The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. TSH, Tg, and TgAb Measurements
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eustatia-Rutten, C.F.A.; Smit, J.W.A.; A Romijn, J.; Van Der Kleij-Corssmit, E.P.M.; Pereira, A.M.; Stokkel, M.P.; Kievit, J. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured meta-analysis. Clin. Endocrinol. 2004, 61, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Tennant, S.; Perros, P. Serum thyroglobulin in the monitoring of differentiated thyroid cancer. Scand. J. Clin. Lab. Investig. 2016, 76, S119–S123. [Google Scholar] [CrossRef] [PubMed]
- Gianoukakis, A.G. Thyroglobulin antibody status and differentiated thyroid cancer. Curr. Opin. Oncol. 2015, 27, 26–32. [Google Scholar] [CrossRef] [PubMed]
- De Meer, S.G.A.; Vorselaars, W.M.C.M.; Kist, J.W.; Stokkel, M.P.M.; De Keizer, B.; Valk, G.D.; Rinkes, I.H.M.B.; Vriens, M.R. Follow-up of patients with thyroglobulin-antibodies: Rising Tg-Ab trend is a risk factor for recurrence of differentiated thyroid cancer. Endocr. Res. 2017, 42, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.; Lopresti, J.; Fatemi, S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 394–404. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Robbins, R.J.; Spencer, C.A.; Braverman, L.E.; Pacini, F.; Wartofsky, L.; Haugen, B.R.; Sherman, S.I.; Cooper, D.S.; Braunstein, G.D.; et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 1433–1441. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Domínguez, J.M.; Nilo, F.; Contreras, T.; Carmona, R.; Droppelmann, N.; González, H.; Iturrieta, V.; Tuttle, R.M. Neck Sonography and Suppressed Thyroglobulin Have High Sensitivity for Identifying Recurrent/Persistent Disease in Patients with Low-risk Thyroid Cancer Treated With Total Thyroidectomy and Radioactive Iodine Ablation, Making Stimulated Thyroglobulin Unne. J. Ultrasound Med. 2017, 36, 2299–2307. [Google Scholar] [CrossRef]
- Giovanella, L.; Clark, P.M.; Chiovato, L.; Duntas, L.; Elisei, R.; Feldt-Rasmussen, U.; Leenhardt, L.; Luster, M.; Schalin-Jäntti, C.; Schott, M.; et al. DIAGNOSIS OF ENDOCRINE DISEASE: Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: A clinical position paper. Eur. J. Endocrinol. 2014, 171, R33–R46. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; Sadeghi, R.; Trimboli, P.; Ceriani, L.; Verburg, F.A. Unstimulated Highly Sensitive Thyroglobulin in Follow-up of Differentiated Thyroid Cancer Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 440–447. [Google Scholar] [CrossRef]
