Cardiac Dose Predicts the Response to Concurrent Chemoradiotherapy in Esophageal Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Study Design
2.2. Radiation Treatment and Dosimetric Analysis
2.3. Hematological Parameters
2.4. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Hematological Parameters and Toxicities
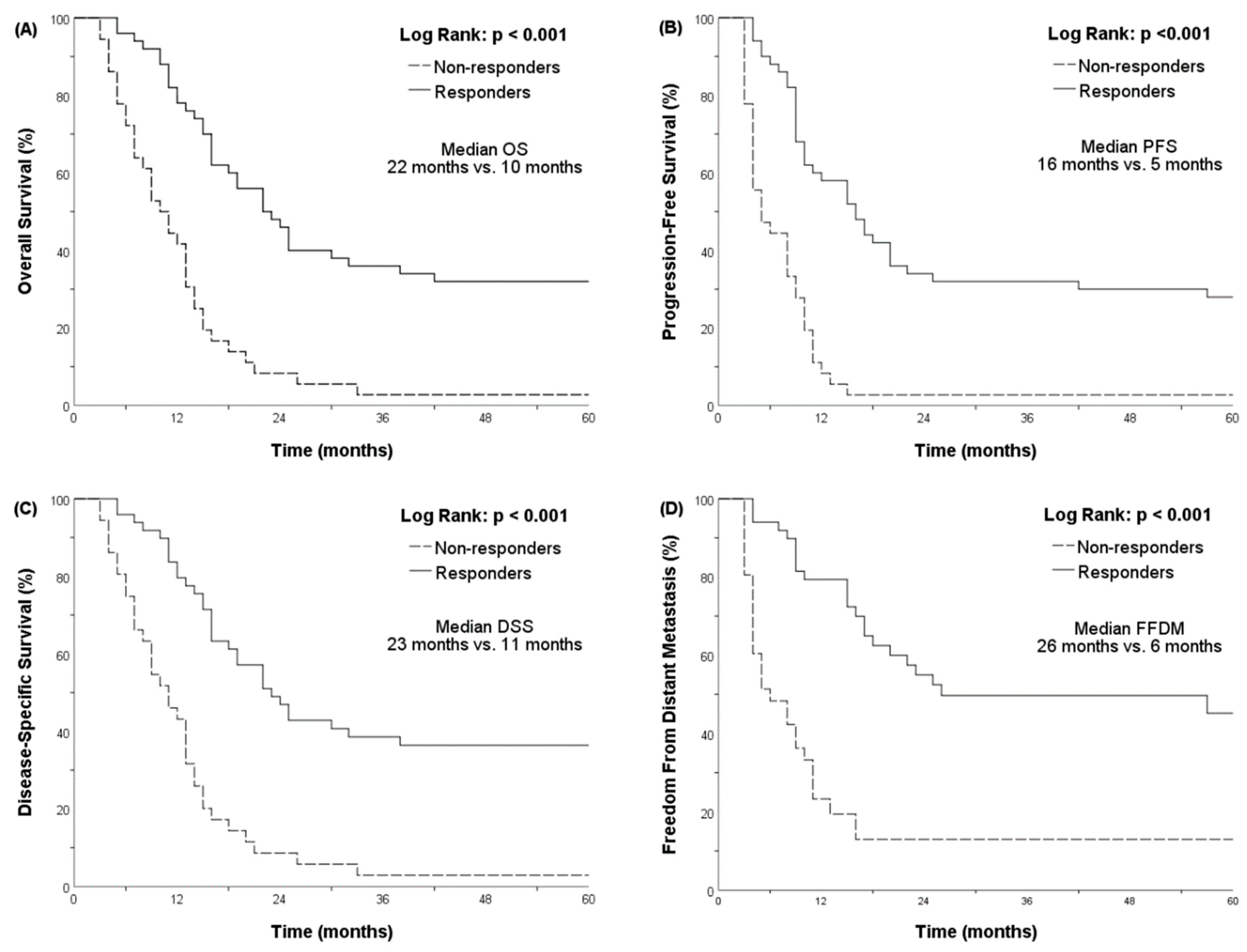
3.3. A Poor Initial Response Was Associated with Lower OS
3.4. Patient Characteristics and Dosimetric Parameters Correlated with the Initial Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Chou, Y.C.; Lee, Y.K.; Hsu, W.L.; Tang, C.S.; Chen, S.Y.; Huang, S.P.; Chen, Y.C.; Lee, J.M. Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers 2022, 14, 5844. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lievre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Herskovic, A.; Martz, K.; al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.; Gronnier, C.; Duhamel, A.; Pasquer, A.; Thereaux, J.; du Rieu, M.C.; Lefevre, J.H.; Turner, K.; Luc, G.; Mariette, C. Salvage Surgery after Chemoradiotherapy in the Management of Esophageal Cancer: Is It a Viable Therapeutic Option? J. Clin. Oncol. 2015, 33, 3866–3873. [Google Scholar] [CrossRef]
- Vincent, J.; Mariette, C.; Pezet, D.; Huet, E.; Bonnetain, F.; Bouche, O.; Conroy, T.; Roullet, B.; Seitz, J.F.; Herr, J.P.; et al. Early surgery for failure after chemoradiation in operable thoracic oesophageal cancer. Analysis of the non-randomised patients in FFCD 9102 phase III trial: Chemoradiation followed by surgery versus chemoradiation alone. Eur. J. Cancer 2015, 51, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Obermannova, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Eyck, B.M.; van der Wilk, B.J.; Noordman, B.J.; Wijnhoven, B.P.L.; Lagarde, S.M.; Hartgrink, H.H.; Coene, P.; Dekker, J.W.T.; Doukas, M.; van der Gaast, A.; et al. Updated protocol of the SANO trial: A stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials 2021, 22, 345. [Google Scholar] [CrossRef]
- Nilsson, M.; Olafsdottir, H.; Alexandersson von Dobeln, G.; Villegas, F.; Gagliardi, G.; Hellstrom, M.; Wang, Q.L.; Johansson, H.; Gebski, V.; Hedberg, J.; et al. Neoadjuvant Chemoradiotherapy and Surgery for Esophageal Squamous Cell Carcinoma Versus Definitive Chemoradiotherapy with Salvage Surgery as Needed: The Study Protocol for the Randomized Controlled NEEDS Trial. Front. Oncol. 2022, 12, 917961. [Google Scholar] [CrossRef]
