Calcium Signaling in the Thyroid: Friend and Foe
Abstract
Simple Summary
Abstract
1. Introduction
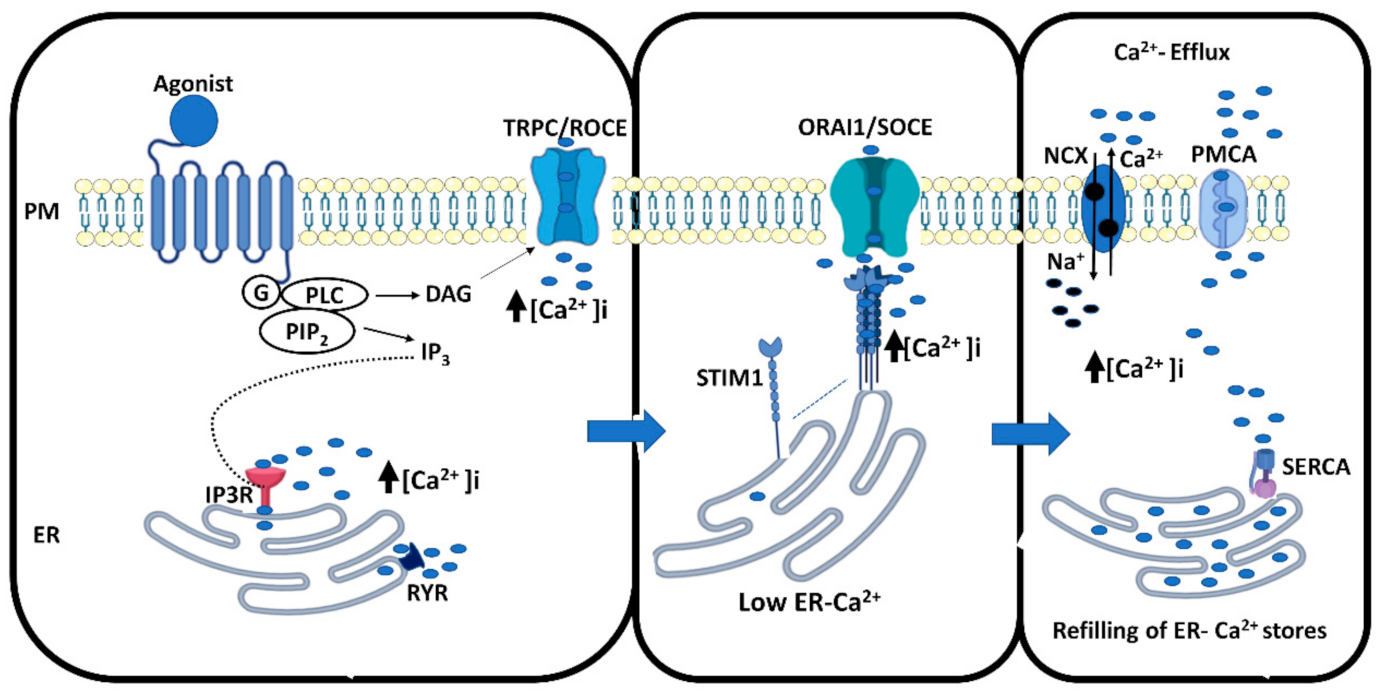
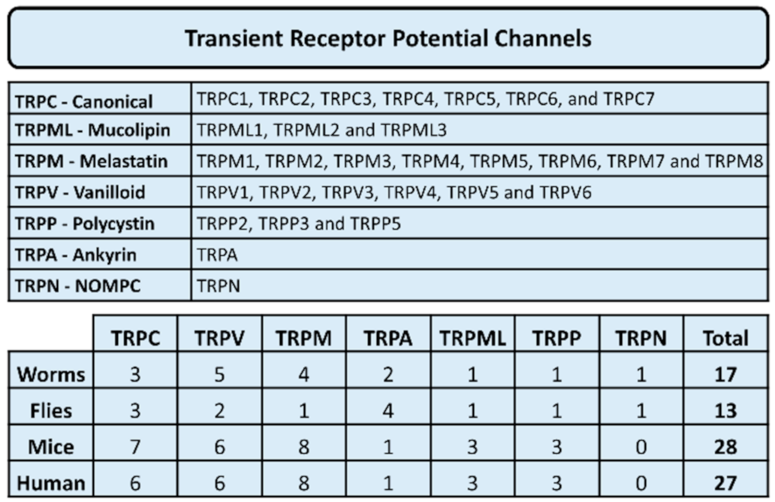
2. Thyroid and Calcium Signaling
3. Calcium Signaling in Thyroid Pathologies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berridge, J.M.; Lipp, P.; Bootman, M.D. The versatility and universtility of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Berridge, J.M.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Shuttleworth, T.J. Exploring the unique features of the ARC channel, a store independent Orai channel. Channels 2013, 7, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hogan, G.P.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.G.; Zeng, W.; Kim, J.Y.; Yan, J.P.; Han, L.; Muallem, S.; Worley, P.F. STIM1 carboxyl-terminus activates native SOC I (CRAC) and TRPC1 channels. Nat. Cell Biol. 2006, 8, 1003–1010. [Google Scholar] [CrossRef]
- Smani, T.; Shapocaloc, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta 2015, 1853, 1772–1782. [Google Scholar] [CrossRef]
- Kimura, T.; van Keymeulen, A.; Golstein, J.; Fusco, A.; Dumont, J.E.; Roger, P.P. Regulation of thyroid cell proliferation by TSH and other factors: A critical evaluation of in vitro models. Endocr. Rev. 2001, 22, 631–656. [Google Scholar] [CrossRef]
- Takada, K.; Amino, N.; Tada, H.; Miyai, K. Relationship between proliferation and cell cycle-dependent Ca2+ influx induced by a combination of thyrotropin and insulin-like growth factor-1 in rat thyroid cells. J. Clin. Investig. 1990, 86, 1548–1555. [Google Scholar] [CrossRef]
- Törnquist, K.; Ekokoski, E.; Dugué, B. ATP functions as a comitogen in thyroid FRTL-5 cells. J. Cell. Physiol. 1996, 166, 241–248. [Google Scholar] [CrossRef]
- Ekokoski, E.; Webb, T.E.; Simon, J.; Törnquist, K. Mechanism of P2 receptor-evoked DNA synthesis in thyroid FRTL-5 cells. J. Cell. Physiol. 2001, 187, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, P.; Lof, C.; Pulli, I.; Kemppainen, K.; Viitanen, T.; Tornquist, K. Significance of the transient receptor potential canonical 2 (TRPC2) channel in the regulation of rat thyroid FRTL-5 cell proliferation, migration, adhesion and invasion. Mol. Cell. Endocrinol. 2013, 374, 10–21. [Google Scholar] [CrossRef]
- Sukumaran, P.; Lof, C.; Kemppainen, K.; Kankaanpaa, P.; Pulli, I.; Nasman, J.; Viitanen, T.; Tornquist, K. Canonical transient receptor potential channel 2 (TRPC2) as a major regulator of calcium homeostasis in rat thyroid FRTL-5 cells: Importance of protein kinase C delta (PKCdelta) and stromal interaction molecule 2 (STIM2). J. Biol. Chem. 2012, 287, 44345–44360. [Google Scholar] [CrossRef] [PubMed]
- Okajima, F.; Tokumitsu, Y.; Kondo, Y.; Ui, M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J. Biol. Chem. 1987, 262, 13483–13490. [Google Scholar] [CrossRef]
- Sho, K.; Okajima, F.; Majid, M.A.; Kondo, Y. Reciprocal modulation of thyrotropin actions by P1-purinergic agonists in FRTL-5 thyroid cells. Inhibition of cAMP pathway and stimulation of phospholipase C-Ca2+ pathway. J. Biol. Chem. 1991, 266, 12180–12184. [Google Scholar] [CrossRef]
