Impact of Adjuvant Radiotherapy in Patients with Central Neurocytoma: A Multicentric International Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
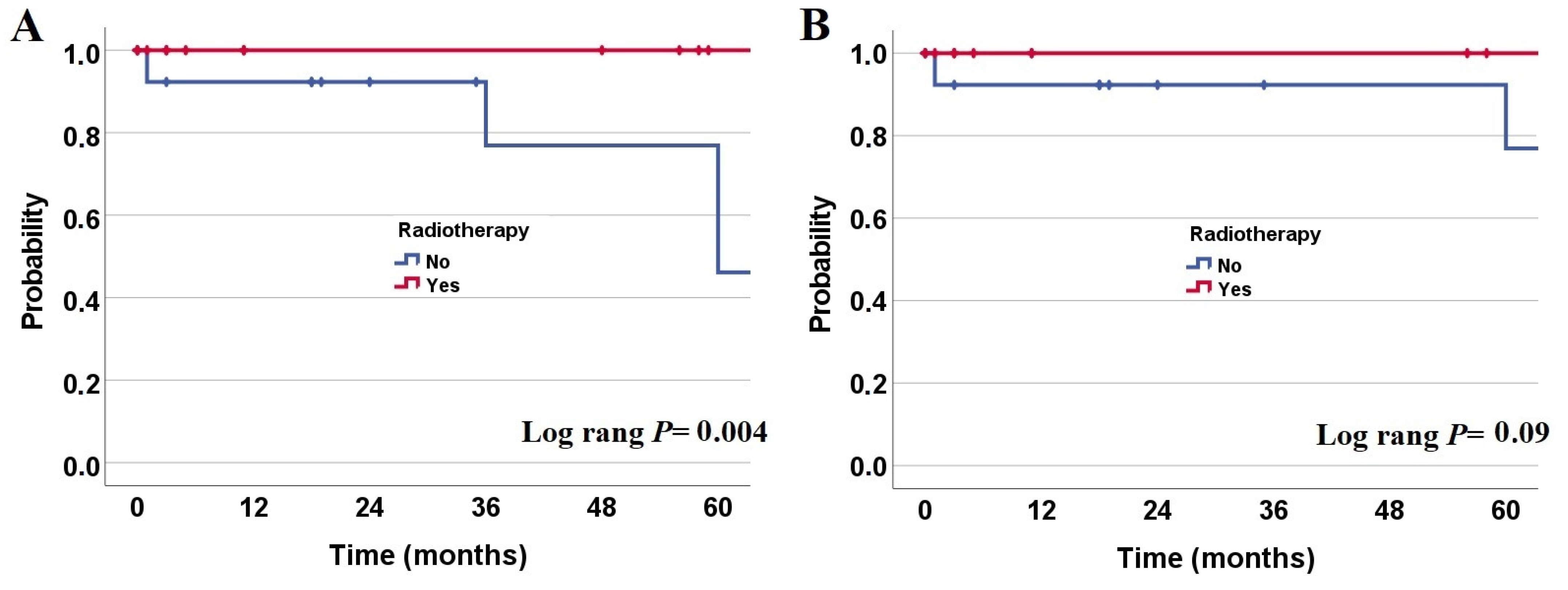
3. Results
3.1. Overall and Progression-Free Survival Rates
3.2. Radiotherapy Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, M.C.; Deb, P.; Sharma, S.; Sarkar, C. Neurocytoma: A comprehensive review. Neurosurg. Rev. 2006, 29, 270–285, discussion 285. [Google Scholar] [CrossRef]
- Hassoun, J.; Gambarelli, D.; Grisoli, F.; Pellet, W.; Salamon, G.; Pellissier, J.F.; Toga, M. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982, 56, 151–156. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassoun, J.; Söylemezoglu, F.; Gambarelli, D.; Figarella-Branger, D.; von Ammon, K.; Kleihues, P. Central neurocytoma: A synopsis of clinical and histological features. Brain Pathol. 1993, 3, 297–306. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Gottfried, O.N.; von Koch, C.S.; Chang, S.M.; McDermott, M.W. Central neurocytoma: A review. J. Neurooncol. 2004, 66, 377–384. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A. Central neurocytoma. Cancer 1999, 85, 1606–1610. [Google Scholar] [CrossRef]
- Mozes, P.; Szanto, E.; Tiszlavicz, L.; Barzo, P.; Cserhati, A.; Fodor, E.; Hideghety, K. Clinical course of central neurocytoma with malignant transformation-an indication for craniospinal irradiation. Pathol. Oncol. Res. 2014, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, A.; François, P.; Loundou, A.; Fèvre-Montange, M.; Jouvet, A.; Roche, P.-H.; Figarella-Branger, D. Prognostic factors in central neurocytomas: A multicenter study of 71 cases. Am. J. Surg. Pathol. 2012, 36, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Schild, S.E. Treatment recommendations for the various subgroups of neurocytomas. J. Neurooncol. 2006, 77, 305–309. [Google Scholar] [CrossRef]
- Hallock, A.; Hamilton, B.; Ang, L.C.; Tay, K.Y.; Meygesi, J.F.; Fisher, B.J.; Watling, C.J.; Macdonald, D.R.; Bauman, G.S. Neurocytomas: Long-term experience of a single institution. Neuro Oncol. 2011, 13, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenstra, J.L.; Rodriguez, F.J.; Frechette, C.M.; Giannini, C.; Stafford, S.L.; Pollock, B.E.; Schild, S.E.; Scheithauer, B.W.; Jenkins, R.B.; Buckner, J.C.; et al. Central neurocytoma: Management recommendations based on a 35-year experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1145–1154. [Google Scholar] [CrossRef]
- Lenzi, J.; Salvati, M.; Raco, A.; Frati, A.; Piccirilli, M.; Delfini, R. Central neurocytoma: A novel appraisal of a polymorphic pathology. Our experience and a review of the literature. Neurosurg. Rev. 2006, 29, 286–292. [Google Scholar] [CrossRef]
