Radiobiological Evaluation of Combined Gamma Knife Radiosurgery and Hyperthermia for Pediatric Neuro-Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
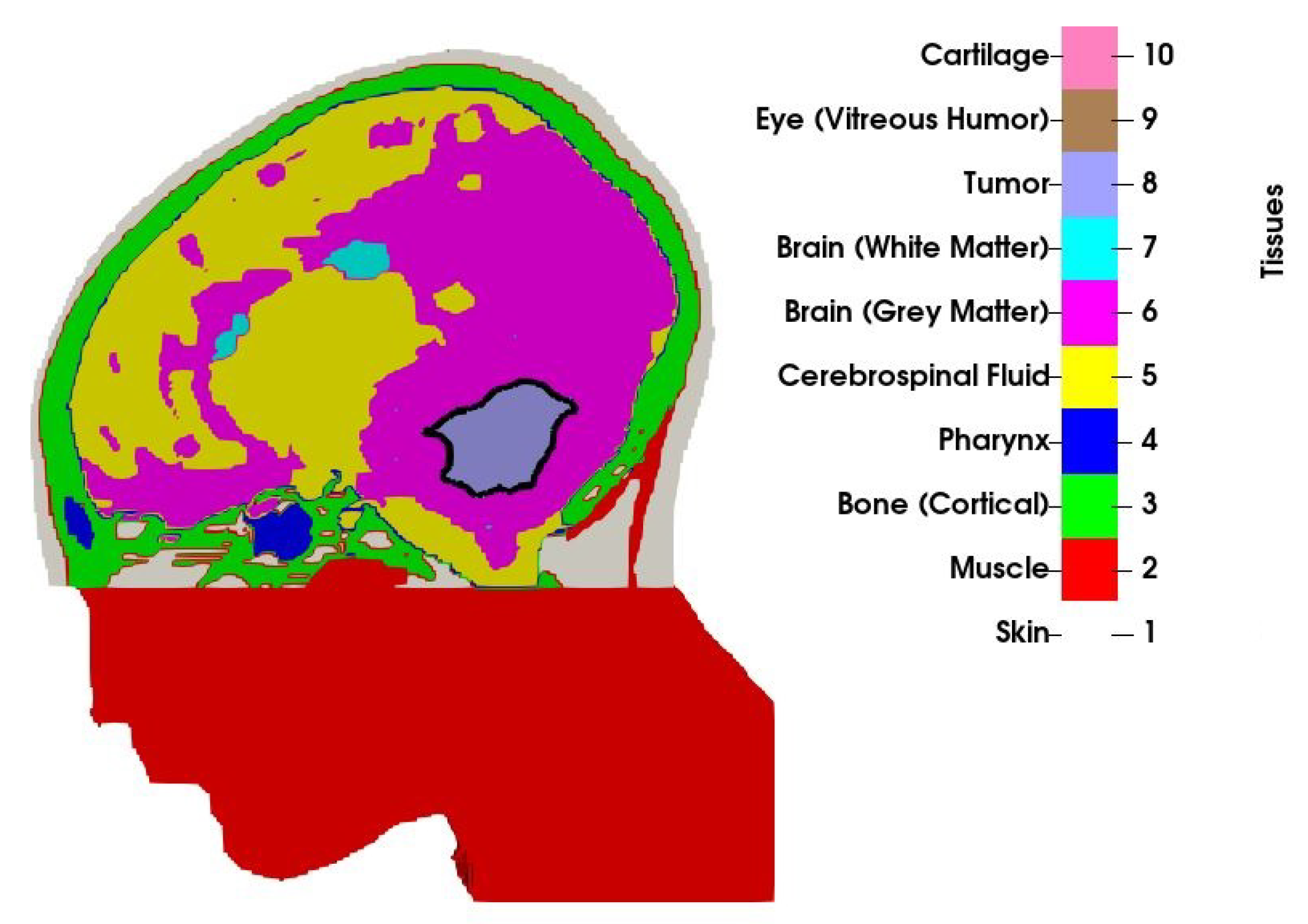
2.1. Patient Model
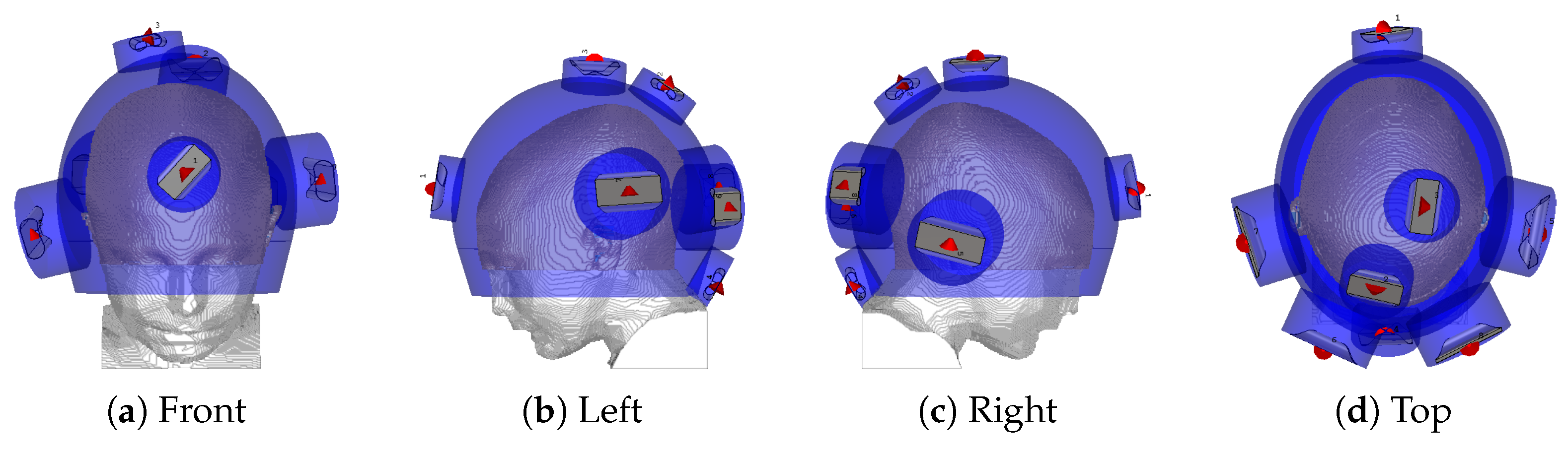
2.2. Hyperthermia Treatment Planning with Novel Applicator
2.3. Stereotactic Radiosurgery Treatment Planning
2.4. Radiosensitivity Modelling
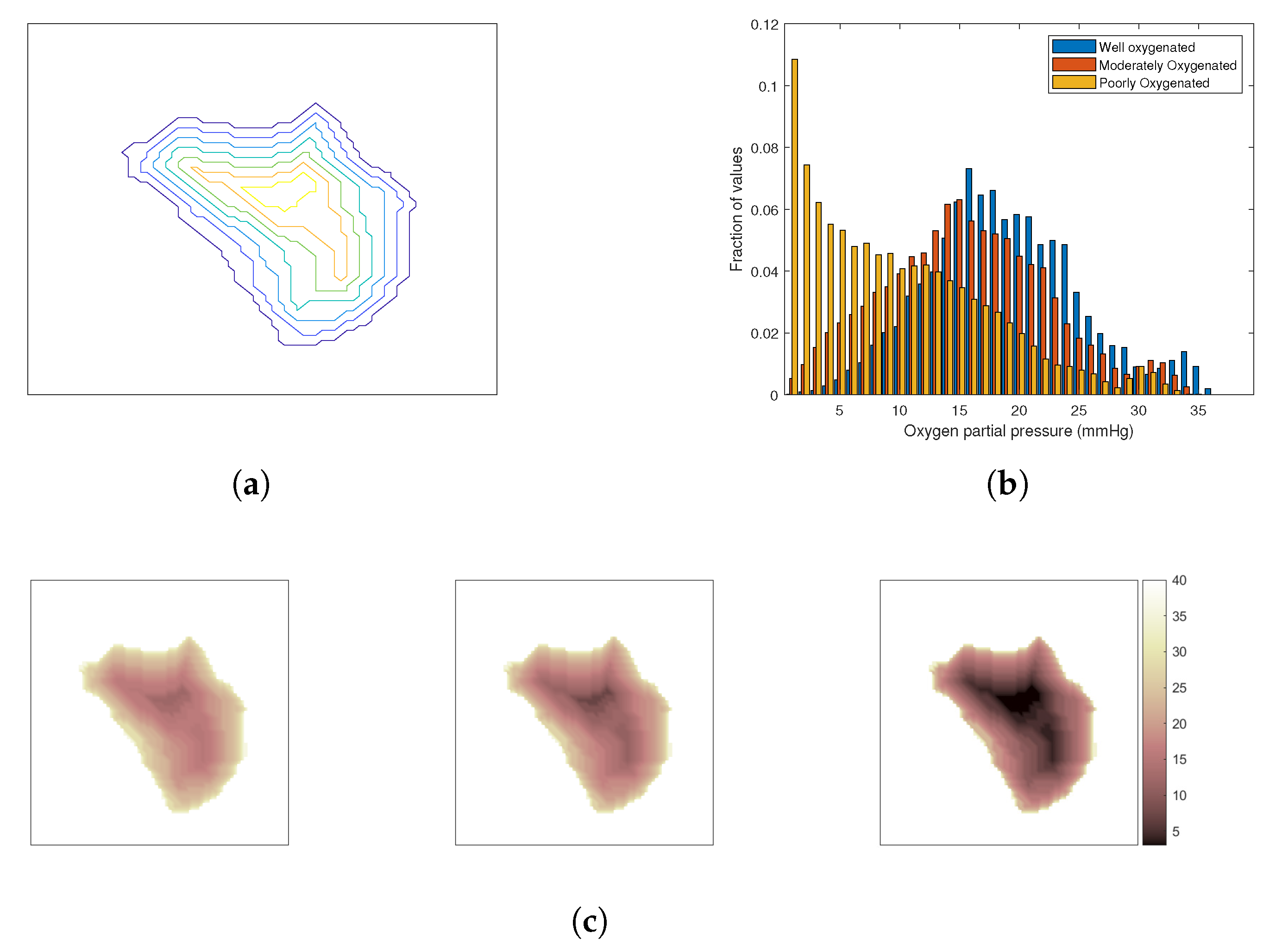
The Oxygen Effect
2.5. Evaluation of Effect of the Combined Treatment
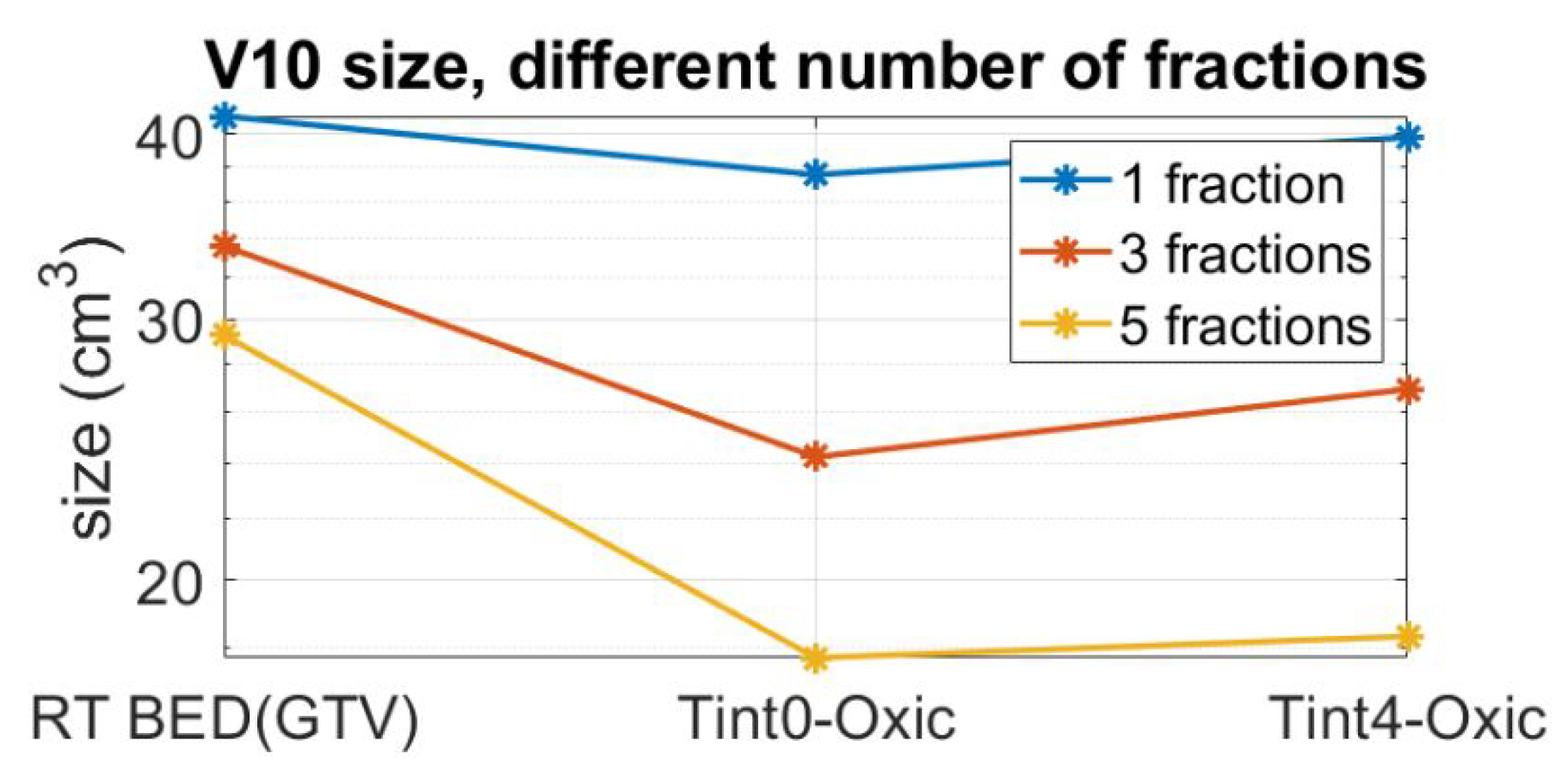
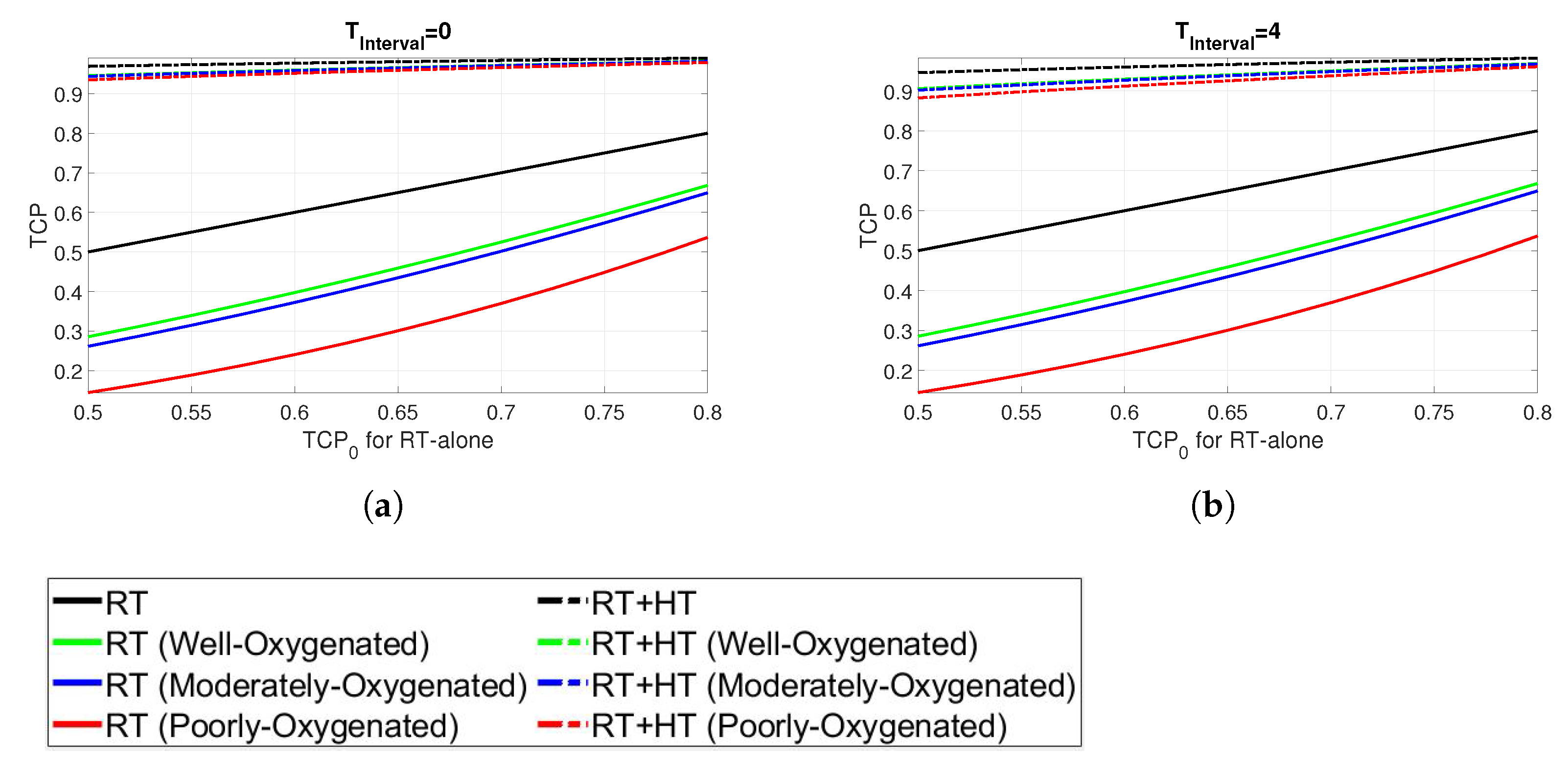
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTP | Hyperthermia Treatment Planning |
| PLD | Power Loss Density |
| SAR | Specific Absorption Rate |
| GTV | Gross tumour Volume |
| DVH | Dose Volume Histogram |
| EQD | EQuivalent radiation Dose |
| BED | Biologically Effective Dose |
| TCP | tumour Control Probability |
References
- Rosychuk, R.J.; Witol, A.; Wilson, B.; Stobart, K. Central nervous system (CNS) tumor trends in children in a western Canadian province: A population-based 22-year retrospective study. J. Neurol. 2012, 259, 1131–1136. [Google Scholar] [CrossRef]
- Prigorowsky, M. Cancerfondsrapporten 2009; Cancerfonden: Stockholm, Sweden, 2009. [Google Scholar]
