18F-FDOPA PET/CT SUV-Derived Indices and Volumetric Parameters Correlation in Patients with Primary Brain Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Selection
- 60 patients were affected by astrocytoma (45.1%), of which one patient was affected by Grade I pilocytic astrocytoma, 28 patients were affected by Grade II astrocytoma, and 15 patients were affected by Grade III astrocytoma;
- 4 were affected by Grade III anaplastic astrocytoma (3%);
- 11 were affected by glioma (8.3%) of which 3 patients were affected by Grade II glioma, 1 patient was affected by Grade III glioma, and 1 patient was affected by glioma (grading not identified/not reported);
- 44 were affected by oligodendroglioma (33.1%) of which 12 patients were affected by Grade II oligodendroglioma, and 8 patients were affected by Grade III Oligodendroglioma;
- 6 were affected by by Grade II oligoastrocytoma (4.5%);
- 7 were affected by Grade IV glioblastoma (5.2%);
- 1 was affected by neurocytoma (0.8%).
2.2. F-FDOPA PET Image Acquisition, Parameters, and Image Evaluation
2.3. Statistical Analyses
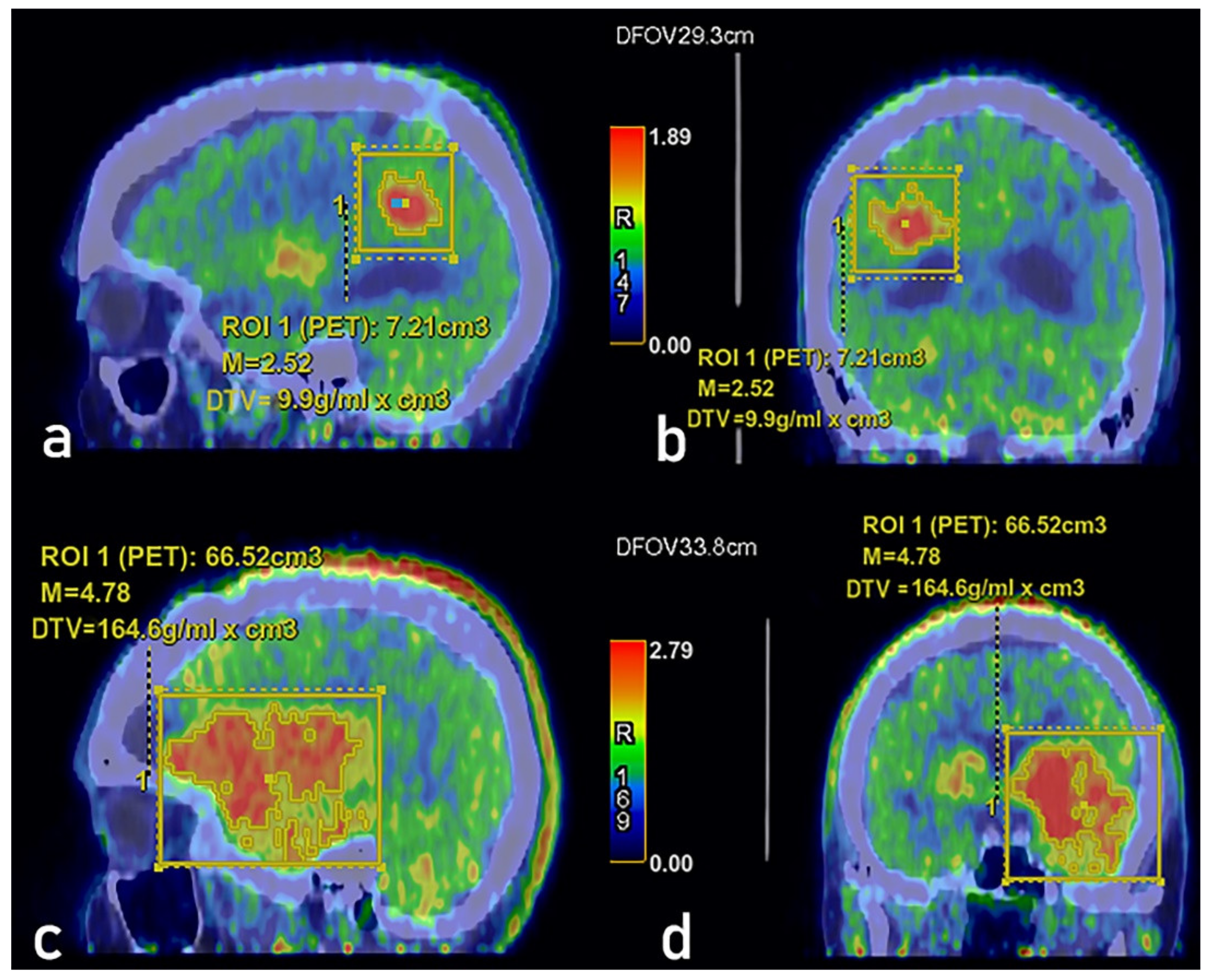
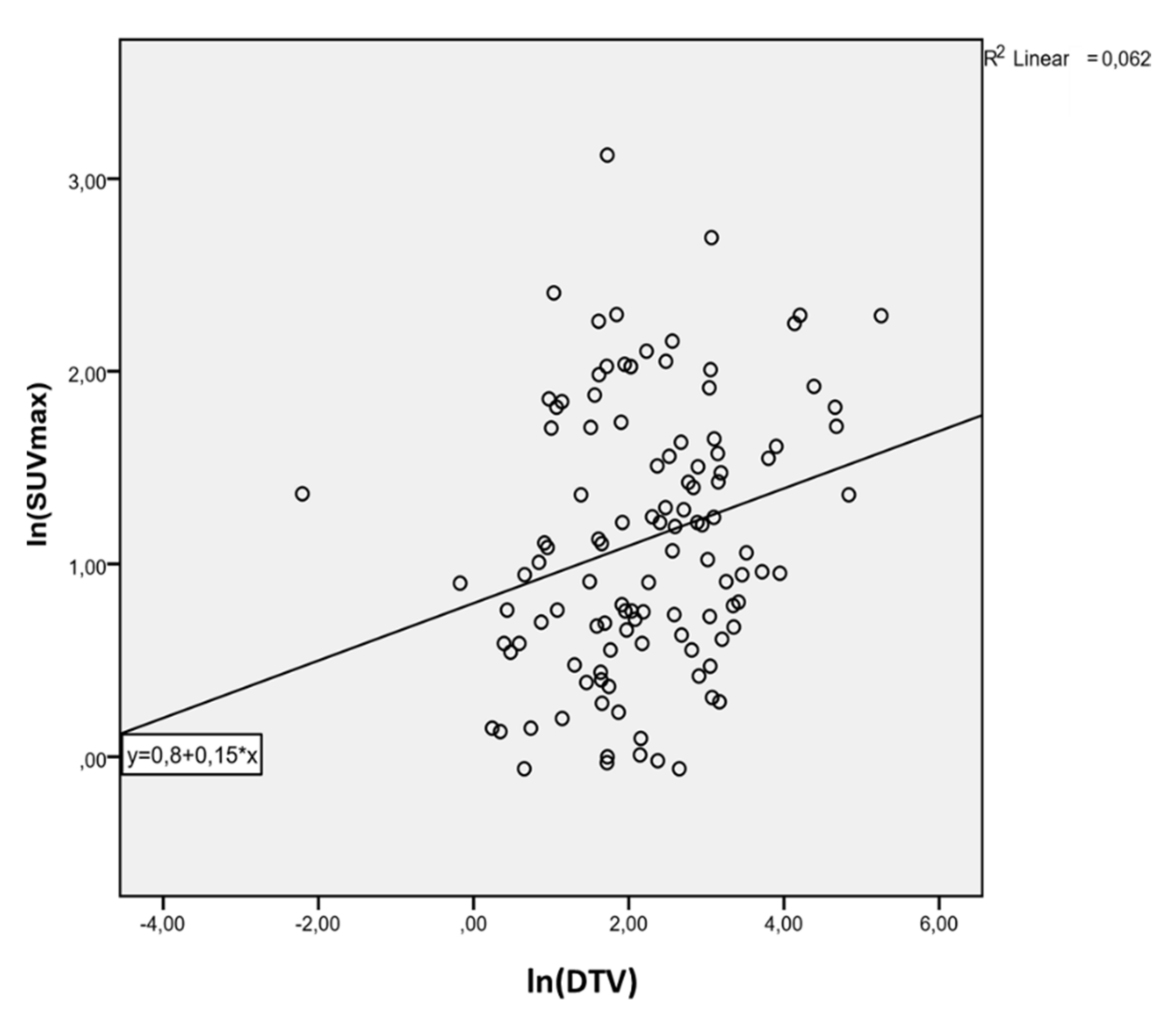
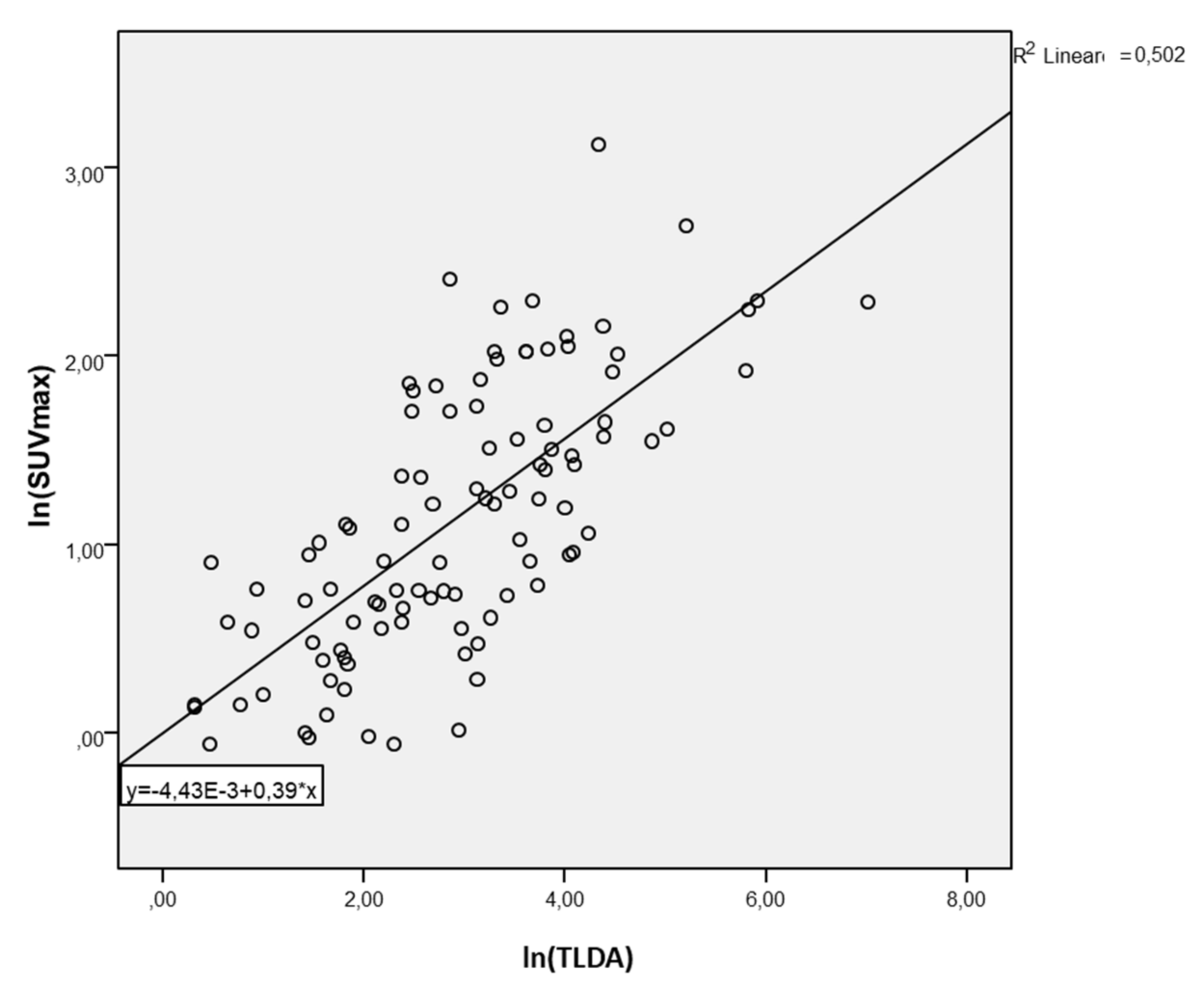
