The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Description of Present RSSs
1.1.1. Chilean TIRADS (2009)
1.1.2. BTA Classification (British Thyroid Association) (2014)
1.1.3. AACE (American Association of Clinical Endocrinologists) Grading System (2016)
1.1.4. ATA (American Thyroid Association) Grading System (2016)
1.1.5. K-TIRADS (Korean-TIRADS) (2016)
1.1.6. EU-TIRADS (European-TIRADS) (2017)
1.1.7. ACR-TIRADS (American College of Radiology-TIRADS) (2017)
1.1.8. C-TIRADS (Chinese-TIRADS) (2020)
1.2. Pattern-Based and Point Based Systems
1.3. Other Similarities and Differences
1.3.1. Lexicon
1.3.2. Classification
1.3.3. Patterns
1.4. Raw Diagnostic Values in Comparative Studies (before Applying Size Cut-Offs for the Decision to Perform FNA)
1.5. Inter-Observer Agreement
2. Indications for FNA and Diagnostic Values of RSSs after Applying Size Cut-Offs for FNA
2.1. Dimensional Cut-Offs
2.2. Diagnostic Value after Applying Cut-Offs: Decision Guidance, Avoided FNAs, and Missed Carcinomas
3. Weaknesses of TIRADSs
3.1. Insufficient Sensitivity for the Diagnosis of Follicular Thyroid Carcinoma and Follicular Variant of PTC
3.2. Insufficient Specificity to Rule-Out Autonomously Functioning/Hot Thyroid Nodules from FNA
3.3. High Rates of Nodules Classified at Intermediate Risk (Usually TI-RADS 4)
3.4. Thyroid Diffuse Masses
- First, several etiological hypotheses should always be mentioned in the US report, including anaplastic carcinoma, lymphoma, metastases from non-thyroidal origin, and large differentiated papillary and follicular carcinomas. Riedel’s thyroiditis could be added to this list. In this case, marked hypoechogenicity and absorption of the US beam, absence of vascularity, and high stiffness are relatively characteristic features.
- The context helps refining the hypotheses. Knowledge of a prior renal cell carcinoma is for instance in favor of a metastasis and rapid development in an elderly subject with severe pressure symptoms in favor of an anaplastic carcinoma.
- Core-needle biopsy or surgical biopsy, depending on the center’s habits, should systematically be added to FNA, due to its low diagnostic power in this situation.
- Quick referral to a tertiary care center is advised.
3.5. Absence of Validation in Large Non-Specialized Medical Communities
4. Pitfalls
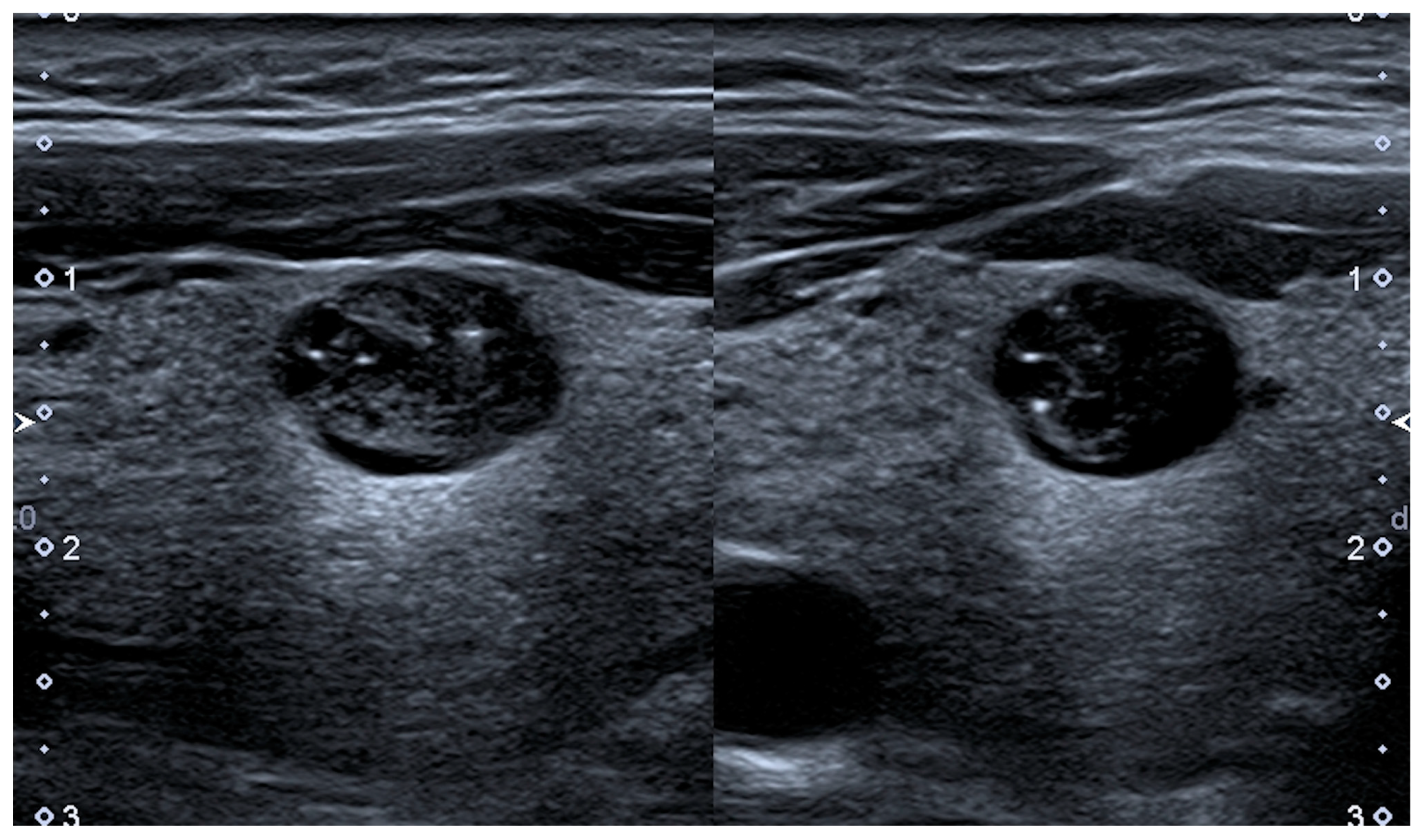
4.1. Shrinking Nodules
4.2. Subacute Thyroiditis
4.3. Confusion or Absence of Clear Distinction between Nodular Disease and Hyperplasia
5. Suggestions for the Future
5.1. Absence of Classification for TNs Treated with Thermal Ablation
5.2. Incorporating in the Algorithm the Number of Nodules Especially If They Belong to the Same Category
5.3. Taking into Account Age, Sex, Time Since Discovery, Results of Previous FNAs
5.4. Taking into Account the Serum Value of TSH (to Exclude a AFTN) and Calcitonin (to Detect a Medullary Cancer), When Available
