174].
Nanomedicines combining chemotherapy and photothermal therapy can compensate for the deficiencies of the traditional approaches to osteosarcoma treatment. When individual photothermal therapy is insufficient to ablate the tumor cells completely, the residual tumor cells can also be effectively killed by increasing the drug release using the rising temperature [181]. Multifunctional magnetic microcapsules PB@(Fe3O4@PEG-PLGA) MCs and (PB+DOX)@(Fe3O4@PEG-PLGA)MCs were developed for osteosarcoma therapy. They possessed magnetic targeting ability and chemo-photothermal therapeutic efficiency. Simultaneous NIR laser irradiation and magnetic field could enhance the drug release rate from PB@(Fe3O4@PEG-PLGA) MCs and (PB+DOX)@(Fe3O4@PEG-PLGA)MCs. PB@(Fe3O4@PEG-PLGA) MCs could effectively target the tumor tissue against the invasion of osteosarcoma and the alleviation of osteolytic lesions [177].
6. Conclusions
In this review, we have introduced the preparation methods of Fe3O4 NPs and presented the drug and superparamagnetic Fe3O4 NPs encapsulated into the artificial cells as PLGA micro/nanocapsules. More importantly, we summarized the applications of superparamagnetic artificial cells (PLGA-Fe3O4) micro/nanocapsules in cancer-targeted therapy. At present, superparamagnetic artificial cell PLGA-Fe3O4 micro/nanocapsules have many advantages, such as high drug loading and controllable release behavior, magnetic targeting, and combination with other therapy methods. Moreover, superparamagnetic artificial cell PLGA-Fe3O4 micro/nanocapsules can also integrate multiple drugs as multifunctional platforms to treat cancer.
The size and distribution of superparamagnetic artificial cells PLGA-Fe3O4 micro/nanocapsules will affect the drug loading capacity and release rate of the drugs from artificial cells PLGA micro/nanocapsules, biodistribution, and bioavailability together. Therefore, to obtain uniform superparamagnetic artificial cells, PLGA-Fe3O4-drug micro/nanocapsules are essential for their applications in anti-tumor treatment. However, many current studies are only focused on some tumor models and small active molecules, and there are still many difficulties in achieving clinical applications, such as how to overcome the cytotoxicity of materials, prepare uniform particles to increase the uptake of cells, and achieve precise control and personalized treatment. Therefore, we can extrapolate the application of artificial PLGA micro/nanocapsules (Fe3O4NPs) in the delivery of the gene, cell, stem cell, oxygen carrier, NO donor, or other complex biological substances. We could extend the simple core-shell structure of artificial cell PLGA-Fe3O4 nano/microcapsules to multi-compartments to allow the integration of multiple active substances in a single carrier to complete synergistic delivery for cancer treatment. Moreover, we could also pay more attention to the development of preparation technology for complex magnetic PLGA-drug artificial cells and the large-scale production and reproductivity of prepared technology for artificial cell PLGA-Fe3O4-drug micro/nanocapsules in the future.
Author Contributions
T.W. carried out the literature review and laboratory research and wrote the manuscript; T.M.S.C. is the research director who conceived the central idea and contributed to the discussion of the needed results and modifications. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The donation by Proheme Biotechnology to McGill University’s Advancement Program as unrestricted funding to Chang’s laboratory is gratefully acknowledged. We thank Muntasirul Hoq and ChenHui Zhao for editing the English and grammar of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef] [PubMed]
- Somatilaka, B.N.; Sadek, A.; McKay, R.M.; Le, L.Q. Malignant peripheral nerve sheath tumor: Models, biology, and translation. J. Oncogene 2022, 41, 2405–2421. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, Z.; Xiao, L.; Hu, W.; Zhang, L.; Xie, B.; Zhou, Q.; He, J.; Qiu, Y.; Wen, M. Glutamine synthetase promotes radiation resistance via facilitating nucleotide metabolism and subsequent DNA damage repair. J. Cell. Rep. 2019, 28, 1136–1143.e1134. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Chang, T.M. Semipermeable microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef]
- Chang, T.M.S. Monograph ARTIFICIAL CELLS: Biotechnology, nanotechnology, blood Substitutes, regenerative medicine, bioencapsulation, cell/stem Cell Therapy. Imp. Coll. Press. Optim. Ser. 2007, 35, 545–554. [Google Scholar]
- Chang, T.M.S. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. J. Artif. Cells. Nanomed. Biotechnol. 2019, 47, 997–1013. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. The role of artificial cells in the fight against COVID-19: Deliver vaccine, hemoperfusion removes toxic cytokines, nanobiotherapeutics lower free radicals and pCO2 and replenish blood supply. J. Artif. Cells. Nanomed. Biotechnol. 2022, 50, 240–251. [Google Scholar] [CrossRef]