- Roger, C.; Chikh, K.; Raverot, V.; Claustrat, F.; Borson-Chazot, F.; Bournaud-Salinas, C.; Charrié, A. New-generation thyroglobulin assay: Performance and implications for follow-up of differentiated thyroid carcinoma. Ann. d’Endocrinologie 2014, 75, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.C.; Howard, R.S.; Stojadinovic, A.; Gaitonde, D.Y.; Wallace, M.K.; Ahmed, J.; Burch, H.B. The Utility of Serum Thyroglobulin Measurement at the Time of Remnant Ablation for Predicting Disease-Free Status in Patients with Differentiated Thyroid Cancer: A Meta-Analysis Involving 3947 Patients. J. Clin. Endocrinol. Metab. 2012, 97, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Gadawska-Juszczyk, K.; Kowalska, A. Comparison of the usefulness of post-ablative and post-operative thyroglobulin concentration measuring in prognostic assessment of patients with differentiated thyroid cancer. Endokrynol. Pol. 2015, 66, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Jarząb, M.; Czarniecka, A.; Roskosz, J.; Kukulska, A.; Handkiewicz-Junak, D.; Puch, Z.; Wygoda, Z.; Paliczka-Cieślik, E.; Kropińska, A.; et al. Ciągła stratyfikacja ryzyka w zróżnicowanym raku tarczycy (DTC) — stymulowane stężenie tyreoglobuliny (Tg) w surowicy, przed leczeniem uzupełniającym radiojodem (RAI), najważniejszym czynnikiem ryzyka nawrotu raka u pacjentów M0. Endokrynol. Polska 2016, 67, 2–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orlov, S.; Salari, F.; Kashat, L.; Freeman, J.L.; Vescan, A.D.; Witterick, I.; Walfish, P.G. Post-operative stimulated thyroglobulin and neck ultrasound as personalized criteria for risk stratification and radioactive iodine selection in low- and intermediate-risk papillary thyroid cancer. Endocrine 2015, 50, 130–137. [Google Scholar] [CrossRef]
- Pitoia, F.; Abelleira, E.; Cross, G. Thyroglobulin levels measured at the time of remnant ablation to predict response to treatment in differentiated thyroid cancer after thyroid hormone withdrawal or recombinant human TSH. Endocrine 2016, 55, 200–208. [Google Scholar] [CrossRef]
- Trevizam, P.G.C.; Tagliarini, J.V.; Castilho, E.C.; Marques, M.D.A.; Kiy, Y.; Mazeto, G.M.F.D.S. Thyroglobulin levels and thyroglobulin/thyrotropin ratio could predict the success of the ablative/therapeutic 131I in the differentiated thyroid cancers. Endocr. Res. 2016, 42, 42–48. [Google Scholar] [CrossRef]
- Yang, X.; Liang, J.; Li, T.-J.; Yang, K.; Liang, D.-Q.; Yu, Z.; Lin, Y.-S. Postoperative Stimulated Thyroglobulin Level and Recurrence Risk Stratification in Differentiated Thyroid Cancer. Chin. Med. J. 2015, 128, 1058–1064. [Google Scholar] [CrossRef]
- Yang, X.; Liang, J.; Li, T.; Zhao, T.; Lin, Y. Preablative Stimulated Thyroglobulin Correlates to New Therapy Response System in Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 1307–1313. [Google Scholar] [CrossRef]