- Crosby, T.; Hurt, C.N.; Falk, S.; Gollins, S.; Mukherjee, S.; Staffurth, J.; Ray, R.; Bashir, N.; Bridgewater, J.A.; Geh, J.I.; et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, M.; Winter, K.; Ilson, D.; Dicker, A.P.; Kachnic, L.; Konski, A.; Chakravarthy, A.B.; Anker, C.J.; Thakrar, H.; Horiba, N.; et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients with Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Lai, Y.C.; Lin, H.Y.; Ko, M.H.; Wang, S.H.; Yang, S.J.; Lin, P.J.; Chou, T.W.; Hung, L.C.; Huang, C.C.; et al. Low cardiac dose and neutrophil-to-lymphocyte ratio predict overall survival in inoperable esophageal squamous cell cancer patients after chemoradiotherapy. Sci. Rep. 2021, 11, 6644. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Hung, L.C.; Cheng, C.Y.; Chien, Y.A.; Lee, C.H.; Huang, C.C.; Chou, T.W.; Ko, M.H.; Lai, Y.C.; Liu, M.T.; et al. Prognostic significance of lung radiation dose in patients with esophageal cancer treated with neoadjuvant chemoradiotherapy. Radiat. Oncol. 2019, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, L.; Liao, Z.; Wang, Y.; Liu, X.; Zhao, S.; Wang, J.; Yuan, Z.; Wang, P.; Lin, S.H. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin. Transl. Radiat. Oncol. 2019, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, D.; Wu, S.; Chen, Y.; Li, R.; Miao, H.; Wen, Z. Prognostic significance of neutrophil-to-lymphocyte ratio in middle thoracic esophageal squamous cell carcinoma patients undergoing radical esophagectomy. J. Thorac. Dis. 2020, 12, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Tian, Q.; Yu, G.; Shui, Y.; Jiang, H.; Wei, Q. Severe Radiation-Induced Lymphopenia Affects the Outcomes of Esophageal Cancer: A Comprehensive Systematic Review and Meta-Analysis. Cancers 2022, 14, 3024. [Google Scholar] [CrossRef] [PubMed]
- Pao, T.H.; Chang, W.L.; Chiang, N.J.; Chang, J.S.; Lin, C.Y.; Lai, W.W.; Tseng, Y.L.; Yen, Y.T.; Chung, T.J.; Lin, F.C. Cardiac radiation dose predicts survival in esophageal squamous cell carcinoma treated by definitive concurrent chemotherapy and intensity modulated radiotherapy. Radiat. Oncol. 2020, 15, 221. [Google Scholar] [CrossRef]
- Cai, G.; Li, C.; Li, J.; Yang, J.; Li, C.; Sun, L.; Li, J.; Yu, J.; Meng, X. Cardiac Substructures Dosimetric Predictors for Cardiac Toxicity After Definitive Radiotherapy in Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 366–381. [Google Scholar] [CrossRef]
- Wang, X.; Palaskas, N.L.; Hobbs, B.P.; Abe, J.I.; Nead, K.T.; Yusuf, S.W.; Hermann, J.; Deswal, A.; Lin, S.H. The Impact of Radiation Dose to Heart Substructures on Major Coronary Events and Patient Survival after Chemoradiation Therapy for Esophageal Cancer. Cancers 2022, 14, 1304. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Norman, A.R.; Cunningham, D.; Waters, J.S.; Oates, J.; Ross, P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 2004, 22, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.A.; Meunier, B.; Boige, V.; Pezet, D.; Robb, W.B.; Le Brun-Ly, V.; et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol. 2014, 32, 2416–2422. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Marson, E.J.; Zhou, D.; Wyn-Griffiths, F.; Lin, A.; Evans, R.P.T.; Bundred, J.R.; Singh, P.; Griffiths, E.A. Meta-analysis of prognostic factors of overall survival in patients undergoing oesophagectomy for oesophageal cancer. Dis. Esophagus 2020, 33, doaa038. [Google Scholar] [CrossRef]