- Wang, J.; Dong, R.; Wu, H.; Cai, Y.; Ren, B. A review on artificial micro/nanomotors for cancer-targeted delivery, diagnosis, and therapy. Nano-Micro Lett. 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, L.; Simmchen, J.; Magdanz, V. Nano-and micromotors designed for cancer therapy. Molecules 2019, 24, 3410. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M. Semipermeable aqueous microcapsules (“Artificial Cells”): With emphasis on experiments in an extracorporeal shunt system. ASAIO J. 1966, 12, 13–19. [Google Scholar]
- Chang, T.M. Biodegradables semipermeable microcapsules containning enzymes, hormones, vaccines, and other biologicals. J. Bioeng. 1976, 1, 25–32. [Google Scholar] [PubMed]
- Grumezescu, V.; Gherasim, O.; Negut, I.; Banita, S.; Holban, A.M.; Florian, P.; Icriverzi, M.; Socol, G. Nanomagnetite-embedded PLGA spheres for multipurpose medical applications. Materials 2019, 12, 2521. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Cham-Fai Leung, K. Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. J. Mater. 2020, 13, 4147. [Google Scholar] [CrossRef]
- Gu, B.; Du, Y.; Fang, S.; Chen, X.; Li, X.; Xu, Q.; Lu, H. Fabrication of UV-stable perovskite solar cells with compact Fe2O3 electron transport layer by FeCl3 solution and Fe3O4 nanoparticles. J. Nanomater. 2022, 12, 4415. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. J. Gels 2023, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yuan, M.; Yuan, H.; Huang, X.; Sui, X.; Cui, X.; Tang, F.; Peng, J.; Chen, J.; Lu, S. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int. J. Nanomed. 2012, 7, 1697. [Google Scholar]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Seenuvasan, M.; Vinodhini, G.; Malar, C.G.; Balaji, N.; Kumar, K.S. Magnetic nanoparticles: A versatile carrier for enzymes in bio-processing sectors. IET Nanobiotechnol. 2018, 12, 535–548. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid. Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Ruirui, Z.; He, J.; Xu, X.; Li, S.; Peng, H.; Deng, Z.; Huang, Y. PLGA-based drug delivery system for combined therapy of cancer: Research progress. Mater. Res. Express. 2021, 8, 122002. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly (lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Mirakabad, F.S.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Tan, W.S.; Ho, K.L.; Mariatulqabtiah, A.R.; Abu Kasim, N.H.; Abd Rahman, N.; Wong, T.W.; Chee, C.F. Challenges and Complications of Poly (lactic-co-glycolic acid)-Based Long-Acting Drug Product Development. Pharmaceutics 2022, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Patel, M.; Patel, R. PLGA core-shell nano/microparticle delivery system for biomedical application. Polymers 2021, 13, 3471. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.-L.; Hamid, Z.A.; Mariatti, M.; Yahaya, B.; Lim, K.; Bee, S.-T.; Sin, L.T. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: A review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Opin. Green. Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Tadic, M.; Kralj, S.; Kopanja, L. Synthesis, particle shape characterization, magnetic properties and surface modification of superparamagnetic iron oxide nanochains. Mater. Charact. 2019, 148, 123–133. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Wang, H. Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Wu, K.; Kuo, P.; Yao, Y.; Tsai, E. Magnetic and Optical Properties of Fe3O4 Nanoparticle Ferrofluids Prepared by Coprecipitation Technique. IEEE Trans. Magn. 2001, 37, 2651–2653. [Google Scholar] [CrossRef]
- Hua, C.C.; Zakaria, S.; Farahiyan, R.; Khong, L.T. Size-controlled synthesis and characterization of Fe. Sains Malays. 2008, 37, 389–394. [Google Scholar]
- Zhang, L.; Wu, J.; Liao, H.; Hou, Y.; Gao, S. Octahedral Fe3O4 nanoparticles and their assembled structures. Chem. Commun. 2009, 4378–4380. [Google Scholar] [CrossRef] [PubMed]
- Bateer, B.; Qu, Y.; Tian, C.; Du, S.; Ren, Z.; Wang, R.; Pan, K.; Fu, H. Facile synthesis of stable magnetic fluid using size-controlled Fe3O4 nanoparticles. Mater. Res. Bull. 2014, 56, 34–38. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion synthesis of superparamagnetic nanoparticles for bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cui, L.; Tong, N.; Gu, H. Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J. Am. Chem. Soc. 2006, 128, 15582–15583. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, R. Hydrothermal synthesis and characterization of nanocrystalline Fe3O4 powders. Mater. Res. Bull. 1998, 33, 1015–1021. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Ayyanaar, S.; Kesavan, M.P.; Sivaraman, G.; Maddiboyina, B.; Annaraj, J.; Rajesh, J.; Rajagopal, G. A novel curcumin-loaded PLGA micromagnetic composite system for controlled and pH-responsive drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 188–195. [Google Scholar] [CrossRef]