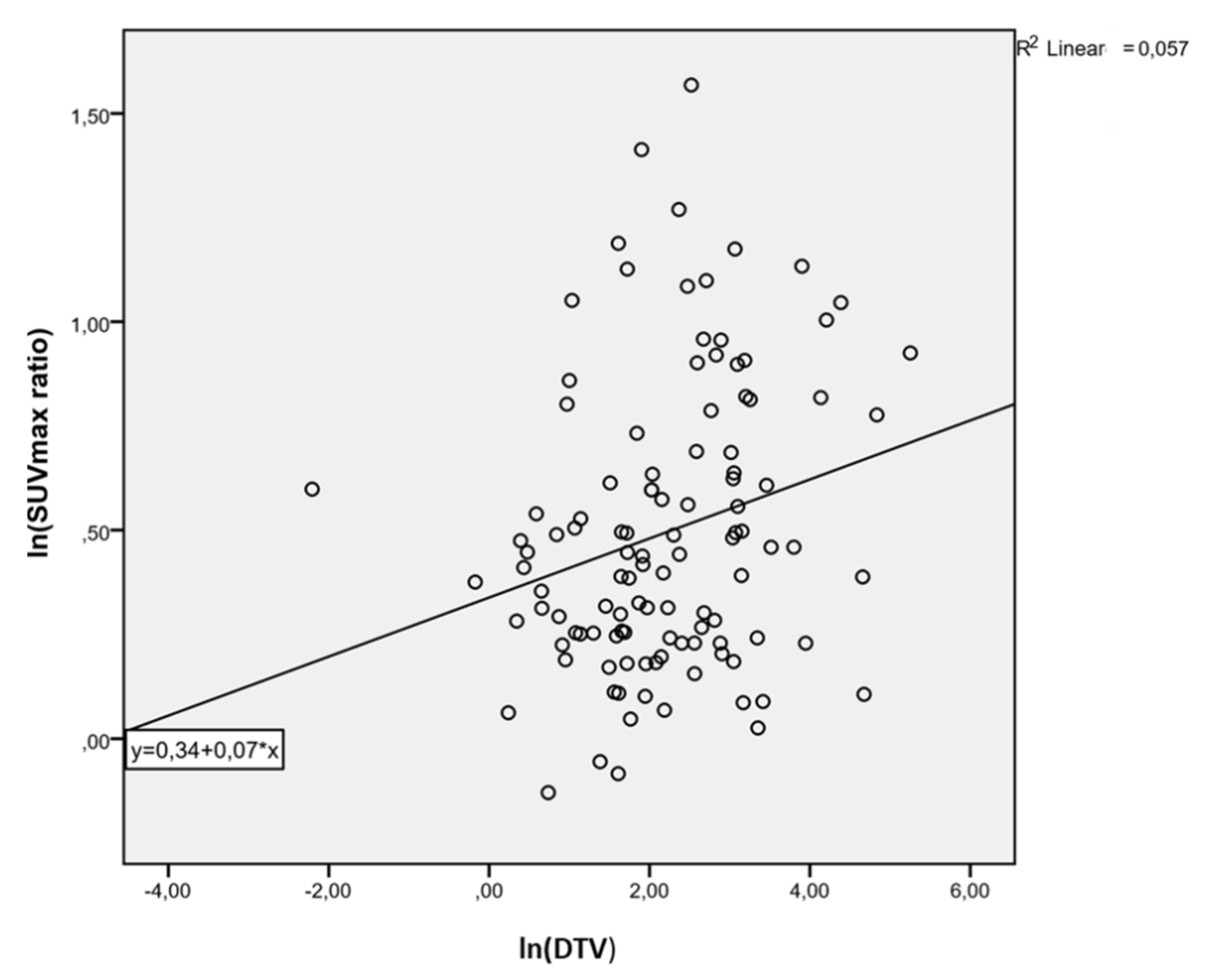
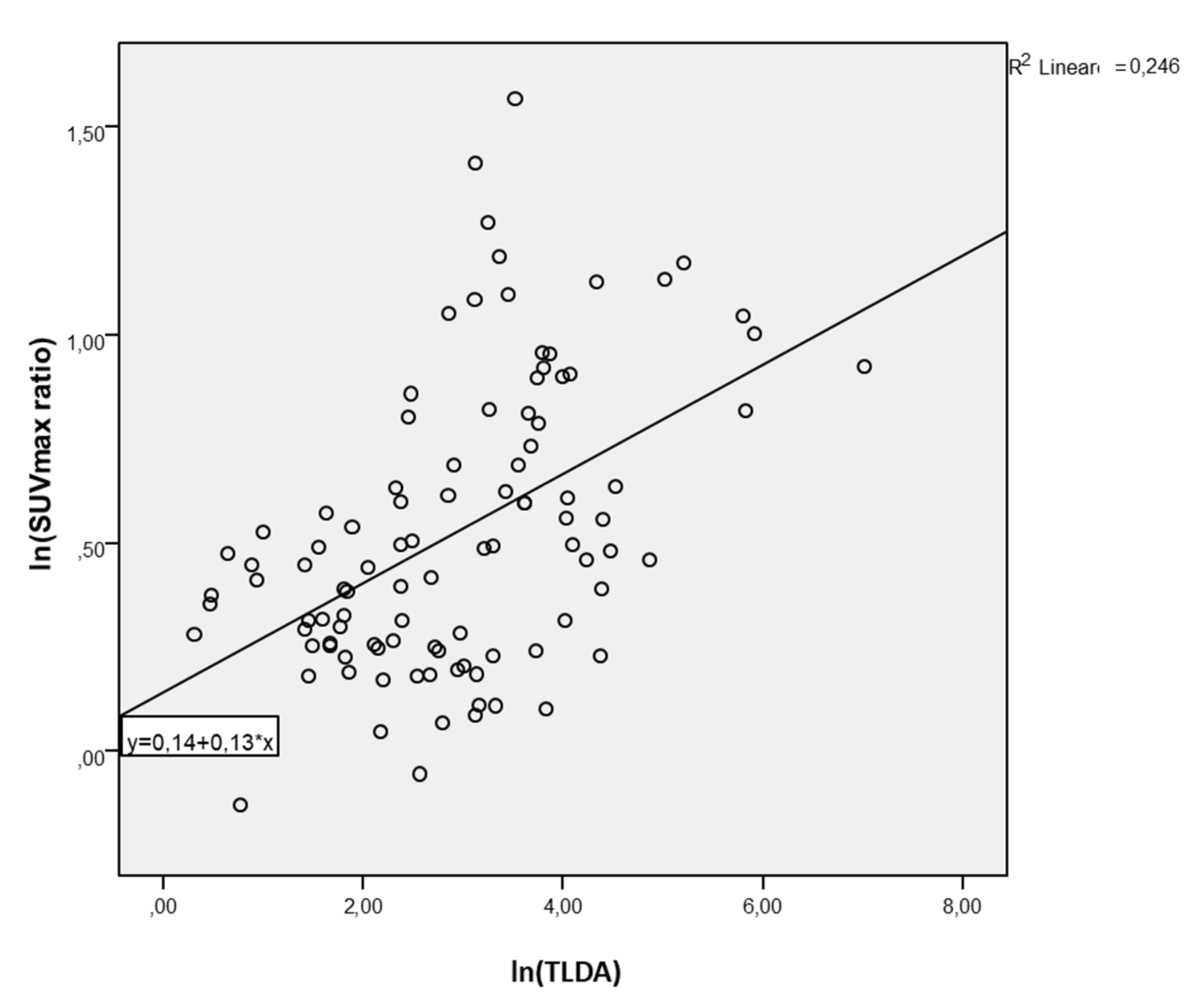
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fourgère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The role of LAT1 in 18F-DOPA uptake in malignant gliomas. J. Neuro-Oncol. 2012, 111, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fiebrich, H.B.; de Jong, J.R.; Kema, I.P.; Koopmans, K.P.; Sluiter, W.; Dierckx, R.A.; Walenkamp, A.M.; Links, T.P.; Brouwers, A.H.; de Vries, E.G. Total 18F-dopa PET tumour uptake reflects metabolic endocrine tumour activity in patients with a carcinoid tumour. Eur. J. Nucl. Mol. Imaging 2011, 38, 1854–1861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Fueger, B.J.; Czernin, J.; Cloughesy, T.; Silverman, D.H.; Geist, C.L.; Walter, M.A.; Schiepers, C.; Nghiemphu, P.; Lai, A.; Phelps, M.E.; et al. Correlation of 6-18F-Fluoro-L-Dopa PET Uptake with Proliferation and Tumor Grade in Newly Diagnosed and Recurrent Gliomas. J. Nucl. Med. 2010, 51, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Fazzari, E.; Chakhoyan, A.; Yao, J.; Raymond, C.; Nguyen, H.; Manoukian, J.; Nguyen, N.; Pope, W.; Cloughesy, T.H.; et al. 18 F-FDOPA PET and MRI characteristics corre-late with degree of malignancy and predict survival in treatment-naïve gliomas: A cross-sectional study. J. Neurooncol. 2018, 139, 399–409. [Google Scholar] [CrossRef]