- Törnquist, K.; Saarinen, P.; Vainio, M.; Ahlström, M. Sphingosine 1-phosphate mobilizes sequestered calcium, activates calcium entry, and stimulates DNA synthesis in thyroid FRTL-5 cells. Endocrinology 1997, 138, 4049–4057. [Google Scholar] [CrossRef]
- Jiménez, E.; Pavía, J.; Morell, V.; Martín, E.; Montiel, M. Muscarinic receptor subtypes and calcium signaling in Fischer rat thyroid cells. Biochem. Pharmacol. 2001, 61, 337–342. [Google Scholar] [CrossRef]
- Wang, D.X.; Kiang, J.G.; Atwa, M.A.; Smallridge, R.C. Evidence for the involvement of protein kinase C isoforms in alpha-1 adrenergic activation of phospholipase A2 in FRTL-5 thyroid cells. J. Investig. Med. 1996, 44, 566–674. [Google Scholar]
- Marsigliante, S.; Elia, M.G.; di Jeso, B.; Greco, S.; Muscella, A.; Storelli, C. Increase of [Ca(2+)](i) via activation of ATP receptors in PC-Cl3 rat thyroid cell line. Cell Signal. 2002, 14, 61–67. [Google Scholar] [CrossRef]
- Raspe’, E.; Laurent, E.; Andry, G.; Dumont, J.E. ATP, bradykinin, TRH and TSH activates the Ca2+-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol. Cell. Endocrinol. 1991, 81, 175–183. [Google Scholar] [CrossRef]
- Raspé, E.; Laurent, E.; Erjans, B.C.; Erneux, C.; Dumont, J.E. Control of intracellular Ca2+-concentration and the inositol phosphate accumulation in dog thyrocyte primary culture: Evidence for different kinetics of Ca2+-phosphatidylinositol cascade activation and for involvement in the regulation of H2O2. J. Cell. Physiol. 1991, 146, 242–250. [Google Scholar] [CrossRef]
- Törnquist, K. Modulatory effect of protein kinase C on thapsigargin-induced calcium entry in FRTL-5 cells. Biochem. J. 1993, 290, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Törnquist, K.; Ekokoski, E.; Forss, L.; Matsson, M. Importance of arachidonic acid metabolites in regulating ATP-induced calcium fluxes in thyroid FRTL-5 cells. Cell Calcium 1994, 15, 153–161. [Google Scholar] [CrossRef]
- Corvilain, B.; Laurent, E.; Lecomte, M.; Vansande, J.; Dumont, J.E. Role of the cyclic adenosine 3′,5′-monophosphate and the phosphatidylinositol-Ca2+ cascades in mediating the effects of thyrotropin and iodide on hormone synthesis and secretion in human thyroid slices. J. Clin. Endocrinol. Investig. 1994, 79, 152–159. [Google Scholar]
- Törnquist, K.; Ahlström, M. Modulatory effects of cyclic AMP on calcium fluxes in FRTL-5 cells. J. Cell. Physiol. 1993, 157, 625–630. [Google Scholar] [CrossRef]
- Lorenz, S.; Eszlinger, M.; Paschke, R.; Aust, G.; Weick, M.; Führer, D.; Krohn, K. Calcium signaling of thyrocytes is modulated by TSH through calcium binding protein expression. Biochim. Biophys. Acta 2010, 1803, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.J.A.; Pantazaka, E.; Shelley, K.L.; Taylor, C.W. Prostaglandin E(2) inhibits histamine.evoked Ca(2+) release in human aortic smooth muscle cells through cAMP signaling junctions and protein inase A. Mol. Pharmacol. 2017, 92, 533–545. [Google Scholar] [CrossRef]