- Zanella, A.; Scheffel, R.S.; Pasa, M.W.; Dora, J.M.; Maia, A.L.; Pasa, M.M. Role of Postoperative Stimulated Thyroglobulin as Prognostic Factor for Differentiated Thyroid Cancer in Children and Adolescents. Thyroid 2017, 27, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hua, W.; Zhang, X.; Peng, J.; Liang, J.; Gao, Z. The predictive value for excellent response to initial therapy in differentiated thyroid cancer. Nucl. Med. Commun. 2018, 39, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.J.; Chua, E.; Gargya, A.; Clark, J.; Gao, K.; Elliott, M. Elevated serum thyroglobulin levels at the time of ablative radioactive iodine therapy indicate a worse prognosis in thyroid cancer: An Australian retrospective cohort study. J. Laryngol. Otol. 2016, 130, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.J.; Pasternak, J.D.; Xiong, M.; Li, N.; Gosnell, J.E.; Yeh, M.W.; Chiu, H.K. Pre-Ablation Thyroglobulin and Thyroglobulin to Thyroid-Stimulating Hormone Ratio May Be Associated with Pulmonary Metastases in Children with Differentiated Thyroid Cancer. Endocr. Pract. 2016, 22, 1259–1266. [Google Scholar] [CrossRef]
- Toubeau, M.; Touzery, C.; Arveux, P.; Chaplain, G.; Vaillant, G.; Berriolo, A.; Riedinger, J.-M.; Boichot, C.; Cochet, A.; Brunotte, F. Predictive value for disease progression of serum thyroglobulin levels measured in the postoperative period and after (131)I ablation therapy in patients with differentiated thyroid cancer. J. Nucl. Med. 2004, 45, 988–994. [Google Scholar]
- Bandeira, L.; Padovani, R.D.P.; Ticly, A.L.; Cury, A.N.; Scalissi, N.M.; Marone, M.M.S.; Ferraz, C. Thyroglobulin levels before radioactive iodine therapy and dynamic risk stratification after 1 year in patients with differentiated thyroid cancer. Arch. Endocrinol. Metab. 2017, 61, 590–599. [Google Scholar] [CrossRef]
- Liu, N.; Meng, Z.; Jia, Q.; Tan, J.; Zhang, G.; Zheng, W.; Wang, R.; Li, X.; Hu, T.; Upadhyaya, A.; et al. Multiple-factor analysis of the first radioactive iodine therapy in post-operative patients with differentiated thyroid cancer for achieving a disease-free status. Sci. Rep. 2016, 6, 34915. [Google Scholar] [CrossRef]
- Mousa, U.; Yikilmaz, A.S.; Nar, A. Stimulated thyroglobulin values above 5.6 ng/ml before radioactive iodine ablation treatment following levothyroxine withdrawal is associated with a 2.38-fold risk of relapse in Tg-ab negative subjects with differentiated thyroid cancer. Clin. Transl. Oncol. 2017, 19, 1028–1034. [Google Scholar] [CrossRef]