5.5. D Vascularity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Horvath, E.; Silva, C.F.; Majlis, S.; Rodriguez, I.; Skoknic, V.; Castro, A.; Rojas, H.; Niedmann, J.P.; Madrid, A.; Capdeville, F.; et al. Prospective validation of the ultrasound based TIRADS (Thyroid Imaging Reporting And Data System) classification: Results in surgically resected thyroid nodules. Eur. Radiol. 2017, 27, 2619–2628. [Google Scholar] [CrossRef]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81 (Suppl. 1), 1–122. [Google Scholar] [CrossRef]
- Arambewela, M.H.; Wijesinghe, A.M.; Randhawa, K.; Bull, M.; Wadsley, J.; Balasubramanian, S.P. A pragmatic assessment of the British Thyroid Association "U classification" of thyroid nodules with a focus on their follow-up. Clin. Radiol. 2020, 75, 466–473. [Google Scholar] [CrossRef]
- Weller, A.; Sharif, B.; Qarib, M.H.; St Leger, D.; De Silva, H.S.; Lingam, R.K. British Thyroid Association 2014 classification ultrasound scoring of thyroid nodules in predicting malignancy: Diagnostic performance and inter-observer agreement. Ultrasound 2020, 28, 4–13. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedus, L.; Paschke, R.; Valcavi, R.; Vitti, P. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules—2016 Update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [Green Version]
- Negro, R.; Greco, G.; Colosimo, E. Ultrasound Risk Categories for Thyroid Nodules and Cytology Results: A Single Institution’s Experience after the Adoption of the 2016 Update of Medical Guidelines by the American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi. J. Thyroid Res. 2017, 2017, 8135415. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.L.; Falciglia, M.; Yang, H.; Mark, J.R.; Steward, D.L. Validation of American Thyroid Association Ultrasound Risk Assessment of Thyroid Nodules Selected for Ultrasound Fine-Needle Aspiration. Thyroid 2017, 27, 1077–1082. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.J.; Moon, W.J.; Na, D.G.; Lee, Y.H.; Choi, N.; Kim, S.J.; Kim, J.K. A Multicenter Prospective Validation Study for the Korean Thyroid Imaging Reporting and Data System in Patients with Thyroid Nodules. Korean J. Radiol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Trimboli, P.; Ngu, R.; Royer, B.; Giovanella, L.; Bigorgne, C.; Simo, R.; Carroll, P.; Russ, G. A multicentre validation study for the EU-TIRADS using histological diagnosis as a gold standard. Clin. Endocrinol. 2019, 91, 340–347. [Google Scholar] [CrossRef]
- Castellana, M.; Grani, G.; Radzina, M.; Guerra, V.; Giovanella, L.; Deandrea, M.; Ngu, R.; Durante, C.; Trimboli, P. Performance of EU-TIRADS in malignancy risk stratification of thyroid nodules: A meta-analysis. Eur. J. Endocrinol. 2020, 183, 255–264. [Google Scholar] [CrossRef]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Hoang, J.K.; Middleton, W.D.; Farjat, A.E.; Langer, J.E.; Reading, C.C.; Teefey, S.A.; Abinanti, N.; Boschini, F.J.; Bronner, A.J.; Dahiya, N.; et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 2018, 287, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Middleton, W.D.; Teefey, S.A.; Reading, C.C.; Langer, J.E.; Beland, M.D.; Szabunio, M.M.; Desser, T.S. Multiinstitutional Analysis of Thyroid Nodule Risk Stratification Using the American College of Radiology Thyroid Imaging Reporting and Data System. Am. J. Roentgenol. 