- Bootdee, K.; Nithitanakul, M.; Grady, B.P. Synthesis and encapsulation of magnetite nanoparticles in PLGA: Effect of amount of PLGA on characteristics of encapsulated nanoparticles. Polym. Bull. 2012, 69, 795–806. [Google Scholar] [CrossRef]
- Enriquez, G.G.; Rizvi, S.A.; D’Souza, M.J.; Do, D.P. Formulation and evaluation of drug-loaded targeted magnetic microspheres for cancer therapy. Int. J. Nanomed. 2013, 8, 1393. [Google Scholar]
- Schliehe, C.; Schliehe, C.; Thiry, M.; Tromsdorf, U.I.; Hentschel, J.; Weller, H.; Groettrup, M. Microencapsulation of inorganic nanocrystals into PLGA microsphere vaccines enables their intracellular localization in dendritic cells by electron and fluorescence microscopy. J. Control. Release 2011, 151, 278–285. [Google Scholar] [CrossRef]
- Gun, S.; Edirisinghe, M.; Stride, E. Encapsulation of superparamagnetic iron oxide nanoparticles in poly-(lactide-co-glycolic acid) microspheres for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, H.; Yao, J.; Zhu, Z.; Sun, D.; Zhang, M. A doxorubicin and vincristine drug release system based on magnetic PLGA microspheres prepared by coaxial electrospray. ACS Appl. Mater. Interfaces 2019, 54, 9689–9706. [Google Scholar] [CrossRef]
- Faramarzi, A.-R.; Barzin, J.; Mobedi, H. Producing PLGA fine particles containing high magnetite nanoparticles by using the electrospray technique. J. Polym. Res. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Nosrati, Z.; Li, N.; Michaud, F.; Ranamukhaarachchi, S.; Karagiozov, S.; Soulez, G.; Martel, S.; Saatchi, K.; Häfeli, U.O. Development of a coflowing device for the size-controlled preparation of magnetic-polymeric microspheres as embolization agents in magnetic resonance navigation technology. ACS Biomater. Sci. Eng. 2018, 4, 1092–1102. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Q.; Han, S.; Xu, W.; Zhong, F.; Yuan, S.; Dwivedi, P.; Si, T.; Xu, R.X. Rapid production of single-and multi-compartment polymeric microcapsules in a facile 3D microfluidic process for magnetic separation and synergistic delivery. Sens. Actuators B Chem. 2018, 275, 190–198. [Google Scholar] [CrossRef]
- He, C.; Zeng, W.; Su, Y.; Sun, R.; Xiao, Y.; Zhang, B.; Liu, W.; Wang, R.; Zhang, X.; Chen, C. Microfluidic-based fabrication and characterization of drug-loaded PLGA magnetic microspheres with tunable shell thickness. Drug Deliv. 2021, 28, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, L.; Zhong, Y.; Luo, Y. Preparation of magnetic poly (lactic-co-glycolic acid) microspheres featuring monodispersity and controllable particle size using a microchannel device. Polym. Int. 2015, 64, 1425–1432. [Google Scholar] [CrossRef]
- Hu, L.; Huang, M.; Wang, J.; Zhong, Y.; Luo, Y. Preparation of magnetic poly (lactic-co-glycolic acid) microspheres with a controllable particle size based on a composite emulsion and their release properties for curcumin loading. J. Appl. Polym. Sci. 2016, 133, 3. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, J.; Luo, S. Generation of well-defined micro/nanoparticles via advanced manufacturing techniques for therapeutic delivery. Materials 2018, 11, 623. [Google Scholar] [CrossRef]
- Chang, T. Report on “Method for Preparing Artificial Hemoglobin Corpuscles”. Bachelor’s Thesis, McGill University, Montreal, QC, Canada, 1957. Available online: http://www.medicine.mcgill.ca/artcell/514.pdf (accessed on 9 November 2023).
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Nt. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Oster, C.; Kissel, T. Comparative study of DNA encapsulation into PLGA microparticles using modified double emulsion methods and spray drying techniques. J. Microencapsul. 2005, 22, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Furtmann, B.; Tang, J.; Kramer, S.; Eickner, T.; Luderer, F.; Fricker, G.; Gomez, A.; Heemskerk, B.; Jähn, P.S. Electrospray synthesis of poly (lactide-co-glycolide) nanoparticles encapsulating peptides to enhance proliferation of antigen-specific CD8+ T cells. J. Pharm. Sci. 2017, 106, 3316–3327. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenorio, M.K.; Pineda, E.A.G.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Juríková, A.; Csach, K.; Koneracká, M.; Závišová, V.; Múčková, M.; Tomašovičová, N.; Lancz, G.; Kopčanský, P.; Timko, M.; Miškuf, J. Magnetic polymer nanospheres for anticancer drug targeting. J. Phys. Conf. Ser. 2023, 42, 122004. [Google Scholar] [CrossRef]
- Sivakumar, B.; Aswathy, R.G.; Romero-Aburto, R.; Mitcham, T.; Mitchel, K.A.; Nagaoka, Y.; Bouchard, R.R.; Ajayan, P.M.; Maekawa, T.; Sakthikumar, D.N. Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agents. Biomater. Sci. 2017, 5, 432–443. [Google Scholar] [CrossRef]
- Chang, T.M.S. Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, and Cell/Stem Cell Therapy; World Scientific: Hackensack, NJ, USA, 2007; Available online: http://www.medicine.mcgill.ca/artcell/504.pdf (accessed on 9 November 2023).