- Piccardo, A.; Arecco, F.; Puntoni, M.; Foppiani, L.; Cabria, M.; Corvisieri, S.; Arlandini, A.; Altrinetti, V.; Bandelloni, R.; Orlandi, F. Focus on High-Risk DTC Patients. Clin. Nucl. Med. 2013, 38, 18–24. [Google Scholar] [CrossRef]
- Rosario, P.W.; Mourão, G.F.; Calsolari, M.R. Low Postoperative Nonstimulated Thyroglobulin as a Criterion for the Indication of Low Radioiodine Activity in Patients with Papillary Thyroid Cancer of Intermediate Risk “with Higher Risk Features”. Clin. Endocrinol. 2016, 85, 453–458. [Google Scholar] [CrossRef]
- Robenshtok, E.; Grewal, R.K.; Fish, S.A.; Sabra, M.; Tuttle, R.M. A Low Postoperative Nonstimulated Serum Thyroglobulin Level Does Not Exclude the Presence of Radioactive Iodine Avid Metastatic Foci in Intermediate-Risk Differentiated Thyroid Cancer Patients. Thyroid 2013, 23, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourão, G.F.; Calsolari, M.R. Efficacy of adjuvant therapy with 3.7 GBq radioactive iodine in intermediate-risk patients with ‘higher risk features’ and predictive value of postoperative nonstimulated thyroglobulin. Nucl. Med. Commun. 2016, 37, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Trimboli, P.; Verburg, F.A.; Treglia, G.; Piccardo, A.; Foppiani, L.; Ceriani, L. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Giannoula, E.; Melidis, C.; Papadopoulos, N.; Bamidis, P.; Raftopoulos, V.; Iakovou, I. Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. J. Clin. Med. 2020, 9, 2708. [Google Scholar] [CrossRef] [PubMed]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
| Parameter | n (%) | ||
|---|---|---|---|
| All | Papillary | Follicular | |
| n = 222 | n = 172 (77) | n = 50 (23) | |
| Age (years) * | |||
| Median (min–max) | 48 (15–91) | 44 (15–82) | 55 (19–91) |
| Sex | |||
| Female | 158 (71) | 120 (70) | 38 (76) |
| Male | 64 (29) | 52 (30) | 12 (24) |
| Histology | |||
| Papillary | - | 172 | - |
| Classical variant | - | 134 (78) | - |
| Follicular variant | - | 29 (17) | - |
| Sclerosing variant | - | 3 (2) | - |
| Tall-cell variant | - | 2 (1) | - |
| Trabecular variant | - | 4 (2) | - |
| Follicular | - | - | 50 |
| Classical variant | - | - | 41 (82) |
| Hurthle cell variant | - | - | 7 (14) |
| Insular variant | - | - | 1 (2) |
| Trabecular variant | - | - | 1 (2) |
| T stage * | |||
| T1 | 77 (35) | 69 (41) | 8 (16) |
| T2 | 51 (23) | 31 (18) | 20 (40) |
| T3 | 69 (30) | 54 (32) | 15 (30) |
| T4 | 22 (10) | 15 (9) | 7 (14) |
| N stage * | |||
| N0 | 151 (68) | 107 (62) | 44 (88) |
| N1 | 71 (32) | 65 (38) | 6 (12) |
| M stage * | |||
| M0 | 207 (93) | 164 (95) | 43 (86) |
| M1 | 15 (7) | 8 (5) | 7 (14) |
| Histological Types and Tumor Stages | |||||||||||||
| Therapeutic Response | All | PTC | FTC | T1 | T2 | T3 | T4 | N0 | N1 | M0 | M1 | ||