- Gavin, A.T.; Francisci, S.; Foschi, R.; Donnelly, D.W.; Lemmens, V.; Brenner, H.; Anderson, L.A.; Group, E.-W. Oesophageal cancer survival in Europe: A EUROCARE-4 study. Cancer Epidemiol. 2012, 36, 505–512. [Google Scholar] [CrossRef]
- Backemar, L.; Wikman, A.; Djarv, T.; Johar, A.; Lagergren, P. Co-morbidity after oesophageal cancer surgery and recovery of health-related quality of life. Br. J. Surg. 2016, 103, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cao, X.; Wen, J.; Yang, H.; Luo, K.; Liu, Q.; Huang, Q.; Chen, J.; Fu, J. Smoking affects treatment outcome in patients with resected esophageal squamous cell carcinoma who received chemotherapy. PLoS ONE 2015, 10, e0123246. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Yan, L.B.; Liu, Z.Z.; Zhao, W.J.; Zhang, C.X.; Chen, Y.M.; Lao, X.Q.; Liu, X. Dietary factors and risk of mortality among patients with esophageal cancer: A systematic review. BMC Cancer 2020, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Mattsson, F.; Lindblad, M.; Lagergren, J. Association between education level and prognosis after esophageal cancer surgery: A Swedish population-based cohort study. PLoS ONE 2015, 10, e0121928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Wang, X.; Geng, X.; Zhu, L.; Li, M. Clinical response to chemoradiotherapy in esophageal carcinoma is associated with survival and benefit of consolidation chemotherapy. Cancer Med. 2020, 9, 5881–5888. [Google Scholar] [CrossRef] [PubMed]
- Noordman, B.J.; Spaander, M.C.W.; Valkema, R.; Wijnhoven, B.P.L.; van Berge Henegouwen, M.I.; Shapiro, J.; Biermann, K.; van der Gaast, A.; van Hillegersberg, R.; Hulshof, M.; et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): A prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018, 19, 965–974. [Google Scholar] [CrossRef]
- Barbour, A.P.; Walpole, E.T.; Mai, G.T.; Barnes, E.H.; Watson, D.I.; Ackland, S.P.; Martin, J.M.; Burge, M.; Finch, R.; Karapetis, C.S.; et al. Preoperative cisplatin, fluorouracil, and docetaxel with or without radiotherapy after poor early response to cisplatin and fluorouracil for resectable oesophageal adenocarcinoma (AGITG DOCTOR): Results from a multicentre, randomised controlled phase II trial. Ann. Oncol. 2020, 31, 236–245. [Google Scholar] [CrossRef]
- van der Wilk, B.J.; Noordman, B.J.; Neijenhuis, L.K.A.; Nieboer, D.; Nieuwenhuijzen, G.A.P.; Sosef, M.N.; van Berge Henegouwen, M.I.; Lagarde, S.M.; Spaander, M.C.W.; Valkema, R.; et al. Active Surveillance Versus Immediate Surgery in Clinically Complete Responders after Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Multicenter Propensity Matched Study. Ann. Surg. 2021, 274, 1009–1016. [Google Scholar] [CrossRef]