- Yen, C.P.; Sheehan, J.; Patterson, G.; Steiner, L. Gamma knife surgery for neurocytoma. JNS 2007, 107, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.A.; Niranjan, A.; Lunsford, L.D. The management of central neurocytoma: Radiosurgery. Neurosurg. Clin. N. Am. 2015, 26, 37–44. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, D.G.; Kim, I.K.; Kim, Y.H.; Choi, S.H.; Han, J.H.; Park, C.-K.; Chung, H.-T.; Park, S.-H.; Paek, S.H.; et al. Central neurocytoma: Long-term outcomes of multimodal treatments and management strategies based on 30 years’ experience in a single institute. Neurosurgery 2013, 72, 407–413, discussion 413–414. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Paek, S.H.; Jeong, S.S.; Chung, H.-T.; Han, J.H.; Park, C.-K.; Jung, H.-W.; Kim, D.G. Gamma knife radiosurgery for central neurocytoma: Primary and secondary treatment. Cancer 2007, 110, 2276–2284. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shuto, T.; Suenaga, J.; Inomori, S.; Fujino, H. Gamma knife radiosurgery for central neurocytomas. Neurol. Med. Chir. 2010, 50, 107–112, discussion 112–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genc, A.; Bozkurt, S.U.; Karabagli, P.; Seker, A.; Bayri, Y.; Konya, D.; Kilic, T. Gamma knife radiosurgery for cranial neurocytomas. J. Neurooncol. 2011, 105, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Guo, W.-Y.; Kejia, T.; Dinesh, N.; Pan, D.H.-C.; Jokura, H.; Kawagishi, J.; van Eck, A.T.C.J.; Horstmann, G.A.; Yeo, T.T.; et al. Gamma Knife surgery for central neurocytomas. JNS 2012, 117, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.M.; Ivan, M.E.; Oh, T.; Barani, I.; Parsa, A.T. Intraventricular neurocytomas: A systematic review of stereotactic radiosurgery and fractionated conventional radiotherapy for residual or recurrent tumors. Clin. Neurol. Neurosurg. 2014, 117, 55–64. [Google Scholar] [CrossRef]
- Barani, I.J.; Raleigh, D.R.; Larson, D. The management of central neurocytoma: Radiotherapy. Neurosurg. Clin. 2015, 26, 45–56. [Google Scholar] [CrossRef]
- Virbel, G.; Cebula, H.; Coca, A.; Lhermitte, B.; Bauchet, L.; Noël, G. Optimisation du choix de la technique d’irradiation des neurocytomes centraux à partir des données de la littérature. Cancer Radiother. 2020, 24, 882–891. [Google Scholar] [CrossRef]
- Mahavadi, A.K.; Patel, P.M.; Kuchakulla, M.; Shah, A.H.; Eichberg, D.; Luther, E.M.; Komotar, R.J.; Ivan, M.E. Central Neurocytoma Treatment Modalities: A Systematic Review Assessing the Outcomes of Combined Maximal Safe Resection and Radiotherapy with Gross Total Resection. World Neurosurg. 2020, 137, e176–e182. [Google Scholar] [CrossRef]
- Sharlvla, M.C.; Rathore, A.; Karak, A.K.; Sarkar, C. A study of proliferative markers in central neurocytoma. Pathol. 1998, 30, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-D.; Li, W.-B.; Feng, J.; Qiu, X.-G. Long-term outcomes of adjuvant radiotherapy after surgical resection of central neurocytoma. Radiat. Oncol. 2014, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.; Hong, S.H.; Yoon, M.J.; Kwon, S.M.; Cho, Y.H.; Kim, J.H.; Kim, C.J. Prognosis and treatment outcomes of central neurocytomas: Clinical interrogation based on a single center experience. J. Neuro-Oncol. 2018, 140, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Laack, N.N.; Brown, P.D.; Ivnik, R.J.; Furth, A.F.; Ballman, K.V.; Hammack, J.E.; Arusell, R.M.; Shaw, E.G.; Buckner, J.C. Cognitive function after radiotherapy for supratentorial low-grade glioma: A North Central Cancer Treatment Group prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1175–1183. [Google Scholar] [CrossRef]