- Cicone, F.; Carideo, L.; Scaringi, C.; Arcella, A.; Giangaspero, F.; Scopinaro, F.; Minniti, G. 18F-DOPA uptake does not correlate with IDH mutation status and 1p/19q co-deletion in glioma. Ann. Nucl. Med. 2019, 33, 295–302. [Google Scholar] [CrossRef]
- Cimini, A.; Chiaravalloti, A.; Ricci, M.; Villani, V.; Vanni, G.; Schillaci, O. MGMT Promoter Methylation and IDH1 Mutations Do Not Affect [18 F]FDOPA Uptake in Primary Brain Tumors. Int. J. Mol. Sci. 2020, 21, 7598. [Google Scholar] [CrossRef]
- Scott, G.; Mahmud, M.; Owen, D.; Johnson, M.R. Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure 2017, 44, 42–47. [Google Scholar] [CrossRef]
- Janvier, L.; Olivier, P.; Blonski, M.; Morel, O.; Vignaud, J.M.; Karcher, G.; Taillandier, L.; Verger, A. Correlation of SUV-Derived Indices With Tumoral Aggressiveness of Gliomas in Static 18F-FDOPA PET: Use in Clinical Practice. Clin. Nucl. Med. 2015, 40, e429–e435. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Esposito, V.; Ursini, F.; Di Giorgio, E.; Zinzi, M.; Calabria, F.; Cimini, A.; Schillaci, O. Overall survival and progression-free survival in patients with primary brain tumors after treatment: Is the outcome of [18F] FDOPA PET a prog-nostic factor in these patients? Ann. Nucl. Med. 2019, 33, 471–480. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Fiorentini, A.; Villani, V.; Carapella, C.; Pace, A.; Di Pietro, B.; Di Russo, C.; Palumbo, B.; Floris, R.; Schil-laci, O. Factors affecting 1⁸F FDOPA standardized uptake value in patients with primary brain tumors after treatment. Nucl. Med. Biol. 2015, 42, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xie, G.; Liao, G.; Wang, B.; Yan, M.; Li, H.; Yuan, Y. Prognostic value of 18F-FDG-PET/CT in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 33884–33896. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, M.; Sun, X.; Li, W.; Xing, L.; Yu, J. Prognostic Value of 18F-FDG PET/CT in Surgical Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0146195. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Xuan, D.; Hu, Y.; Li, X.; Liu, L.; Xu, D. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with breast cancer: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225959. [Google Scholar] [CrossRef]