2017, 208, 1331–1341. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wen, J.; Zhang, L.; Sun, Y. Diagnostic Performance of American College of Radiology TI-RADS: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2021, 216, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yin, L.; Wei, X.; Zhang, S.; Song, Y.; Luo, B.; Li, J.; Qian, L.; Cui, L.; Chen, W.; et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: The C-TIRADS. Endocrine 2020, 70, 256–279. [Google Scholar] [CrossRef]
- Zhou, J.; Song, Y.; Zhan, W.; Wei, X.; Zhang, S.; Zhang, R.; Gu, Y.; Chen, X.; Shi, L.; Luo, X.; et al. Thyroid imaging reporting and data system (TIRADS) for ultrasound features of nodules: Multicentric retrospective study in China. Endocrine 2021, 72, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Rahal, A.J.; Falsarella, P.M.; Rocha, R.D.; Lima, J.P.; Iani, M.J.; Vieira, F.A.; Queiroz, M.R.; Hidal, J.T.; Francisco, M.J.N.; Garcia, R.G.; et al. Correlation of Thyroid Imaging Reporting and Data System [TI-RADS] and fine needle aspiration: Experience in 1000 nodules. Einstein 2016, 14, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildman-Tobriner, B.; Buda, M.; Hoang, J.K.; Middleton, W.D.; Thayer, D.; Short, R.G.; Tessler, F.N.; Mazurowski, M.A. Using Artificial Intelligence to Revise ACR TI-RADS Risk Stratification of Thyroid Nodules: Diagnostic Accuracy and Utility. Radiology 2019, 292, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Na, D.G.; Yoon, S.J.; Gwon, H.Y.; Paik, W.; Kim, T.; Kim, J.Y. Ultrasound malignancy risk stratification of thyroid nodules based on the degree of hypoechogenicity and echotexture. Eur. Radiol. 2020, 30, 1653–1663. [Google Scholar] [CrossRef]
- Persichetti, A.; Di Stasio, E.; Guglielmi, R.; Bizzarri, G.; Taccogna, S.; Misischi, I.; Graziano, F.; Petrucci, L.; Bianchini, A.; Papini, E. Predictive Value of Malignancy of Thyroid Nodule Ultrasound Classification Systems: A Prospective Study. J. Clin. Endocrinol. Metab. 2018, 103, 1359–1368. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Zhang, S.; Gao, M. Meta-analysis of thyroid imaging reporting and data system in the ultrasonographic diagnosis of 10,437 thyroid nodules. Head Neck 2016, 38, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Suh, C.H.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Diagnostic Performance of Four Ultrasound Risk Stratification Systems: A Systematic Review and Meta-Analysis. Thyroid 2020, 30, 1159–1168. [Google Scholar] [CrossRef]
- Magri, F.; Chytiris, S.; Croce, L.; Molteni, M.; Bendotti, G.; Gruosso, G.; Tata Ngnitejeu, S.; Agozzino, M.; Rotondi, M.; Chiovato, L. Performance of the ACR TI-RADS and EU TI-RADS scoring systems in the diagnostic work-up of thyroid nodules in a real-life series using histology as reference standard. Eur. J. Endocrinol. 2020, 183, 521–528. [Google Scholar] [CrossRef]
- Yim, Y.; Na, D.G.; Ha, E.J.; Baek, J.H.; Sung, J.Y.; Kim, J.H.; Moon, W.J. Concordance of Three International Guidelines for Thyroid Nodules Classified by Ultrasonography and Diagnostic Performance of Biopsy Criteria. Korean J. Radiol. 2020, 21, 108–116. [Google Scholar] [CrossRef]