- Taylor, C.W. Regulation of IP 3 receptors by cyclic AMP. Cell Calcium 2017, 63, 48–52. [Google Scholar] [CrossRef]
- Saji, M.; Ikuyama, S.; Akamizu, T.; Kohn, L.D. Increases in cytosolic Ca++ down regulates thyrotropin receptor gene expression by a mechanism different from the cAMP signal. Biochem. Biophys. Res. Commun. 1991, 176, 94–101. [Google Scholar] [CrossRef]
- Lof, C.; Sukumaran, P.; Viitanen, T.; Vainio, M.; Kemppainen, K.; Pulli, I.; Nasman, J.; Kukkonen, J.P.; Tornquist, K. Communication between the calcium and cAMP pathways regulate the expression of the TSH receptor: TRPC2 in the center of action. Mol. Endocrinol. 2012, 26, 2046–2057. [Google Scholar] [CrossRef][Green Version]
- Weiss, S.; Philp, N.J.; Grollman, E.F. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology 1984, 114, 1090–1098. [Google Scholar] [CrossRef]
- Corda, D.; Marocci, R.; Kohn, L.D.; Axelrod, J.; Luini, A. Association of the changes in cytosolic Ca and iodide efflux induced by thyrotropin and by the stimulation of alpha 1-adrenergic receptors in cultured rat thyroid cells. J. Biol. Chem. 1985, 260, 9230–9236. [Google Scholar] [CrossRef]
- Marcocci, C.; Luini, A.; Santisteban, P.; Grollman, E.F. Norepinephrine and thyrotropin stimulation of iodide efflux in FRTL-5 thyroid cells involves metabolites of arachidonic acid and is associated with the iodination of thyroglobulin. Endocrinology 1987, 120, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Raspé, E.; Dumont, J.E. Control of dog thyrocyte plasma membrane iodide permeability by the Ca2+-phosphatidylinositol and adenosine 3′,5′-monophosphate cascades. Endocrinology 1994, 135, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Hattori, K.; Hisatome, I.; Taniguchi, S.; Ueta, Y.; Hukui, H.; Santo, Y.; Igawa, O.; Shigemasa, C.; Kosugi, S.; et al. A TSH/dibutyryl cAMP activated Cl-/I- channel in FRTL-5 cells. Biochem. Biophys. Res. Commun. 1999, 259, 631–635. [Google Scholar] [CrossRef]
- Yoshida, A.; Taniguchi, S.; Hisatome, I.; Royaux, I.E.; Green, E.D.; Kohn, L.D.; Suzuki, K. Pendrin is a iodide-specific apical porter responsible for iodide efflux from thyroid cells. J. Clin. Endocrinol. Metab 2002, 87, 3356–3361. [Google Scholar] [CrossRef] [PubMed]
- Twyffels, L.; Massart, C.; Golstein, P.E.; Raspe, E.; van Sande, J.; Dumont, J.E.; Beauwens, R.; Kruys, V. Pendrin: The thyrocyte apical membrane iodide transporter? Cell Physiol. Biochem. 2011, 28, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, T.M.; Sukumaran, P.; Lof, C.; Tornquist, K. Functional coupling of TRPC2 cation channels and the calcium-activated anion channels in rat thyroid cells: Implications for iodide homeostasis. J. Cell. Physiol. 2013, 228, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Twyffels, L.; Strickaert, A.; Virreira, M.; Massart, C.; van Sande, J.; Wauquier, C.; Beauwens, R.; Dumont, J.E.; Galietta, L.J.; Boom, A.; et al. Anoctamin-1/TMEM16A is the major apical iodide channel of the thyrocyte. Am. J. Physiol 2014, 307, C1102–C1112. [Google Scholar] [CrossRef] [PubMed]