- Han, S.; Kim, Y.J.; Woo, S.; Suh, C.H.; Lee, J.J. Prognostic Value of Volumetric Parameters of Pretreatment 18F-FDG PET/CT in Esophageal Cancer: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2018, 43, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mauler, J.; Maudsley, A.A.; Langen, K.-J.; Nikoubashman, O.; Stoffels, G.; Sheriff, S.; Lohmann, P.; Filss, C.; Galldiks, N.; Kops, E.R.; et al. Spatial Relationship of Glioma Volume Derived from 18F-FET PET and Volumetric MR Spectroscopy Imaging: A Hybrid PET/MRI Study. J. Nucl. Med. 2018, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Jaymanne, D.T.; Kaushal, S.; Chan, D.; Schembri, G.; Brazier, D.; Bailey, D.; Wheeler, H.; Back, M. Utilizing 18F-fluoroethyl-l -tyrosine positron emission tomography in high grade glioma for radiation treatment planning in patients with contraindications to MRI. J. Med. Imaging Radiat. Oncol. 2017, 62, 122–127. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Jang, S. Prognostic Value of the Metabolic and Volumetric Parameters of 11C-Methionine Positron-Emission Tomography for Gliomas: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2018, 39, 1629–1634. [Google Scholar] [CrossRef]