- Okada, M.; Kato, K.; Cho, B.C.; Takahashi, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin. Cancer Res. 2022, 28, 3277–3286. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, F.; Cao, L.; Wang, S.; Zhou, W.; Ma, W. A retrospective study: The prevalence and prognostic value of anemia in patients undergoing radiotherapy for esophageal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, 244. [Google Scholar] [CrossRef]
- Kapoor, R.; Bansal, A.; Kumar, S.; Miriyala, R.T. Factors Influencing Compliance to Radical Treatment of Middle Thoracic Esophageal Cancer: An Audit from a Regional Cancer Centre. Indian J. Palliat. Care 2016, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Pirozzolo, G.; Gisbertz, S.S.; Castoro, C.; van Berge Henegouwen, M.I.; Scarpa, M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res. 1990, 123, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Talebian Yazdi, M.; Schinkelshoek, M.S.; Loof, N.M.; Taube, C.; Hiemstra, P.S.; Welters, M.J.; van der Burg, S.H. Standard radiotherapy but not chemotherapy impairs systemic immunity in non-small cell lung cancer. Oncoimmunology 2016, 5, e1255393. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-C.; Lai, Y.-C.; Lin, H.-Y.; Ko, M.-H.; Wang, S.-H.; Yang, S.-J.; Lin, P.-J.; Chou, T.-W.; Hung, L.-C.; Huang, C.-C.; et al. The volume of low-dose thoracic irradiation influences systemic inflammation-immunity status after chemoradiation in esophageal cancer. Ther. Radiol. Oncol. 2021, 5, 9. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Moey, M.Y.Y.; Tomdio, A.N.; McCallen, J.D.; Vaughan, L.M.; O’Brien, K.; Naqash, A.R.; Cherry, C.; Walker, P.R.; Carabello, B.A. Characterization of Immune Checkpoint Inhibitor-Related Cardiotoxicity in Lung Cancer Patients from a Rural Setting. JACC CardioOncol. 2020, 2, 491–502. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef]
- Bradley, J.D.; Hu, C.; Komaki, R.R.; Masters, G.A.; Blumenschein, G.R.; Schild, S.E.; Bogart, J.A.; Forster, K.M.; Magliocco, A.M.; Kavadi, V.S.; et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy with or without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, A.; Abravan, A.; Banfill, K.; Faivre-Finn, C.; van Herk, M. Demystifying the Results of RTOG 0617: Identification of Dose Sensitive Cardiac Subregions Associated with Overall Survival. J. Thorac. Oncol. 2023, 18, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Palaskas, N.L.; Yusuf, S.W.; Abe, J.I.; Lopez-Mattei, J.; Banchs, J.; Gladish, G.W.; Lee, P.; Liao, Z.; Deswal, A.; et al. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. J. Thorac. Oncol. 2020, 15, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Isacsson, U.; Lennernas, B.; Grusell, E.; Jung, B.; Montelius, A.; Glimelius, B. Comparative treatment planning between proton and x-ray therapy in esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Komaki, R.; Liao, Z.; Wei, C.; Myles, B.; Guo, X.; Palmer, M.; Mohan, R.; Swisher, S.G.; Hofstetter, W.L.; et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e345–e351. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Hobbs, B.P.; Verma, V.; Tidwell, R.S.; Smith, G.L.; Lei, X.; Corsini, E.M.; Mok, I.; Wei, X.; Yao, L.; et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 1569–1579. [Google Scholar] [CrossRef]