- Persichetti, A.; Di Stasio, E.; Coccaro, C.; Graziano, F.; Bianchini, A.; Di Donna, V.; Corsello, S.; Valle, D.; Bizzarri, G.; Frasoldati, A.; et al. Inter- and Intraobserver Agreement in the Assessment of Thyroid Nodule Ultrasound Features and Classification Systems: A Blinded Multicenter Study. Thyroid 2020, 30, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.; Rosenkrantz, A.B.; Bennett, G.L.; Dane, B.; Jacobs, J.E.; Slywotzky, C.; Smereka, P.N.; Tong, A.; Sheth, S. Interreader Concordance of the TI-RADS: Impact of Radiologist Experience. Am. J. Roentgenol. 2020, 214, 1152–1157. [Google Scholar] [CrossRef]
- Seifert, P.; Gorges, R.; Zimny, M.; Kreissl, M.C.; Schenke, S. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine 2020, 67, 143–154. [Google Scholar] [CrossRef]
- Sych, Y.P.; Fadeev, V.V.; Fisenko, E.P.; Kalashnikova, M. Reproducibility and Interobserver Agreement of Different Thyroid Imaging and Reporting Data Systems (TIRADS). Eur. Thyroid J. 2021, 10, 161–167. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Na, D.G.; Kim, J.H.; Kim, D.S.; Kim, S.J. Thyroid nodules with minimal cystic changes have a low risk of malignancy. Ultrasonography 2016, 35, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, X.V.; Choudhury, K.R.; Eastwood, J.D.; Lyman, G.H.; Esclamado, R.M.; Werner, J.D.; Hoang, J.K. Incidental thyroid nodules on CT: Evaluation of 2 risk-categorization methods for work-up of nodules. AJNR Am. J. Neuroradiol. 2013, 34, 1812–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koseoglu Atilla, F.D.; Ozgen Saydam, B.; Erarslan, N.A.; Diniz Unlu, A.G.; Yilmaz Yasar, H.; Ozer, M.; Akinci, B. Does the ACR TI-RADS scoring allow us to safely avoid unnecessary thyroid biopsy? single center analysis in a large cohort. Endocrine 2018, 61, 398–402. [Google Scholar] [CrossRef]
- Castellana, M.; Castellana, C.; Treglia, G.; Giorgino, F.; Giovanella, L.; Russ, G.; Trimboli, P. Performance of Five Ultrasound Risk Stratification Systems in Selecting Thyroid Nodules for FNA. J. Clin. Endocrinol. Metab. 2020, 105, 1659–1669. [Google Scholar] [CrossRef]
- Yoon, J.H.; Han, K.; Kim, E.K.; Moon, H.J.; Kwak, J.Y. Diagnosis and Management of Small Thyroid Nodules: A Comparative Study with Six Guidelines for Thyroid Nodules. Radiology 2017, 283, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.J.; Na, D.G.; Moon, W.J.; Lee, Y.H.; Choi, N. Diagnostic Performance of Ultrasound-Based Risk-Stratification Systems for Thyroid Nodules: Comparison of the 2015 American Thyroid Association Guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology Guidelines. Thyroid 2018, 28, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Middleton, W.D.; Teefey, S.A.; Reading, C.C.; Langer, J.E.; Beland, M.D.; Szabunio, M.M.; Desser, T.S. Comparison of Performance Characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association Guidelines. Am. J. Roentgenol. 2018, 210, 1148–1154. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Wu, R.X.; Zhang, Y.Z.; Gu, J.Y.; Ye, X.H.; Tang, W.; Xu, S.H.; Liu, C.; Wu, X.H. Validation and comparison of three newly-released Thyroid Imaging Reporting and Data Systems for cancer risk determination. Endocrine 2019, 64, 299–307. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 2019, 104, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Betel, C.; Burton, K.R.; Higgins, K.M.; Ghorab, Z.; Halperin, I.J. Retrospective Application of the 2015 American Thyroid Association Guidelines for Ultrasound Classification, Biopsy Indications, and Follow-up Imaging of Thyroid Nodules: Can Improved Reporting Decrease Testing? Can. Assoc. Radiol. J. 2019, 70, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.L.; Yang, H.Y.; Liu, R.B.; Liang, M.; Han, P.; Xu, X.L.; Luo, B.M. Fine needle aspiration biopsy indications for thyroid nodules: Compare a point-based risk stratification system with a pattern-based risk stratification system. Eur. Radiol. 2019, 29, 4871–4878. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Du, J.R.; Wang, H.; Jin, C.X.; Sui, G.Q.; Yang, D.Y.; Lin, Y.Q.; Luo, Q.; Fu, P.; Li, H.Q.; et al. Comparison and preliminary discussion of the reasons for the differences in diagnostic performance and unnecessary FNA biopsies between the ACR TIRADS and 2015 ATA guidelines. Endocrine 2019, 65, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Na, D.G.; Baek, J.H.; Sung, J.Y.; Kim, J.H.; Kang, S.Y. US Fine-Needle Aspiration Biopsy for Thyroid Malignancy: Diagnostic Performance of Seven Society Guidelines Applied to 2000 Thyroid Nodules. Radiology 2018, 287, 893–900. [Google Scholar] [CrossRef]
- Yoon, S.J.; Na, D.G.; Gwon, H.Y.; Paik, W.; Kim, W.J.; Song, J.S.; Shim, M.S. Similarities and Differences Between Thyroid Imaging Reporting and Data Systems. Am. J. Roentgenol. 2019, 213, W76–W84. [Google Scholar] [CrossRef]
- Baek, H.J.; Kim, D.W.; Shin, G.W.; Heo, Y.J.; Baek, J.W.; Lee, Y.J.; Cho, Y.J.; Park, H.K.; Ha, T.K.; Kim, D.H.; et al. Ultrasonographic Features of Papillary Thyroid Carcinomas According to Their Subtypes. Front. Endocrinol. 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellana, M.; Piccardo, A.; Virili, C.; Scappaticcio, L.; Grani, G.; Durante, C.; Giovanella, L.; Trimboli, P. Can ultrasound systems for risk stratification of thyroid nodules identify follicular carcinoma? Cancer Cytopathol. 2020, 128, 250–259. [Google Scholar] [CrossRef]
- Castellana, M.; Virili, C.; Paone, G.; Scappaticcio, L.; Piccardo, A.; Giovanella, L.; Trimboli, P. Ultrasound systems for risk stratification of thyroid nodules prompt inappropriate biopsy in autonomously functioning thyroid nodules. Clin. Endocrinol. 2020, 93, 67–75. [Google Scholar] [CrossRef]
- Russ, G.; Royer, B.; Bigorgne, C.; Rouxel, A.; Bienvenu-Perrard, M.; Leenhardt, L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur. J. Endocrinol. 2013, 168, 649–655. [Google Scholar] [CrossRef]