- Liu, C.-J.; Lu, M.-Y.; Liu, Y.-L.; Ko, C.-L.; Ko, K.-Y.; Tzen, K.-Y.; Chang, H.-H.; Yang, Y.-L.; Jou, S.-T.; Hsu, W.-M.; et al. Risk Stratification of Pediatric Patients With Neuroblastoma Using Volumetric Parameters of 18F-FDG and 18F-DOPA PET/CT. Clin. Nucl. Med. 2017, 42, e142–e148. [Google Scholar] [CrossRef] [PubMed]
- Ginet, M.; Zaragori, T.; Marie, P.Y. Integration of dynamic parameters in the analysis of 18F-FDopa PET imaging im-proves the prediction of molecular features of gliomas. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Zaragori, T.; Ginet, M.; Marie, P.-Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of static and dynamic [18F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tatekawa, H.; Yao, J.; Oughourlian, T.C.; Hagiwara, A.; Wang, C.; Raymond, C.; Lai, A.; Cloughesy, T.F.; Nghiemphu, P.L.; Liau, L.M.; et al. Maximum Uptake and Hypermetabolic Volume of 18F-FDOPA PET Estimate Molecular Status and Overall Survival in Low-Grade Gliomas: A PET and MRI Study. Clin. Nucl. Med. 2020, 45, e505–e511. [Google Scholar] [CrossRef]
- Gauvain, K.; Ponisio, M.R.; Barone, A.; Grimaldi, M.; Parent, E.; Leeds, H.; Goyal, M.; Rubin, J.; McConathy, J. 18F-FDOPA PET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neuro-Oncol. Pr. 