- Han, J.; Kwon, S.; Won, S.; Shin, Y.; Ko, J.; Lyu, C. Comprehensive clinical follow-up of late effects in childhood cancer survivors shows the need for early and well-timed intervention. Ann. Oncol. 2009, 20, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.; Kesari, S. Brain irradiation and long-term cognitive disability: Current concepts. Nat. Rev. Neurol. 2017, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, J.; Bentzen, S.; Gonzalez, D.G.; Hulshof, M.; Arcangeli, G.; Dahl, O.; Mella, O. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- van der Zee, J.; González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordóñez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Klingbiel, D.; Gómez, S.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperth. 2016, 32, 809–821. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating hyperthermia into modern radiation oncology: What evidence is necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Ott, O.J. Hyperthermia in Oncology: Principles and Therapeutic Outlook; UNI-MED Verlag: Bremen, Germany, 2010. [Google Scholar]
- Wessalowski, R.; Schneider, D.T.; Mils, O.; Friemann, V.; Kyrillopoulou, O.; Schaper, J.; Matuschek, C.; Rothe, K.; Leuschner, I.; Willers, R.; et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: An open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013, 14, 843–852. [Google Scholar] [CrossRef]
- Hulshof, M.; Raaymakers, B.; Lagendijk, J.; Koot, R.; Crezee, H.; Stalpers, L.; Gonzalez Gonzalez, D. A feasibility study of interstitial hyperthermia plus external beam radiotherapy in glioblastoma multiforme using the multi electrode current source (MECS) system. Int. J. Hyperth. 2004, 20, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Salcman, M.; Samaras, G.M. Interstitial microwave hyperthermia for brain tumors. J. Neuro-Oncol. 1983, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Laing, J.; Paglione, R.; Sterzer, F. Microwave hyperthermia for brain tumors. Neurosurgery 1985, 17, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Sneed, P.K.; Stauffer, P.R.; McDermott, M.W.; Diederich, C.J.; Lamborn, K.R.; Prados, M.D.; Chang, S.; Weaver, K.A.; Spry, L.; Malec, M.K.; et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 287–295. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an adjuvant cancer therapy in combination with radiotherapy versus radiotherapy alone for recurrent/progressive glioblastoma: A systematic review. J. Neuro-Oncol. 2021, 152, 419–428. [Google Scholar] [CrossRef]