- Chung, T.-W.; Huang, Y.-Y.; Liu, Y.-Z. Effects of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int. J. Pharm. 2001, 212, 161–169. [Google Scholar] [CrossRef]
- Khoee, S.; Yaghoobian, M. An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. Eur. J. Med. Chem. 2009, 44, 2392–2399. [Google Scholar] [CrossRef]
- Li, W.; Zaloga, J.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, S.; Park, K.; Kim, K.; Woo, D.G.; Kwon, I.C.; Chung, H.-M.; Park, K.-H. Heparin/poly (L-lysine) nanoparticle-coated polymeric microspheres for stem-cell therapy. J. Am. Chem. Soc. 2007, 129, 5788–5789. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Fujimori, H.; Makino, K. Incorporation of water-soluble drugs in PLGA microspheres. Colloids Surf. B Biointerfaces 2007, 54, 173–178. [Google Scholar] [CrossRef]
- Marante, T.; Viegas, C.; Duarte, I.; Macedo, A.S.; Fonte, P. An overview on spray-drying of protein-loaded polymeric nanoparticles for dry powder inhalation. Pharmaceutics 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Kavadiya, S.; Biswas, P. Electrospray deposition of biomolecules: Applications, challenges, and recommendations. J. Aerosol Sci. 2018, 125, 182–207. [Google Scholar] [CrossRef]
- Morais, A.Í.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.; Cambrussi, A.N.; Santos, J.A.; Bezerra, R.D.; Furtini, J.A.; Silva-Filho, E.C. Fabrication of polymeric microparticles by electrospray: The impact of experimental parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef]
- Jeyhani, M.; Mak, S.Y.; Sammut, S.; Shum, H.C.; Hwang, D.K.; Tsai, S.S. Controlled electrospray generation of nonspherical alginate microparticles. ChemPhys Chem. 2018, 19, 2113–2118. [Google Scholar] [CrossRef]
- Neisiany, R.E.; Aminoroaya, A.; Farzi, G.; Das, O. Encapsulation: Electrospray. In Principles of Biomaterials Encapsulation: Volume One; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–212. [Google Scholar]
- Zhao, M.L.; Yang, Y.; Yu, D.; Yan, P. Advances in droplet-based microfluidic technology and its applications. Chin. J. Anal. Chem. 2017, 45, 282–296. [Google Scholar]
- Xu, Z.; Lu, C.; Riordon, J.; Sinton, D.; Moffitt, M.G. Microfluidic manufacturing of polymeric nanoparticles: Comparing flow control of multiscale structure in single-phase staggered herringbone and two-phase reactors. Langmuir 2016, 32, 12781–12789. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Controlled and tunable polymer particles’ production using a single microfluidic device. Appl. Nanosci. 2018, 8, 905–914. [Google Scholar] [CrossRef]
- Watanabe, T.; Motohiro, I.; Ono, T. Microfluidic formation of hydrogel microcapsules with a single aqueous core by spontaneous cross-linking in aqueous two-phase system droplets. Langmuir 2019, 35, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Sparago, C.; Palocci, C. A modular microfluidic platform for the synthesis of biopolymeric nanoparticles entrapping organic actives. J. Nanopart Res. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Yi, H.; Lu, S.; Wu, J.; Wang, Y.; Luo, G. Parallelized microfluidic droplet generators with improved ladder–tree distributors for production of monodisperse γ-Al2O3 microspheres. Particuology 2022, 62, 47–54. [Google Scholar] [CrossRef]
- Huang, Y.; Han, T.; Xuan, J.; Xu, H.; Wang, Y.; Zhang, L. Design criteria and applications of multi-channel parallel microfluidic module. J. Micromech. Microeng. 2018, 28, 105021. [Google Scholar] [CrossRef]
- Ramos Payán, M.a.; Santigosa, E.; Fernández Torres, R.; Bello Lopez, M.A. A new microchip design. A versatile combination of electromembrane extraction and liquid-phase microextraction in a single chip device. J. Anal. Chem. 2018, 90, 10417–10424. [Google Scholar] [CrossRef] [PubMed]
- Bokharaei, M.; Schneider, T.; Dutz, S.; Stone, R.C.; Mefford, O.T.; Häfeli, U.O. Production of monodispersed magnetic polymeric microspheres in a microfluidic chip and 3D simulation. J. Microfluid. Nanofluidics 2016, 20, 1–14. [Google Scholar] [CrossRef]
- Chou, W.-L.; Lee, P.-Y.; Yang, C.-L.; Huang, W.-Y.; Lin, Y.-S. Recent advances in applications of droplet microfluidics. J. Micromachines 2015, 6, 1249–1271. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.; Zhao, C.-X. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 2019, 59, 4134–4149. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291. [Google Scholar] [CrossRef]