- Yang, B.R.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Park, V.Y.; Kwak, J.Y. Qualitative and Semiquantitative Elastography for the Diagnosis of Intermediate Suspicious Thyroid Nodules Based on the 2015 American Thyroid Association Guidelines. J. Ultrasound Med. 2018, 37, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimboli, P.; Piccardo, A.; Alevizaki, M.; Virili, C.; Naseri, M.; Sola, S.; Paone, G.; Russ, G.; Giovanella, L. Dedicated neck (18) F-FDG PET/CT: An additional tool for risk assessment in thyroid nodules at ultrasound intermediate risk. Clin. Endocrinol. 2019, 90, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, S.; Zhang, Z.; Chen, Y.; Wang, K.; Shang, M.; Chen, B. The associated factors for spontaneous intranodular hemorrhage of partially cystic thyroid nodules: A retrospective study of 101 thyroid nodules. Medicine 2020, 99, e23846. [Google Scholar] [CrossRef] [PubMed]
- Lacout, A.; Chevenet, C.; Marcy, P.Y. Mummified Thyroid Syndrome. Am. J. Roentgenol. 2016, 206, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Jung, C.K.; Lee, J.H. Degenerating Thyroid Nodules: Ultrasound Diagnosis, Clinical Significance, and Management. Korean J. Radiol. 2019, 20, 947–955. [Google Scholar] [CrossRef]
- Bernardi, S.; Palermo, A.; Grasso, R.F.; Fabris, B.; Stacul, F.; Cesareo, R. Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review. Cancers 2021, 13, 2746. [Google Scholar] [CrossRef]
- Pan, F.S.; Wang, W.; Wang, Y.; Xu, M.; Liang, J.Y.; Zheng, Y.L.; Xie, X.Y.; Li, X.X. Sonographic features of thyroid nodules that may help distinguish clinically atypical subacute thyroiditis from thyroid malignancy. J. Ultrasound Med. 2015, 34, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Gorges, R.; Schmid, K.W. Hyperplasia of the thyroid gland. Pathologe 2003, 24, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, Y.; Xu, G.; Fan, Z.; Ren, W. Causes of misdiagnoses by thyroid fine-needle aspiration cytology (FNAC): Our experience and a systematic review. Diagn Pathol 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedus, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyroid J. 2020, 9, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Trimboli, P.; Mormile, A.; Cont, A.T.; Milan, L.; Buffet, C.; Giovanella, L.; Limone, P.P.; Poiree, S.; Leenhardt, L.; et al. Determining an energy threshold for optimal volume reduction of benign thyroid nodules treated by radiofrequency ablation. Eur. Radiol. 2021, 31, 5189–5197. [Google Scholar] [CrossRef] [PubMed]
- Elsayes, K.M.; Hooker, J.C.; Agrons, M.M.; Kielar, A.Z.; Tang, A.; Fowler, K.J.; Chernyak, V.; Bashir, M.R.; Kono, Y.; Do, R.K.; et al. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics 2017, 37, 1994–2017. [Google Scholar] [CrossRef]
- Angell, T.E.; Maurer, R.; Wang, Z.; Kim, M.I.; Alexander, C.A.; Barletta, J.A.; Benson, C.B.; Cibas, E.S.; Cho, N.L.; Doherty, G.M.; et al. A Cohort Analysis of Clinical and Ultrasound Variables Predicting Cancer Risk in 20,001 Consecutive Thyroid Nodules. J. Clin. Endocrinol. Metab. 2019, 104, 5665–5672. [Google Scholar] [CrossRef]