- Jordan, A.; Maier-Hauff, K. Magnetic nanoparticles for intracranial thermotherapy. J. Nanosci. Nanotechnol. 2007, 7, 4604–4606. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Gagliardo, C.; Marrone, S.; Paolini, F.; Gerardi, R.M.; Umana, G.E.; Yağmurlu, K.; Chaurasia, B.; Scalia, G.; Midiri, F.; et al. Focused Ultrasound in Neuroscience. State of the Art and Future Perspectives. Brain Sci. 2021, 11, 84. [Google Scholar] [CrossRef]
- Turner, P.; Tumeh, A.; Schaefermeyer, T. BSD-2000 approach for deep local and regional hyperthermia: Physics and technology. Strahlenther. Onkol. 1989, 165, 738–741. [Google Scholar]
- Paulides, M.; Bakker, J.; Neufeld, E.; Zee, J.v.d.; Jansen, P.; Levendag, P.; Van Rhoon, G. The HYPERcollar: A novel applicator for hyperthermia in the head and neck. Int. J. Hyperth. 2007, 23, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Takook, P.; Persson, M.; Trefná, H.D. Performance evaluation of hyperthermia applicators to heat deep-seated brain tumors. IEEE J. Electromagn. RF Microwaves Med. Biol. 2018, 2, 18–24. [Google Scholar] [CrossRef]