- Damen, P.J.J.; Kroese, T.E.; van Hillegersberg, R.; Schuit, E.; Peters, M.; Verhoeff, J.J.C.; Lin, S.H.; van Rossum, P.S.N. The Influence of Severe Radiation-Induced Lymphopenia on Overall Survival in Solid Tumors: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 936–948. [Google Scholar] [CrossRef]
- Fang, P.; Shiraishi, Y.; Verma, V.; Jiang, W.; Song, J.; Hobbs, B.P.; Lin, S.H. Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int. J. Part. Ther. 2018, 4, 23–32. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Fang, P.; Xu, C.; Song, J.; Krishnan, S.; Koay, E.J.; Mehran, R.J.; Hofstetter, W.L.; Blum-Murphy, M.; Ajani, J.A.; et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother. Oncol. 2018, 128, 154–160. [Google Scholar] [CrossRef]
- Routman, D.M.; Garant, A.; Lester, S.C.; Day, C.N.; Harmsen, W.S.; Sanheuza, C.T.; Yoon, H.H.; Neben-Wittich, M.A.; Martenson, J.A.; Haddock, M.G.; et al. A Comparison of Grade 4 Lymphopenia with Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv. Radiat. Oncol. 2019, 4, 63–69. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Deng, W.; Xu, C.; Liu, A.; van Rossum, P.S.N.; Deng, W.; Liao, Z.; Koong, A.C.; Mohan, R.; Lin, S.H. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother. Oncol. 2019, 133, 9–15. [Google Scholar] [CrossRef]
| Variables (n = 86) | Number or Median | Standard Deviation or % | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | |||
| Age at diagnosis (years) | 60 | 10.4 | 0.992 [0.971–1.013] | 0.444 | ||
| Gender | ||||||
| Female | 10 | 11.6 | 1 | 1 | ||
| Male | 76 | 88.4 | 2.497 [1.077–5.791] | 0.033 | 9.105 [2.379–34.853] | 0.001 |
| Smoker | ||||||
| No (never smoked) | 14 | 16.3 | 1 | 1 | ||
| Yes (current smoker or quitted) | 60 | 69.8 | 2.020 [1.026–3.979] | 0.042 | 0.422 [0.155–1.151] | 0.092 |
| Unknown | 12 | 13.9 | ||||
| Alcohol drinker | ||||||
| No (never or not regular) | 8 | 9.3 | 1 | 1 | ||
| Yes (current use or ever regularly use) | 67 | 77.9 | 3.102 [1.122–8.578] | 0.029 | 1.982 [0.506–7.771] | 0.326 |
| Unknown | 11 | 12.8 | ||||
| Betel-nuts chewer | ||||||
| No (never or not regular) | 26 | 30.2 | 1 | |||
| Yes (current use or ever regularly use) | 45 | 52.3 | 1.161 [0.684–1.969] | 0.580 | ||
| Unknown | 15 | 17.5 | ||||
| Eastern Cooperative Oncology Group—Performance Status, n (%) | ||||||
| 0 and 1 | 79 | 91.9 | 1 | 1 | ||
| 2 | 7 | 8.1 | 2.201 [1.000–4.846] | 0.050 | 3.755 [1.477–9.547] | 0.005 |