- Talischi, M.H.; Mohamadnia, A.; Mahmoodi, M.; Bahrami, N.; Nasab, A.F. Predictive Molecular Blood Biomarkers in Non-Small Cell Lung Cancer. J. Cell. Mol. Anesth. 2022, 7, 116–121. [Google Scholar]
- Mirza, S.; Ahmad, M.S.; Shah, M.I.A.; Ateeq, M. Magnetic nanoparticles: Drug delivery and bioimaging applications. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–213. [Google Scholar]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Gao, X.; Liu, W.; Hong, T.; Chen, C. Drug-loaded microbubbles combined with ultrasound for thrombolysis and malignant tumor therapy. Biomed. Res. Int. 2019, 2019, 6792465. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Cheah, P.; Qu, J.; Liu, L.; Zhao, Y. Applications of Nanomaterials for Theranostics of Melanoma. J. Nanotheranostics 2020, 1, 39–55. [Google Scholar] [CrossRef]
- Feng, X.; Dixon, H.; Glen-Ravenhill, H.; Karaosmanoglu, S.; Li, Q.; Yan, L.; Chen, X. Smart nanotechnologies to target tumor with deep penetration depth for efficient cancer treatment and imaging. Adv. Ther. 2019, 2, 1900093. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 2020, 25, 150–159. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Khurana, R.K.; Kesharwani, P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef]
- Sun, H.; Dong, Y.; Feijen, J.; Zhong, Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Control. Release 2018, 290, 11–27. [Google Scholar] [CrossRef]
- Wang, K.; Hu, H.; Zhang, Q.; Zhang, Y.; Shi, C. Synthesis, purification, and anticancer effect of magnetic Fe3O4-loaded poly (lactic-co-glycolic) nanoparticles of the natural drug tetrandrine. J. Microencapsul. 2019, 36, 356–370. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Q.; Hu, H.; Yang, F.; Li, K.; Zhang, Y.; Shi, C. Enhancing cellular morphological changes and ablation of cancer cells via the interaction of drug co-loaded magnetic nanosystems in weak rotating magnetic fields. RSC Adv. 2020, 10, 14471–14481. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mei, C.; He, L.; Xiao, Z.; Chan, L.; Zhang, D.; Shi, C.; Chen, T.; Luo, L. Designing multifunctional cancer-targeted nanosystem for magnetic resonance molecular imaging-guided theranostics of lung cancer. Drug Deliv. 2018, 25, 1811–1825. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Li, W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016, 7, 40800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Zhao, F.; Yang, G.; Wang, J.J. Tetrandrine suppresses lung cancer growth and induces apoptosis, potentially via the VEGF/HIF-1α/ICAM-1 signaling pathway. Oncol. Lett. 2018, 15, 7433–7437. [Google Scholar] [CrossRef] [PubMed]
- Amirsaadat, S.; Pilehvar-Soltanahmadi, Y.; Zarghami, F.; Alipour, S.; Ebrahimnezhad, Z.; Zarghami, N. Silibinin-loaded magnetic nanoparticles inhibit hTERT gene expression and proliferation of lung cancer cells. J. Artif. Cells. Nanomed. Biotechnol. 2017, 45, 1649–1656. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Dariushnejad, H.; Sanjarian, F.; Zarghami, N. The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Anticancer. Agents Med. 2017, 17, 1363–1373. [Google Scholar] [CrossRef]
- Salmani Javan, E.; Lotfi, F.; Jafari-Gharabaghlou, D.; Mousazadeh, H.; Dadashpour, M.; Zarghami, N. Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pac. J. Cancer Prev. 2022, 23, 519–527. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Alipour, S.; Montaseri, H. Fabrication and in vitro evaluation of magnetic PLGA nanoparticles as a potential Methotrexate delivery system for breast cancer. J. Drug. Deliv. Sci. Technol. 2018, 44, 467–474. [Google Scholar] [CrossRef]
- Basu, T.; Singh, S.; Pal, B. Fe3O4@PLGA-PEG Nanocomposite for Improved Delivery of Methotrexate in Cancer Treatment. ChemistrySelect 2018, 3, 8522–8528. [Google Scholar] [CrossRef]
- Liang, C.; Li, N.; Cai, Z.; Liang, R.; Zheng, X.; Deng, L.; Feng, L.; Guo, R.; Wei, B. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biocompatible PLGA-PEG nanocarriers for early detection and treatment of tumours. J. Artif. Cells. Nanomed. Biotechnol. 2019, 47, 4211–4221. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Zhu, Y.; Zhang, M.; Tang, S.; Li, J.; Pei, W.; Huang, B.; Niu, C. Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 42102–42114. [Google Scholar] [CrossRef] [PubMed]