2018, 5, 28–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaravalloti, A.; Ricci, M.; Cimini, A.; Russo, F.; Ursini, F.; Filippi, L.; Schillaci, O. 18F-FDOPA PET/CT SUV-Derived Indices and Volumetric Parameters Correlation in Patients with Primary Brain Tumors. Cancers 2021, 13, 4315. https://doi.org/10.3390/cancers13174315
Chiaravalloti A, Ricci M, Cimini A, Russo F, Ursini F, Filippi L, Schillaci O. 18F-FDOPA PET/CT SUV-Derived Indices and Volumetric Parameters Correlation in Patients with Primary Brain Tumors. Cancers. 2021; 13(17):4315. https://doi.org/10.3390/cancers13174315
Chicago/Turabian StyleChiaravalloti, Agostino, Maria Ricci, Andrea Cimini, Francesca Russo, Francesco Ursini, Luca Filippi, and Orazio Schillaci. 2021. "18F-FDOPA PET/CT SUV-Derived Indices and Volumetric Parameters Correlation in Patients with Primary Brain Tumors" Cancers 13, no. 17: 4315. https://doi.org/10.3390/cancers13174315
APA StyleChiaravalloti, A., Ricci, M., Cimini, A., Russo, F., Ursini, F., Filippi, L., & Schillaci, O. (2021). 18F-FDOPA PET/CT SUV-Derived Indices and Volumetric Parameters Correlation in Patients with Primary Brain Tumors. Cancers, 13(17), 4315. https://doi.org/10.3390/cancers13174315