| Age Adjusted Charlson’s Comorbidity Index (excluding esophageal cancer) | 2 | 1.4 | ||||
| 0–2 | 59 | 68.6 | 1 | |||
| >2 | 27 | 31.4 | 0.862 [0.5331–1.399] | 0.547 | ||
| Primary tumor location, n (%) | ||||||
| Cervical and Upper | 24 | 27.9 | 1 | 0.399 | ||
| Middle and Lower | 62 | 72.1 | 1.300 [0.784–2.157] | 0.309 | ||
| Primary tumor size (cm) | 5 | 3.5 | 1.039 [0.966–1.117] | 0.303 | ||
| Histologic grade, n (%) | ||||||
| Grade 1 | 2 | 2.3 | 1 | 0.901 | ||
| Grade 2 | 60 | 69.8 | 1.390 [0.337–5.729] | 0.648 | ||
| Grade 3 | 5 | 5.8 | 1.352 [0.261–6.989] | 0.719 | ||
| Unknown | 19 | 22.1 | ||||
| Clinical T stage, n (%) | ||||||
| T1 and T2 | 32 | 37.2 | 1 | |||
| T3 and T4 | 54 | 62.8 | 1.384 [0.864–2.217] | 0.176 | ||
| Clinical N stage, n (%) | ||||||
| N0 and N1 | 41 | 47.7 | 1 | |||
| N2 and N3 | 45 | 52.3 | 1.459 [0.926–2.300] | 0.103 | ||
| Clinical TNM stage, n (%) | ||||||
| II | 25 | 29.1 | 1 | |||
| III | 61 | 70.9 | 1.399 [0.845–2.316] | 0.192 | ||
| Baseline Hb (g/dL) | 12.8 | 1.9 | 0.985 [0.875–1.108] | 0.799 | ||
| Baseline WBC (cells/mm3) | 7900 | 2669.1 | 1.062 [0.973–1.159] | 0.180 | ||
| Baseline ANC (cells/mm3) | 5074 | 2492.1 | 1.001 [1.000–1.002] | 0.118 | ||
| Baseline ALC (cells/mm3) | 1635 | 599.8 | 1.000 [1.000–1.000] | 0.831 | ||
| Baseline NLR | 3.32 | 2.2 | 1.066 [0.966–1.176] | 0.207 | ||
| ALC nadir during CCRT (cells/mm3) | 219.4 | 172.3 | 1.000 [ 0.998–1.001] | 0.471 | ||
| Highest NLR during CCRT (NLR-h) | 17.1 | 28.3 | 1.007 [1.000–1.015] | 0.061 | 1.012 [1.002–1.022] | 0.016 |
| Radiotherapy technique | ||||||
| 3D-CRT | 20 | 23.2 | 1 | 0.669 | ||
| IMRT | 33 | 38.3 | 0.801 [0.450–1.429] | 0.453 | ||
| VMAT | 33 | 38.3 | 0.781 [0.438–1.392] | 0.402 | ||
| Radiation dose (cGy) | 5940 | 605.3 | 0.984 [0.947–1.023] | 0.422 | ||
| Mean heart dose (cGy) | 2047 | 692.5 | 1.000 [1.000–1.001] | 0.093 * | ||
| Dose–volume of heart (%) | ||||||
| V10 | 90 | 25.8 | 1.010 [0.999–1.020] | 0.071 * | 1.012 [0.999–1.026] | 0.072 |
| V20 | 44 | 23.1 | 1.007 [0.997–1.017] | 0.182 | ||
| V30 | 15.5 | 12.7 | 1.013 [0.996–1.031] | 0.124 | ||
| V40 | 5 | 5.5 | 1.033 [0.993–1.076] | 0.108 | ||
| Mean lung dose (cGy) | 1511.5 | 295.9 | 1.000 [0.999–1.001] | 0.670 | ||
| Dose–volume of lung (%) | ||||||
| V5 | 91 | 14.9 | 1.005 [0.990–1.021] | 0.509 | ||
| V10 | 69 | 16.8 | 1.001 [0.988–1.015] | 0.836 | ||
| V20 | 21 | 8 | 0.997 [0.967–1.028] | 0.832 | ||
| Radiotherapy and chemotherapy sequence, n (%) | ||||||
| CCRT and +/− adjuvant chemo | 36 | 41.9 | 1 | 0.608 | ||
| Induction chemo, CCRT, and +/− adjuvant chemo | 48 | 55.8 | 0.797 [0.503–1.263] | 0.334 | ||
| Sequential RT and chemo | 2 | 2.3 | 1.053 [0.251–4.413] | 0.944 | ||
| Concurrent cisplatin dose (mg/m2) during CCRT | 75 | 41.6 | 0.998 [0.992–1.003] | 0.430 | ||
| Cumulative cisplatin dose (mg/m2) before CCRT finished | 140 | 54.7 | 0.998 [0.993–1.003] | 0.434 | ||
| Treatment Response | ||||||