- Rodrigues, D.; Ellsworth, J.; Turner, P. Feasibility of heating brain tumors using a 915 MHz annular phased array. IEEE Antennas Wirel. Propag. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Winter, L.; Özerdem, C.; Hoffmann, W.; Santoro, D.; Müller, A.; Waiczies, H.; Seemann, R.; Graessl, A.; Wust, P.; Niendorf, T. Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: Electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla. PLoS ONE 2013, 8, e61661. [Google Scholar] [CrossRef]
- Zanoli, M.; Trefna, H.D. Iterative time-reversal for multi-frequency hyperthermia. Phys. Med. Biol. 2021, 66, 045027. [Google Scholar] [CrossRef] [PubMed]
- Oberacker, E.; Kuehne, A.; Oezerdem, C.; Nadobny, J.; Weihrauch, M.; Beck, M.; Zschaeck, S.; Diesch, C.; Eigentler, T.W.; Waiczies, H.; et al. Radiofrequency applicator concepts for thermal magnetic resonance of brain tumors at 297 MHz (7.0 Tesla). Int. J. Hyperth. 2020, 37, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Trefná, H.D.; Martinsson, B.; Petersson, T.; Renström, N.; Torstensson, M.; Ravanis, J.; Kok, P.; Persson, M. Multifrequency approach in hyperthermia treatment planning: Impact of frequency on SAR distribution in head and neck. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 3710–3712. [Google Scholar]