| Tumor free | 64 | 65 | 62 | 77 | 73 | 54 | 36 | 76 | 39 | 69 | 0 | ||
| Uncertain | 13 | 12 | 14 | 12 | 10 | 19 | 5 | 13 | 11 | 14 | 0 | ||
| Incomplete biochemical response | 7 | 9 | 2 | 8 | 2 | 9 | 14 | 3 | 16 | 7 | 7 | ||
| Structural disease | 16 | 14 | 22 | 4 | 16 | 19 | 46 | 7 | 34 | 10 | 94 | ||
| p-Values of Comparisons of Subgroups | |||||||||||||
| PTC–FTC | T1–T2 | T1–T3 | T1–T4 | T2–T3 | T2–T4 | T3–T4 | N0–N1 | M0–M1 | |||||
| p | 0.569 | 0.440 | 0.007 | <0.001 | 0.063 | 0.005 | 0.030 | <0.001 | <0.001 | ||||
| Histological Types | ||||||||
| Thyroglobulin (ng/mL) | All | PTC | FTC | |||||
| Median (quartiles) | ||||||||
| Postoperative | 15.5 (4.8–39.8) | 14.8 (4.6–39.3) | 17.3 (4.7–47.7) | |||||
| After 9–12 months | 0.0 (0–1.1) | 0.1 (0–1.2) | 0 (0–0.6) | |||||
| At the end of follow-up | 0 (0–0.6) | 0 (0–0.6) | 0 (0–0.6) | |||||
| The lowest | 0 (0–0.2) | 0 (0–0.3) | 0 (0–0) | |||||
| The highest | 0.5 (0.1–4.45) | 0.55 (0.2–4.3) | 0.45 (0–14.6) | |||||
| Tumor Stages | ||||||||
| Thyroglobulin (ng/mL) | T1 | T2 | T3 | T4 | N0 | N1 | M0 | M1 |
| Median (quartiles) | ||||||||
| Postoperative | 12.9 | 13.5 | 14.9 | 71.0 | 11.2 | 25.9 | 13.5 | 638.0 |
| After 9–12 months | 0 | 0 | 0.1 | 5.7 | 0 | 1.3 | 0 | 47.7 |
| At the end of follow-up | 0 | 0 | 0 | 3.5 | 0 | 0.7 | 0 | 22.0 |
| The lowest | 0 | 0 | 0 | 1.3 | 0 | 0.2 | 0 | 15.0 |
| The highest | 0.3 | 0.4 | 0.7 | 9.4 | 0.4 | 2.8 | 0.4 | 159.8 |
| p-Values of Comparisons of Groups | ||||||||
| Thyroglobulin | PTC–FTC | T1–T2 | T1–T3 | T1–T4 | T2–T3 | T2–T4 | T3–T4 | N0–N1 |
| p | ||||||||
| Postoperative | 0.540 | 0.748 | 0.277 | 0.015 | 0.246 | 0.024 | 0.045 | <0.001 |
| After 9–12 months | 0.723 | 0.183 | 0.093 | 0.001 | 0.502 | 0.015 | 0.012 | <0.001 |
| At the end of follow-up | 0.481 | 0.408 | 0.008 | <0.001 | 0.096 | 0.002 | 0.009 | <0.001 |
| The lowest | 0.671 | 0.263 | 0.011 | <0.001 | 0.161 | 0.001 | 0.006 | <0.001 |
| The highest | 0.787 | 0.875 | 0.030 | <0.001 | 0.045 | <0.001 | 0.002 | <0.001 |
| Thyroglobulin (ng/mL) | Tumor Free | Uncertain | Incomplete Biochemical Response | Structural Disease |
|---|---|---|---|---|
| Median (Quartiles) | ||||
| Postoperative | 9.8 (3.2–19.7) | 23.0 (9.7–52.1) | 45.4 (16.3–60.7) | 215.0 (65.0–638.0) |
| After 9–12 months | 0 (0–0) | 0.5 (0–1.0) | 1.7 (1.3–5.7) | 21.6 (4.3–104.4) |
| At the end of follow-up | 0 (0–0) | 0.5 (0.4–0.6) | 2.0 (1.4–3.0) | 19.1 (7.8–117.0) |
| The lowest | 0 (0–0) | 0.1 (0–0.4) | 0.9 (0.6–1.9) | 10.2 (3.0–40.0) |
| The highest | 0.2 (0–0.5) | 1.5 (0.6–2.4) | 5.0 (2.0–8.8) | 63.7 (11.4–1254.0) |
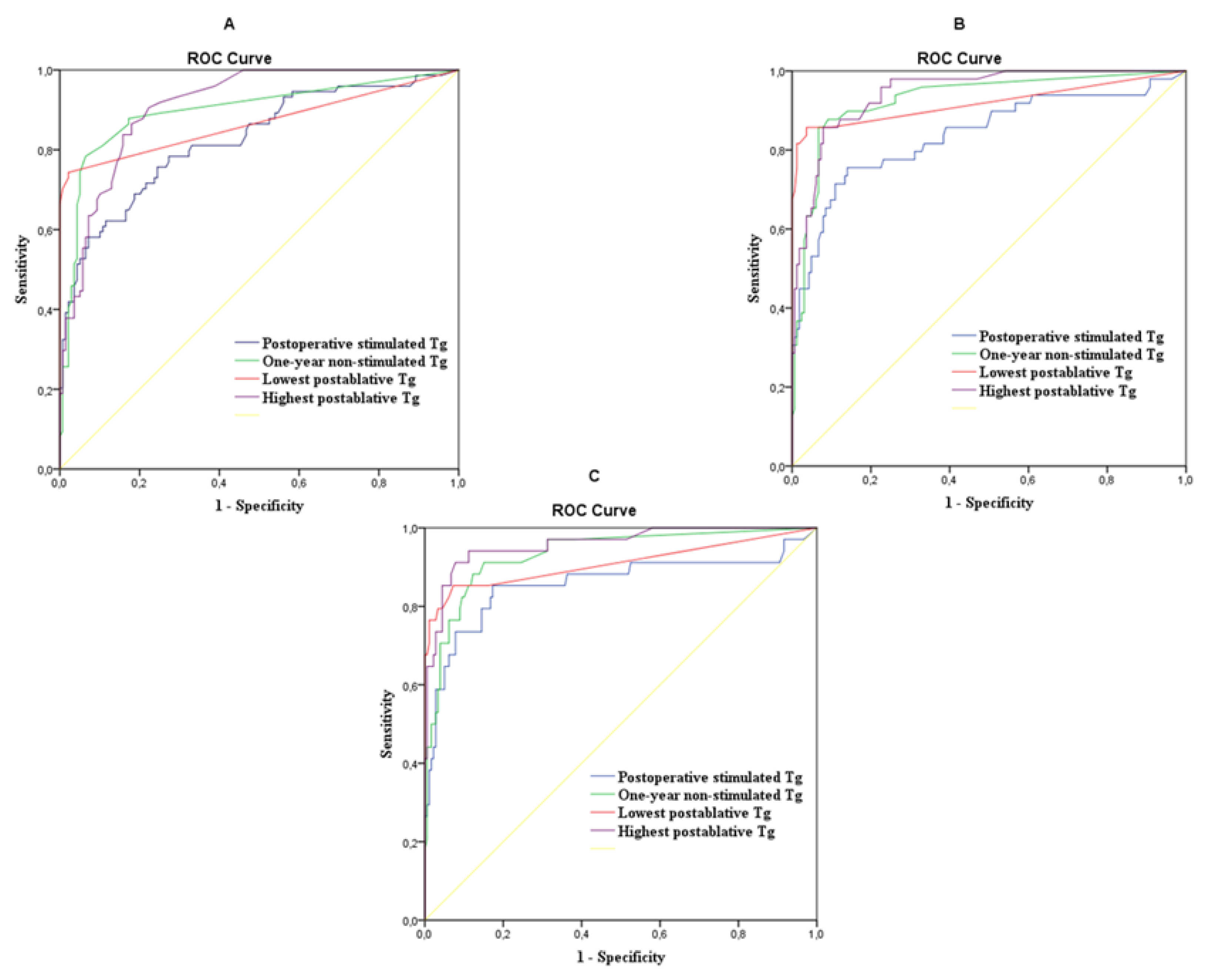