- Mansourizadeh, F.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Tousi, M.S. Designing Salvigenin–loaded mPEG-b-PLGA@ Fe3O4 nanoparticles system for improvement of Salvigenin anti-cancer effects on the breast cancer cells, an in vitro study. J. Drug Deliv. Sci. Technol. 2020, 57, 101619. [Google Scholar] [CrossRef]
- Hamzian, N.; Hashemi, M.; Ghorbani, M.; Toosi, M.H.B.; Ramezani, M. Preparation, optimization and toxicity evaluation of (SPION-PLGA)±PEG nanoparticles loaded with Gemcitabine as a multifunctional nanoparticle for therapeutic and diagnostic applications. Iran. J. Pharm. Res. 2017, 16, 8. [Google Scholar] [PubMed]
- Hamzian, N.; Hashemi, M.; Ghorbani, M.; Aledavood, S.A.; Ramezani, M.; Toosi, M.H.B. In-vitro study of multifunctional plga-spion nanoparticles loaded with gemcitabine as radiosensitizer used in radiotherapy. Iran. J. Pharm. Res. 2019, 18, 1694. [Google Scholar]
- Cui, Y.-N.; Xu, Q.-X.; Davoodi, P.; Wang, D.-P.; Wang, C.-H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef]
- Panda, J.; Satapathy, B.S.; Majumder, S.; Sarkar, R.; Mukherjee, B.; Tudu, B. Engineered polymeric iron oxide nanoparticles as potential drug carrier for targeted delivery of docetaxel to breast cancer cells. J. Magn. Magn. Mater. 2019, 485, 165–173. [Google Scholar] [CrossRef]
- Deng, L.; Liu, M.; Sheng, D.; Luo, Y.; Wang, D.; Yu, X.; Wang, Z.; Ran, H.; Li, P. Low-intensity focused ultrasound-augmented Cascade chemodynamic therapy via boosting ROS generation. Biomaterials 2021, 271, 120710. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Tang, W.; Zhang, Q.; Li, M.; Jin, H.; Huang, Z.; Cui, Z.; Xu, J.; Wang, K. A multifunctional magnetic nanosystem based on “two strikes” effect for synergistic anticancer therapy in triple-negative breast cancer. J. Control. Release 2020, 322, 401–415. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, S.; Guo, Q.; Jing, Z.; Huang, B.; Pan, Y.; Wang, L.; Hu, Y. Zoledronate and SPIO dual-targeting nanoparticles loaded with ICG for photothermal therapy of breast cancer tibial metastasis. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, X.; Huang, J.; Wang, J.; Zhao, J.; Zhang, L.; Wang, Z.; Li, P.; Li, R. Dual-imaging magnetic nanocatalysis based on Fenton-like reaction for tumor therapy. J. Mater. Chem. B 2022, 10, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Riva, R.; Jérôme, C.; Gallez, B.; Danhier, F.; Préat, V. Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J. Drug Target. 2019, 27, 614–623. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M. Co-encapsulation of superparamagnetic nanoparticles and doxorubicin in PLGA nanocarriers: Development, characterization and in vitro antitumor efficacy in glioma cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A biomimetic polymer magnetic nanocarrier polarizing tumor-associated macrophages for potentiating immunotherapy. Small 2020, 16, 2003543. [Google Scholar] [CrossRef] [PubMed]
- Takke, A.; Shende, P. Magnetic-core-based silibinin nanopolymeric carriers for the treatment of renal cell cancer. Life Sci. 2021, 275, 119377. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, Q.; Chen, C.; Liu, Y.; Wang, X. Synergic combination of paclitaxel and irinotecan loaded poly (lactic-co-glycolic acid) nanocomplexes for MRI and the treatment of endometrial cancer. Mater. Express 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Sedki, M.; Khalil, I.A.; El-Sherbiny, I.M. Hybrid nanocarrier system for guiding and augmenting simvastatin cytotoxic activity against prostate cancer. J. Artif. Cells Nanomed. Biotechnol. 2018, 46, 641–650. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Maity, D.; Soni, S.; Sushmita, K.; Veerapu, N.S.; Bose, S.; Tomy, C. Multifunctional magnetic-polymeric nanoparticles based ferrofluids for multi-modal in vitro cancer treatment using thermotherapy and chemotherapy. J. Mol. Liq. 2019, 293, 111549. [Google Scholar] [CrossRef]
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional Fe3O4@ PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. C 2019, 94, 291–302. [Google Scholar] [CrossRef]
- Tang, H.; Guo, Y.; Peng, L.; Fang, H.; Wang, Z.; Zheng, Y.; Ran, H.; Chen, Y. In vivo targeted, responsive, and synergistic cancer nanotheranostics by magnetic resonance imaging-guided synergistic high-intensity focused ultrasound ablation and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15428–15441. [Google Scholar] [CrossRef]