| Responders | 50 | 58.1 | 1 | |||
| Complete response | 4 | 4.6 | 1 | <0.001 | ||
| Partial response | 46 | 53.5 | 0.888 [0.316–2.498] | 0.822 | ||
| Non-responders | 36 | 41.9 | 3.201 [1.985–5.164] | <0.001 | 4.172 [2.296–7.583] | <0.001 |
| Stable disease | 14 | 16.3 | 1.828 [0.592–5.646] | 0.294 | ||
| Progressive disease | 22 | 25.6 | 5.011 [1.664–15.091] | 0.004 | ||
| Variables | Youden’s Index Cutoff Value | OR | 95% CI | Univariate p Value | OR | 95% CI | Multivariate p Value |
|---|---|---|---|---|---|---|---|
| Baseline Hb | 11.25 | 5.750 | 1.673–19.761 | 0.005 | 11.536 | 2.036–65.377 | 0.006 |
| Baseline NLR | 4.34 | 0.370 | 0.141–0.976 | 0.045 * | |||
| Highest NLR during CCRT | 14.48 | 0.264 | 0.101–0.690 | 0.007 * | 0.261 | 0.075–0.910 | 0.035 |
| ALC nadir during CCRT | 137.8 | 2.533 | 1.006–6.381 | 0.049 * | |||
| Mean heart dose (cGy) | 1964 | 0.327 | 0.130–0.826 | 0.018 ** | |||
| Heart V10 | 86.5 | 0.214 | 0.076–0.607 | 0.004 ** | 0.278 | 0.079–0.976 | 0.046 |
| Heart V20 | 58.5 | 0.400 | 0.157–1.022 | 0.055 | |||
| Heart V30 | 15.5 | 0.293 | 0.117–0.734 | 0.009 ** | |||
| Heart V40 | 4.5 | 0.234 | 0.092–0.598 | 0.002 ** | |||
| Mean lung dose (cGy) | 1808 | 0.173 | 0.034–0.888 | 0.036 | 0.353 | 0.030–4.142 | 0.407 |
| Lung V5 | 88.5 | 1.737 | 0.722–4.178 | 0.218 | |||
| Lung V10 | 71.5 | 0.503 | 0.210–1.205 | 0.123 | |||
| Lung V20 | 20.5 | 0.542 | 0.227–1.295 | 0.168 | |||
| Cumulative cisplatin dose (mg/m2) before CCRT finished | 147.5 | 2.857 | 1.118–7.303 | 0.028 | 4.966 | 1.446–17.051 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.-C.; Lai, Y.-C.; Lin, H.-Y.; Ko, M.-H.; Wang, S.-H.; Yang, S.-J.; Chou, T.-W.; Hung, L.-C.; Huang, C.-C.; Chang, T.-H.; et al. Cardiac Dose Predicts the Response to Concurrent Chemoradiotherapy in Esophageal Squamous Cell Carcinoma. Cancers 2023, 15, 4580. https://doi.org/10.3390/cancers15184580
Ho Y-C, Lai Y-C, Lin H-Y, Ko M-H, Wang S-H, Yang S-J, Chou T-W, Hung L-C, Huang C-C, Chang T-H, et al. Cardiac Dose Predicts the Response to Concurrent Chemoradiotherapy in Esophageal Squamous Cell Carcinoma. Cancers. 2023; 15(18):4580. https://doi.org/10.3390/cancers15184580
Chicago/Turabian StyleHo, Yu-Chieh, Yuan-Chun Lai, Hsuan-Yu Lin, Ming-Hui Ko, Sheng-Hung Wang, Shan-Jun Yang, Tsai-Wei Chou, Li-Chung Hung, Chia-Chun Huang, Tung-Hao Chang, and et al. 2023. "Cardiac Dose Predicts the Response to Concurrent Chemoradiotherapy in Esophageal Squamous Cell Carcinoma" Cancers 15, no. 18: 4580. https://doi.org/10.3390/cancers15184580
APA StyleHo, Y.-C., Lai, Y.-C., Lin, H.-Y., Ko, M.-H., Wang, S.-H., Yang, S.-J., Chou, T.-W., Hung, L.-C., Huang, C.-C., Chang, T.-H., Lin, J.-B., & Lin, J.-C. (2023). Cardiac Dose Predicts the Response to Concurrent Chemoradiotherapy in Esophageal Squamous Cell Carcinoma. Cancers, 15(18), 4580. https://doi.org/10.3390/cancers15184580