- Vigliani, M.-C.; Sichez, N.; Poisson, M.; Delattre, J.-Y. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 527–533. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Hunter, J.V.; Ledakis, G.E.; Cohen, B.; Tallent, E.M.; Goldstein, B.H.; Tochner, Z.; Lustig, R.; Judy, K.D.; Pruitt, A.; et al. Late cognitive and radiographic changes related to radiotherapy: Initial prospective findings. Neurology 2002, 59, 40–48. [Google Scholar] [CrossRef]
- Brown, P.D.; Buckner, J.C.; O’Fallon, J.R.; Iturria, N.L.; Brown, C.A.; O’Neill, B.P.; Scheithauer, B.W.; Dinapoli, R.P.; Arusell, R.M.; Curran, W.J.; et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J. Clin. Oncol. 2003, 21, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.J.; Mundt, A.J.; Sweeney, P.J.; Llanes-Macy, S.; Dunaway, L.; Castillo, M.; Macdonald, R.L. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology 2003, 60, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, V.A.; Galarza, M.; Catapano, D.; Monte, V.; Bisceglia, M.; Carosi, I. Lateral ventricle tumors: Surgical strategies according to tumor origin and development—A series of 72 cases. Neurosurgery 2005, 56, 36–45, discussion 36–45. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Meyers, C.A.; Sawaya, R. Neuropsychological effects of third ventricle tumor surgery. Neurosurgery 2003, 52, 791–798, discussion 798. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, D.; Song, J.; Yao, Y.; Mao, Y. Emotion and cognitive function assessment of patients with central neurocytoma resection through transcortical frontal approach: A 5-year postoperative follow-up study. Chin. Med. J. 2011, 124, 2593–2598. [Google Scholar]
- Klein, M.; Drijver, A.J.; van den Bent, M.J.; Bromberg, J.C.; Hoang-Xuan, K.; Taphoorn, M.J.B.; Reijneveld, J.C.; Ben Hassel, M.; Vauleon, E.; Eekers, D.B.P.; et al. Memory in low-grade glioma patients treated with radiotherapy or temozolomide: A correlative analysis of EORTC study 22033-26033. Neuro Oncol. 2021, 23, 803–811. [Google Scholar] [CrossRef]
- Söylemezoglu, F.; Scheithauer, B.W.; Esteve, J.; Kleihues, P. Atypical central neurocytoma. J. Neuropathol. Exp. Neurol. 1997, 56, 551–556. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Nr. (% or Range) | Therapy | ||
|---|---|---|---|---|
| Radiotherapy | No Radiotherapy | p-Value | ||
| Patients | 33 | 19 (58%) | 14 (42%) | |
| Med. age (range) | 25 y (4–58) | 24 (12–58) | 26 (4–50) | 0.5 |
| Sex | M: 17 (51%) F: 16 (49%) | 9 (47%) 10 (53%) | 8 (47%) 6 (53%) | 0.7 |
| Ki67 MIB1 value, median | 8 (1–30) | 7.5 (1–30) | 10 (1–25) | 0.8 |
| Resection | 0.02 | |||
| Gross total resection | 9 (27%) | 2 (10%) | 7 (50%) | |
| Subtotal resection | 24 (73%) | 17 (90%) | 7 (50%) | |
| Chemotherapy | 0.2 | |||
| Yes | 2 (6%) | 0 (0%) | 2 (14%) | |
| No | 31 (94%) | 19 (100%) | 12 (86%) | |
| WHO grade | 0.6 | |||