- Iosco, C.; Cosentino, C.; Sirna, L.; Romano, R.; Cursano, S.; Mongia, A.; Pompeo, G.; di Bernardo, J.; Ceccarelli, C.; Tallini, G.; et al. Anoctamin 1 is apically expressed on thyroid follicular cells and controbutes to ATP- and calcium-activated iodide efflux. Cell Physiol. Biochem. 2014, 34, 966–980. [Google Scholar] [CrossRef]
- Schanze, N.; Jacobi, S.F.; Rijntjes, E.; Mergler, S.; del Omo, M.; Hoefig, C.S.; Khajavi, N.; Lehmphul, I.; Biebermann, H.; Mittag, J.; et al. 3.Iodothyronamine decreases expression of genes involved in iodide metabolism in mouse thyroids and inhibits iodide uptake in PCCL3 thyrocytes. Thyroid 2017, 27, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, N.; Mergler, S.; Biebermann, H. 3-Iodothyronamine, a novel endogenous modulator of transient receptor potential melastatin 8? Front. Endocrinol. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Di Jeso, B.; Pereira, R.; Consiglio, E.; Formisano, S.; Satrustegui, J.; Sandoval, I.V. Demonstration of a Ca2+ requirement for thyroglobulin dimerization and export to the golgi complex. Eur. J. Biochem. 1998, 252, 583–590. [Google Scholar] [CrossRef]
- Rivas, M.; Mellström, B.; Naranjo, J.R.; Santisteban, P. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J. Biol. Chem. 2004, 279, 33114–33122. [Google Scholar] [CrossRef] [PubMed]
- Rigutto, S.; Hoste, C.; Grasberger, H.; Milenkovic, M.; Communi, D.; Dumont, J.E.; Corvilain, B.; Miot, F.; de Deken, X. Activation of dual oxidases Duox1 and Duox2. Differential regulation mediated by cAMP-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009, 284, 6725–6734. [Google Scholar] [CrossRef]
- Vanvooren, V.; Allgeier, A.; Cosson, E.; van Sande, J.; Defer, N.; Pirlot, M.; Hanoune, J.; Dumont, J.E. Expression of multiple adenylyl isoforms in human and dog thyroid. Mol. Cell. Endocrinol. 2000, 170, 185–196. [Google Scholar] [CrossRef]
- Lorenz, S.; Aust, G.; Krohn, K. Ca(2+)-binding protein expression in primary human thyrocytes. Biochim. Biophys. Acta 2013, 1833, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Takasu, N.; Murakami, M.; Nagasawa, Y.; Yamada, T.; Shimizu, Y.; Kojima, I.; Ogata, E. BAY-K-8644, a calcium channel agonist, induces a rise in cytoplasmic free calcium and iodide discharge in thyroid cells. Biochem. Biophys. Res. Commun. 1987, 143, 1107–1111. [Google Scholar] [CrossRef]
- Mignen, O.; Constantin, B.; Potier-Cartereau, M.; Penna, A.; Gautier, M.; Guéguinou, M.; Renaudineau, Y.; Shoji, K.F.; Félix, R.; Bayet, E.; et al. Constitutive calcium entry and cancer: Updated views and insights. Eur. Biophys. J. 2017, 46, 395–413. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Magnusson, M.; Kemppainen, K.; Sukumar, P.; Löf, C.; Pulli, I.; Kalhori, V.; Törnquist, K. Transient Receptor Potential Canonical 1 (TRPC1) Channels as Regulators of Sphingolipid and VEGF Receptor Expression: Implications for Thyroid Cancer Cell Migration And Proliferation. J. Biol. Chem 2015, 290, 16116–16131. [Google Scholar] [CrossRef]