- Zumaya, A.L.V.; Rimpelová, S.; Štějdířová, M.; Ulbrich, P.; Vilčáková, J.; Hassouna, F. Antibody conjugated PLGA nanocarriers and superparmagnetic nanoparticles for targeted delivery of oxaliplatin to cells from colorectal carcinoma. Int. J. Mol. Sci. 2022, 23, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Fu, H.; Huang, H.; Wu, Z.; Zhao, M.; Yang, X.; Guo, Q.; Duan, Y.; Sun, Y. Multifunctional tumor-targeted PLGA nanoparticles delivering Pt (IV)/siBIRC5 for US/MRI imaging and overcoming ovarian cancer resistance. Biomaterials 2021, 269, 120478. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid)(PLGA) copolymers for the design of drug delivery systems. J. Pharm. Investig. 2019, 49, 443–458. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Y.; Huang, Y.; He, Y.; Xu, Y.; Lu, W.; Yu, J. Poly (ethylene glycol) shell-sheddable TAT-modified core cross-linked nano-micelles: TAT-enhanced cellular uptake and lysosomal pH-triggered doxorubicin release. Colloids Surf. B Biointerfaces 2020, 188, 110772. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Xin, H.; Sha, X.; Jiang, X.; Zhang, W.; Chen, L.; Fang, X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33, 8167–8176. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, H.-J.; Huh, Y.-M.; Kim, I.W.; Jeong, J.H.; Kim, J.-C.; Kim, J.-D. Functionalized magnetic PLGA nanospheres for targeting and bioimaging of breast cancer. J. Nanosci. Nanotechnol. 2018, 18, 1542–1547. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, F.; Zhang, L.; Wang, L.; Luo, Y.; Li, N.; Guo, Y.; Wang, Q.; Zou, J. Multifunctional l-arginine-based magnetic nanoparticles for multiple-synergistic tumor therapy. Biomater. Sci. 2021, 9, 2230–2243. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, Q.; Jiang, D.; Feng, X.; Feng, J.; Jiang, T.; Yao, J.; Jing, Y.; Song, Q.; Jiang, X. Synergistic targeting tenascin C and neuropilin-1 for specific penetration of nanoparticles for anti-glioblastoma treatment. Biomaterials 2016, 101, 60–75. [Google Scholar] [CrossRef]
- Jani, K.; Kaushal, N.; Sadoqi, M.; Long, G.; Chen, Z.-S.; Squillante, E. Formulation and characterization of oleic acid magnetic PEG PLGA nanoparticles for targeting glioblastoma multiforme. J. Magn. Magn. Mater. 2021, 533, 167970. [Google Scholar] [CrossRef]
- Prodi, L.; Rampazzo, E.; Rastrelli, F.; Speghini, A.; Zaccheroni, N. Imaging agents based on lanthanide doped nanoparticles. Chem. Soc. Rev. 2015, 44, 4922–4952. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Suman, S.; Subramanian, S.; Korde, A.; Gohel, D.; Singh, R.; Sawant, K. Development of mitochondrial targeted theranostic nanocarriers for treatment of gliomas. J. Drug Deliv. Sci. Technol. 2021, 64, 102648. [Google Scholar] [CrossRef]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. 2019, 11, e1527. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.; Tansi, F.L.; Hilger, I.; Prina-Mello, A. The Effects of Localized Heat on the Hallmarks of Cancer. Adv. Ther. 2021, 4, 2000267. [Google Scholar] [CrossRef]
- Dhas, N.; García, M.C.; Kudarha, R.; Pandey, A.; Nikam, A.N.; Gopalan, D.; Fernandes, G.; Soman, S.; Kulkarni, S.; Seetharam, R.N. Advancements in cell membrane camouflaged nanoparticles: A bioinspired platform for cancer therapy. J. Control. Release 2022, 346, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793. [Google Scholar] [CrossRef]
- Eynali, S.; Khoei, S.; Khoee, S.; Esmaelbeygi, E. Evaluation of the cytotoxic effects of hyperthermia and 5-fluorouracil-loaded magnetic nanoparticles on human colon cancer cell line HT-29. Int. J. Hyperth. 2017, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Niu, C.; An, S.; Tang, S.; Xiao, P.; Peng, Q.; Wang, L. Thermal-sensitive magnetic nanoparticles for dual-modal tumor imaging and therapy. RSC Adv. 2017, 7, 40791–40802. [Google Scholar] [CrossRef]
- Rochani, A.K.; Balasubramanian, S.; Girija, A.R.; Raveendran, S.; Borah, A.; Nagaoka, Y.; Nakajima, Y.; Maekawa, T.; Kumar, D.S. Dual mode of cancer cell destruction for pancreatic cancer therapy using Hsp90 inhibitor loaded polymeric nano magnetic formulation. Int. J. Pharm. 2016, 511, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf. A Physicochem. Eng. 2021, 622, 126648. [Google Scholar] [CrossRef]