| I | 5 (15%) | 2 (10%) | 3 (21%) | |
| II | 25 (76%) | 15 (80%) | 10 (72%) | |
| III | 1 (3%) | 1 (5%) | 0 (0%) | |
| Unknown | 2 (6%) | 1 (5%) | 1 (7%) | |
| Primary tumor site | 0.7 | |||
| Ventricles | 14 (42%) | 7 (37%) | 7 (50%) | |
| Central | 12 (36%) | 7 (37%) | 5 (36%) | |
| Others | 7 (21%) | 5 (26%) | 2 (14%) | |
| Relapse pattern | 0.4 | |||
| Yes | 7 (21%) | 3 (16%) | 4 (29%) | |
| No | 26 (79%) | 16 (84%) | 10 (71%) | |
| Studies | Number of Patients Receiving RT/All Patients | Median RT Dose in Gy (Range) | Local Control Rate |
|---|---|---|---|
| Fractionated Conventional Radiotherapy (FCRT) | |||
| Sharma et al. 1998 [24] | 15/15 | 40–60 | 100% |
| Rades et al. 2006 [9] | 177/350 | 50–60 | 87% |
| Leenstra et al. 2007 [11] | 18/18 | Median: 54.5 (48.6–61.2) | 78% |
| Kim et al. 2013 [15] | 7/58 | Median: 54 (50.4–55.8) | 80% |
| Chen et al. 2014 [25] | 63/63 | Median: 54 (46–60) | 100% |
| Byun et al. 2018 [26] | 10/40 | 54–56 | 69% |
| Current study | 19/33 | Median: 54 (50–60) | 84% |
| Stereotactic Radiosurgery (SRS) | |||
| Yen et al. 2007 [13] | 6/6 | Median: 15.1 (9–20) | 100% |
| Kim et al. 2007 [16] | 7/13 | Median: 15.7 (15–18) | 85% |
| Matsunaga et al. 2010 [17] | 7/7 | Median: 13.9 (12–18) | 88% |
| Genc et al. 2011 [18] | 18/18 | Median: 16.7 (9–20) | 93% |
| Karlsson et al. 2012 [19] | 35/35 | Median: 14.0 (11–25) | 83% |
| Kim et al. 2013 [15] | 17/58 | Median: 16 (9–20) | 80% |
| Monaco at al. 2015 [14] | 8/8 | Median: 14.6 Gy (12–20) | 87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samhouri, L.; Meheissen, M.A.M.; Ibrahimi, A.K.H.; Al-Mousa, A.; Zeineddin, M.; Elkerm, Y.; Hassanein, Z.M.A.; Ismail, A.A.; Elmansy, H.; Al-Hanaqta, M.M.; et al. Impact of Adjuvant Radiotherapy in Patients with Central Neurocytoma: A Multicentric International Analysis. Cancers 2021, 13, 4308. https://doi.org/10.3390/cancers13174308
Samhouri L, Meheissen MAM, Ibrahimi AKH, Al-Mousa A, Zeineddin M, Elkerm Y, Hassanein ZMA, Ismail AA, Elmansy H, Al-Hanaqta MM, et al. Impact of Adjuvant Radiotherapy in Patients with Central Neurocytoma: A Multicentric International Analysis. Cancers. 2021; 13(17):4308. https://doi.org/10.3390/cancers13174308
Chicago/Turabian StyleSamhouri, Laith, Mohamed A. M. Meheissen, Ahmad K. H. Ibrahimi, Abdelatif Al-Mousa, Momen Zeineddin, Yasser Elkerm, Zeyad M. A. Hassanein, Abdelsalam Attia Ismail, Hazem Elmansy, Motasem M. Al-Hanaqta, and et al. 2021. "Impact of Adjuvant Radiotherapy in Patients with Central Neurocytoma: A Multicentric International Analysis" Cancers 13, no. 17: 4308. https://doi.org/10.3390/cancers13174308
APA StyleSamhouri, L., Meheissen, M. A. M., Ibrahimi, A. K. H., Al-Mousa, A., Zeineddin, M., Elkerm, Y., Hassanein, Z. M. A., Ismail, A. A., Elmansy, H., Al-Hanaqta, M. M., AL-Azzam, O. A., Elsaid, A. A., Kittel, C., Micke, O., Stummer, W., Elsayad, K., & Eich, H. T. (2021). Impact of Adjuvant Radiotherapy in Patients with Central Neurocytoma: A Multicentric International Analysis. Cancers, 13(17), 4308. https://doi.org/10.3390/cancers13174308