- Choukair, D.; Eberle, B.; Vick, P.; Hermann, P.; Weiss, B.; Paramasivam, N.; Schlesner, M.; Lornsen, K.; Roeth, R.; Klutman, C.; et al. Identification of transient receptor potential channel 4-associated proteins as novel candidate gene causing congenital primary hypothyroidism. Horm. Res. Paediatr. 2020, 93, 16–29. [Google Scholar] [CrossRef]
- Xu, S.-Z.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin induces mitochondrial dysfunction and apoptosis in anaplastic thyroid carcinoma cells via TRPV1-mediated mitochondrial calcium overload. Cell. Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Peng, J.B.; Tou, L.; Takanaga, H.; Adam, R.M.; Hediger, M.A.; Freeman, M.R. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab. Investig. 2002, 82, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Parma, J.; van Sande, J.; Swillens, S.; Tonacchera, M.; Dumont, J.E.; Vassart, G. Somatic mutations causing constitutive activity of the thyrotropin receptor are the major cause of hyperfunctioning thyroid adenomas: Identification of additional mutations activating both the cytosolic adenosine 3′,5′-monophosphate and inositol phosphate-Ca2+ cascades. Mol. Endocrinol. 1995, 9, 725–733. [Google Scholar]
- Grasberger, H.; van Sande, J.; Hag-Dahood, M.; Tenenbaum-Rakover, Y.; Refetoff, S. A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphate/Ca2+ cascade mediated TSH action on thyroid hormone synthesis. J. Clin. Endocrinol. Metab. 2007, 92, 2816–2820. [Google Scholar] [CrossRef][Green Version]
- Rivas, M.; Mellström, B.; Torres, B.; Cali, G.; Ferrara, A.M.; Terracciano, D.; Zannini, M.; de Escobar, G.M.; Naranjo, J.R. The DREAM protein is associated with thyroid enlargement and nodular development. Mol. Endocrinol. 2009, 23, 862–870. [Google Scholar] [CrossRef]
- Salama, I.; Malone, P.S.; Mihaimeed, F.; Jones, J.L. A review of the S100 proteins in cancer. Eur. J. Surg. Oncol. 2008, 34, 357–364. [Google Scholar] [CrossRef]
- Zhong, J.; Chang, L.; Chen, Y.-J.; Zhang, Q.-H.; Yang, J.; Kang, X.; Chen, S.-R.; Wen, G.-B.; Zu, X.-Y.; Cao, R.-X. The association between S100A13 and HMGA1 in the modulation of thyroid cancer proliferation and invasion. J. Transl. Med. 2016, 14, 80. [Google Scholar] [CrossRef]
- Jia, W.; Gao, X.J.; Zhang, Z.-D.; Yang, Z.-X.; Zhang, G. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur. Rev. Med. Pharmacol. 2013, 17, 1486–1508. [Google Scholar]
- Zhang, K.; Yu, M.; Hao, F.; Dong, A.; Chen, D. Knockdown of S1004A blocks growth and metastasis of anaplastic thyroid cancer cells in vitro and in vivo. Cancer Biomark 2016, 17, 281–291. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, H.; Xu, X.; Yu, Y.; Zhang, H.; Zhang, J.; Ning, L.; Hao, F.; Liu, X.; Niu, M.; et al. S100A4 knockout sensitizes anaplastic thuroid carcinoma cells harbouring BRAFV600E/Mt to vemurafenib. Cell Physiol. Biochem. 2018, 49, 1143–1162. [Google Scholar] [CrossRef]