- Noto, B.; Eveslage, M.; Pixberg, M.; Gonzalez Carvalho, J.M.; Schafers, M.; Riemann, B.; Kies, P. Prevalence of hyperfunctioning thyroid nodules among those in need of fine needle aspiration cytology according to ATA 2015, EU-TIRADS, and ACR-TIRADS. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Schenke, S.; Seifert, P.; Zimny, M.; Winkens, T.; Binse, I.; Gorges, R. Risk Stratification of Thyroid Nodules Using the Thyroid Imaging Reporting and Data System (TIRADS): The Omission of Thyroid Scintigraphy Increases the Rate of Falsely Suspected Lesions. J. Nucl. Med. 2019, 60, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, H.H.; de Groot, J.W.B.; Sluiter, W.J.; Muller Kobold, A.C.; van den Heuvel, E.R.; Plukker, J.T.; Links, T.P. Calcitonin testing for detection of medullary thyroid cancer in people with thyroid nodules. Cochrane Database Syst. Rev. 2020, 3, CD010159. [Google Scholar] [CrossRef] [Green Version]
- Trimboli, P.; Giovanella, L.; Valabrega, S.; Andrioli, M.; Baldelli, R.; Cremonini, N.; Rossi, F.; Guidobaldi, L.; Barnabei, A.; Rota, F.; et al. Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: A retrospective multicenter study. J. Exp. Clin. Cancer Res. 2014, 33, 87. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Guidobaldi, L.; Romanelli, F.; Nigri, G.; Valabrega, S.; Sadeghi, R.; Crescenzi, A.; Faquin, W.C.; Bongiovanni, M.; et al. Detection rate of FNA cytology in medullary thyroid carcinoma: A meta-analysis. Clin. Endocrinol. 2015, 82, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlea, A.; Borcan, F.; Sporea, I.; Dehelean, C.A.; Negrea, R.; Cotoi, L.; Stoian, D. TI-RADS Diagnostic Performance: Which Algorithm is Superior and How Elastography and 4D Vascularity Improve the Malignancy Risk Assessment. Diagnostics 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| RSS | Number of Classes | Meaning of TIRADS 1 | Pattern or Point-Based RSS | Features of High Suspicion | Composition Included in the RSS | ETE Included in the RSS |
|---|---|---|---|---|---|---|
| Chilean TIRADS | 6 TIRADS 4 divided into 2 subclasses | Normal examination | Pattern | Irregular margins Irregular shape Multiple peripheral microcalcifications Penetrating vessels | Yes | No |
| Kwak-TIRADS | 5 TIRADS 4 divided into 3 subclasses | No nodule | Point | Marked hypoechogenicity Irregular margins Microcalcifications Taller than wide | No | No |
| BTA | 5 | Normal | Pattern | In a solid hypoechoic nodule: Irregular margins Microcalcifications Globular calcifications Intranodular vascularity Taller than wide Lymphadenopathy | Yes | No |
| AACE | 3 | Low risk | Pattern | Marked hypoechogenicity Irregular margins Microcalcifications Taller-than-wide Extrathyroidal growth Pathologic lymph node. | No | Yes |
| ATA | 5 | Benign | Pattern | In a solid hypoechoic nodule: Irregular margins Microcalcifications Taller than wide Rim calcifications with small extrusive soft tissue component Extra-thyroidal extension | Yes | Yes |
| K-TIRADS | 5 | Absence of nodule | Pattern | In a solid hypoechoic nodule: Irregular margins Microcalcification Nonparallel orientation | Yes | No |
| EU-TIRADS | 5 | Absence of significant nodule | Pattern | Marked hypoechogenicity Irregular margins Microcalcifications Taller than wide | No | No |