| Tumor Free vs. Uncertain + Incomplete Biochemical Response + Structural Disease at the End of Follow-Up | ||||||||
| Thyroglobulin | AUC | p-Value | Cut-Off (ng/mL) | Sens% | Spec% | PPV% | NPV% | DA% |
| Postoperative | 0.825 | <0.001 | 20.1 | 75.7 | 75.5 | 75.5 | 75.7 | 75.6 |
| After 1 year | 0.897 | <0.001 | 0.45 | 78.4 | 93.5 | 92.3 | 81.2 | 86.0 |
| The lowest | 0.868 | <0,001 | <0.1 | 74.3 | 97.8 | 97.1 | 79.2 | 86.1 |
| The highest | 0.911 | <0.001 | 0.85 | 86.4 | 82.0 | 82.8 | 85.8 | 84.2 |
| Tumor Free + Uncertain vs. Incomplete Biochemical Response + Structural Disease at the End of Follow-Up | ||||||||
| Thyroglobulin | AUC | p-Value | Cut-Off (ng/mL) | Sens% | Spec% | PPV% | NPV% | DA% |
| Postoperative | 0.838 | <0.001 | 41.2 | 71.4 | 89.0 | 86.7 | 75.7 | 80.2 |
| After 1 year | 0.928 | <0.001 | 0.85 | 87.8 | 90.9 | 90.6 | 88.2 | 89.4 |
| The lowest | 0.919 | <0.001 | 0.45 | 85.7 | 96.3 | 95.9 | 87.1 | 91.0 |
| The highest | 0.945 | <0.001 | 3.35 | 85.7 | 92.1 | 91.6 | 86.6 | 88.9 |
| Tumor Free + Uncertain + Incomplete Biochemical Response vs. Structural Disease at the End of Follow-Up | ||||||||
| Thyroglobulin | AUC | p-Value | Cut-Off (ng/mL) | Sens% | Spec% | PPV% | NPV% | DA% |
| Postoperative | 0.857 | <0.001 | 34.6 | 85.3 | 82.7 | 83.1 | 84.9 | 84.0 |
| After 1 year | 0.933 | <0.001 | 0.85 | 91.2 | 84.9 | 85.8 | 90.6 | 88.1 |
| The lowest | 0.909 | <0.001 | 0.75 | 85.3 | 92.7 | 92.1 | 86.3 | 89.0 |
| The highest | 0.958 | <0.001 | 7.7 | 91.2 | 92.2 | 92.1 | 91.3 | 91.7 |
| Thyroglobulin | Cut-Off Values (ng/mL) | Relative Risk (RR) |
|---|---|---|
| Postoperative | 34.6 | 15.87 |
| One-year non-stimulated | 0.85 | 28.53 |
| Lowest non-stimulated | 0.75 | 25.18 |
| Highest non-stimulated | 7.7 | 30.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szujo, S.; Bajnok, L.; Bodis, B.; Nagy, Z.; Nemes, O.; Rucz, K.; Mezosi, E. The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer. Cancers 2021, 13, 310. https://doi.org/10.3390/cancers13020310
Szujo S, Bajnok L, Bodis B, Nagy Z, Nemes O, Rucz K, Mezosi E. The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer. Cancers. 2021; 13(2):310. https://doi.org/10.3390/cancers13020310
Chicago/Turabian StyleSzujo, Szabina, Laszlo Bajnok, Beata Bodis, Zsuzsanna Nagy, Orsolya Nemes, Karoly Rucz, and Emese Mezosi. 2021. "The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer" Cancers 13, no. 2: 310. https://doi.org/10.3390/cancers13020310
APA StyleSzujo, S., Bajnok, L., Bodis, B., Nagy, Z., Nemes, O., Rucz, K., & Mezosi, E. (2021). The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer. Cancers, 13(2), 310. https://doi.org/10.3390/cancers13020310