- Chang, Z.; Cai, C.; Han, D.; Gao, Y.; Li, Q.; Feng, L.; Zhang, W.; Zheng, J.; Jin, J.; Zhang, H.; et al. Anoctamin5 regulates cell migration and invasion in thyroid cancer. Int. J. Oncol. 2017, 51, 1311–1319. [Google Scholar] [CrossRef]
- Williams, M.E.; Washburn, M.S.; Hans, M.; Urrutia, A.; Brust, P.F.; Prodanovich, P.; Harpold, M.M.; Stauderman, K.A. Structure and functional characterization of a novel human low-voltage activated calcium channel. J. Neurochem. 1999, 72, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; James, A.D. Targeting the calcium signalling machinery in cancer. Cancers 2020, 12, 2351. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Galluzi, L.; Pinton, P. Ca2+ fluxes and cancer. Mol. Cell 2020, 78, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Z.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Zhang, L. Capsaicin inhibits the metastasis of human papillary carcinoma BCPAP cells through the modulating of the TRPV1 channel. Food Funct. 2018, 24, 344–354. [Google Scholar] [CrossRef]
- Mace, K.E.; Lussier, M.P.; Boulay, G.; Powers, J.L.T.; Parfrey, H.; Perraud, A.L.; Riches, D.W. TRUSS, TNF-R1, and TRPC ion channels synergistically reverse endoplasmic reticulum Ca2+ storage reduction in response to m1 muscarinic acethylcholine receptor signaling. J. Cell. Physiol. 2010, 225, 444–453. [Google Scholar] [CrossRef]
- Mamaeva, V.; Niemi, R.; Beck, M.; Ozliseli, E.; Desai, D.; Landor, S.; Grönroos, T.; Kronqvist, P.; Pettersen, I.K.N.; McCormack, E.; et al. Inhibiting notch activity in breast cancer stem cells by glucose functionalized nanoparticles carrying g-secretase inhibitors. Mol. Ther. 2016, 24, 926–936. [Google Scholar] [CrossRef]
- Niemelä, E.; Desai, D.; Niemi, R.; Özliseli, E.; Doroszka, M.; Kemppainen, K.; Sahlgren, C.; Törnquist, K.; Eriksson, J.E.; Rosenholm, J. Fingolimod (FTY720) and methotrexate (MTX) multidrug carrying nanoparticles enables targeted induction of cell death and immobilization of invasive thyroid cancer cells. Eur. J. Pharm. Biopharm. 2020, 148, 1–9. [Google Scholar] [CrossRef]
- Prabhakar, N.; Zhang, J.; Desai, D.; Casals, E.; Gulin-Sarfraz, T.; Näreoja, T.; Westermarck, J.; Rosenholm, J.M. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int. J. Nanomed. 2016, 11, 6591–6608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, M.Y.; Lassila, T.; Törnquist, K. Calcium Signaling in the Thyroid: Friend and Foe. Cancers 2021, 13, 1994. https://doi.org/10.3390/cancers13091994
Asghar MY, Lassila T, Törnquist K. Calcium Signaling in the Thyroid: Friend and Foe. Cancers. 2021; 13(9):1994. https://doi.org/10.3390/cancers13091994
Chicago/Turabian StyleAsghar, Muhammad Yasir, Taru Lassila, and Kid Törnquist. 2021. "Calcium Signaling in the Thyroid: Friend and Foe" Cancers 13, no. 9: 1994. https://doi.org/10.3390/cancers13091994
APA StyleAsghar, M. Y., Lassila, T., & Törnquist, K. (2021). Calcium Signaling in the Thyroid: Friend and Foe. Cancers, 13(9), 1994. https://doi.org/10.3390/cancers13091994