| ACR-TIRADS | 5 | Benign | Point | Marked hypoechogenicity All punctate echogenic foci Taller-than-wide Extra-thyroidal extension | Yes | Yes |
| C-TIRADS | 5 TIRADS 4 divided into 3 subclasses | No nodule | Point | Markedly hypoechogenicity Ill-defined and irregular margins Vertical orientation Solid composition Microcalcifications Extra-thyroidal extension | Yes | Yes |
| RSS | TIRADS 2 or Very Low Risk | TIRADS 3 or Low Risk | TIRADS 4 or Intermediate Risk | TIRADS 5 or High Risk | Small Nodules < 10 mm |
|---|---|---|---|---|---|
| Chilean TIRADS | No FNA or follow-up | FNA (no cut-off) or follow-up | FNA (no cut-off) | FNA (no cut-off) | FNA if >3–4 mm and feasible |
| Kwak-TIRADS | No FNA | No FNA | TIRADS 4a: ≥25 mm TIRADS 4B: 15 mm | TIRADS 4 C and 5: ≥10 mm | No FNA |
| BTA | No FNA | All nodules | All nodules | All nodules | - |
| AACE | No FNA | ≥20 mm and growing lesion or risk factors | ≥20 mm | ≥10 mm | <5 mm no FNA 5–10 mm FNA if clinical or US risk factors or PP |
| ATA | ≥20 mm or observation | ≥15 mm | ≥10 mm | ≥10 mm | 5–10 mm FNA if clinical or US risk factors or PP |
| K-TIRADS | ≥20 mm | ≥15 mm | ≥10 mm | ≥10 mm | ≥5 mm selective cases |
| EU-TIRADS | No FNA | >20 mm | >15 mm | >10 mm | FNA or active surveillance, PP |
| ACR-TIRADS | No FNA | ≥25 mm | ≥15 mm | ≥10 mm | No FNA |
| C-TIRADS | No FNA | No FNA | ≥15 mm | ≥10 mm | US risk factors |
| Current Pitfalls of Existent RSSs Variables Not Taken into Account for Risk Stratification | Suggested Correction |
|---|---|
| Modifications of nodules treated by thermal ablation are classified as highly suspect | Incorporate a treatment response (TR) algorithm |
| The number of nodules is an independent predictor of the malignancy risk | Add the number of nodules in the risk stratification algorithm, especially if they look all alike and are of low or intermediate risk |
| Some clinical variables and previous results of FNA(s) are predictors of the malignancy risk | Incorporate age, sex, time since discovery, results of previous FNAs in the risk stratification algorithm |
| TSH and serum calcitonin are predictors of the malignancy risk | Incorporate TSH and serum calcitonin in the risk stratification algorithm |
| Complementary tools not used in most RSSs, such as vascularity and elastography | At least, incorporate these in the lexicon, to allow comparative studies on the subject |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russ, G.; Trimboli, P.; Buffet, C. The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls. Cancers 2021, 13, 4316. https://doi.org/10.3390/cancers13174316
Russ G, Trimboli P, Buffet C. The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls. Cancers. 2021; 13(17):4316. https://doi.org/10.3390/cancers13174316
Chicago/Turabian StyleRuss, Gilles, Pierpaolo Trimboli, and Camille Buffet. 2021. "The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls" Cancers 13, no. 17: 4316. https://doi.org/10.3390/cancers13174316
APA StyleRuss, G., Trimboli, P., & Buffet, C. (2021). The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls. Cancers, 13(17), 4316. https://doi.org/10.3390/cancers13174316