- Niu, C.; Xu, Y.; An, S.; Zhang, M.; Hu, Y.; Wang, L.; Peng, Q. Near-infrared induced phase-shifted ICG/Fe3O4 loaded PLGA nanoparticles for photothermal tumor ablation. Sci. Rep. 2017, 7, 5490. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.; Guo, K.; Liu, Y.; Razzak, M.; Manir, M.S.; Alam, A.M. Dual targeting smart drug delivery system for multimodal synergistic combination cancer therapy with reduced cardiotoxicity. Acta Biomater. 2021, 131, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Tang, L.; Shubhra, Q.T. Homotypic biomimetic coating synergizes chemo-photothermal combination therapy to treat breast cancer overcoming drug resistance. Chem. Eng. J. 2022, 428, 131120. [Google Scholar] [CrossRef]
- Maehara, Y.; Sakaguchi, Y.; Takahashi, I.; Yoshida, M.; Kusumoto, H.; Masuda, H.; Sugimachi, K. 5-Fluorouracil’s cytotoxicity is enhanced both in vitro and in vivo by concomitant treatment with hyperthermia and dipyridamole. Cancer Chemother. Pharmacol. 1992, 29, 257–260. [Google Scholar] [CrossRef]
- Kido, Y.; Kuwano, H.; Maehara, Y.; Mori, M.; Matsuoka, H.; Sugimachi, K. Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother. Pharmacol. 1991, 28, 251–254. [Google Scholar] [CrossRef]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef]
- Kirchherr, A.-K.; Briel, A.; Mäder, K. Stabilization of indocyanine green by encapsulation within micellar systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Petaccia, M.; Condello, M.; Giansanti, L.; La Bella, A.; Leonelli, F.; Meschini, S.; Villalva, D.G.; Pellegrini, E.; Ceccacci, F.; Galantini, L. Inclusion of new 5-fluorouracil amphiphilic derivatives in liposome formulation for cancer treatment. MedChemComm. 2015, 6, 1639–1642. [Google Scholar] [CrossRef]
- Wu, H.; Shen, L.; Zhu, Z.; Luo, X.; Zhai, Y.; Hua, X.; Zhao, S.; Cen, L.; Zhang, Z. A cell-free therapy for articular cartilage repair based on synergistic delivery of SDF-1 & KGN with HA injectable scaffold. Chem. Eng. J. 2020, 393, 124649. [Google Scholar]
- Ayyanaar, S.; Bhaskar, R.; Esthar, S.; Vadivel, M.; Rajesh, J.; Rajagopal, G. Design and development of 5-fluorouracil loaded biodegradable magnetic microspheres as site-specific drug delivery vehicle for cancer therapy. J. Magn. Magn. Mater. 2022, 546, 168853. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Kesavan, M.P.; Balachandran, C.; Rasala, S.; Rameshkumar, P.; Aoki, S.; Rajesh, J.; Webster, T.J.; Rajagopal, G. Iron oxide nanoparticle core-shell magnetic microspheres: Applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102134. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Weidner, A.; Nosrati, Z.; Wöckel, L.; Dellith, J.; Müller, R.; Saatchi, K.; Häfeli, U.O.; Dutz, S. Temperature controlled camptothecin release from biodegradable magnetic PLGA microspheres. J. Magn. Magn. Mater. 2019, 469, 698–703. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Kesavan, M.P.; Sivaraman, G.; Raja, R.P.; Vijayakumar, V.; Rajesh, J.; Rajagopal, G. Reactive oxygen species (ROS)-responsive microspheres for targeted drug delivery of camptothecin. J. Drug Deliv. Sci. Technol. 2019, 52, 722–729. [Google Scholar] [CrossRef]
- Zahn, D.; Weidner, A.; Saatchi, K.; Häfeli, U.O.; Dutz, S. Biodegradable magnetic microspheres for drug targeting, temperature controlled drug release, and hyperthermia. Curr. Dir. Biomed. Eng. 2019, 5, 161–164. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Wang, H.; Yu, H.; Feng, G.; Liu, F.; Ma, M.; Zhang, Y.; Gu, N. Magnetic navigation helps PLGA drug loaded magnetic microspheres achieve precise chemoembolization and hyperthermia. Colloids Surf. A Physicochem. Eng. 2020, 588, 124364. [Google Scholar] [CrossRef]
- Wang, H.; Xu, S.; Fan, D.; Geng, X.; Zhi, G.; Wu, D.; Shen, H.; Yang, F.; Zhou, X.; Wang, X. Multifunctional microcapsules: A theranostic agent for US/MR/PAT multi-modality imaging and synergistic chemo-photothermal osteosarcoma therapy. Bioact. Mater. 2022, 7, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Uemura, T.; Xu, Z.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Rapid oriented fibril formation of fish scale collagen facilitates early osteoblastic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2015, 103, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).