- Schooneveldt, G.; Trefná, H.D.; Persson, M.; De Reijke, T.M.; Blomgren, K.; Kok, H.P.; Crezee, H. Hyperthermia treatment planning including convective flow in cerebrospinal fluid for brain tumour hyperthermia treatment using a novel dedicated paediatric brain applicator. Cancers 2019, 11, 1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, B.E. Complications After Stereotactic Radiosurgery. In Complications in Neurosurgery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–206. [Google Scholar]
- Van Leeuwen, C.; Oei, A.; Ten Cate, R.; Franken, N.; Bel, A.; Stalpers, L.; Crezee, J.; Kok, H. Measurement and analysis of the impact of time-interval, temperature and radiation dose on tumour cell survival and its application in thermoradiotherapy plan evaluation. Int. J. Hyperth. 2018, 34, 30–38. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, C.; Crezee, J.; Oei, A.; Franken, N.; Stalpers, L.; Bel, A.; Kok, H. The effect of time interval between radiotherapy and hyperthermia on planned equivalent radiation dose. Int. J. Hyperth. 2018, 34, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Alper, T.; Howard-Flanders, P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature 1956, 178, 978–979. [Google Scholar] [CrossRef]
- Tissue Properties Database. V3.1. 2016. Available online: http://itis.swiss/virtual-population/tissue-properties/downloads-v3-1/ (accessed on 29 January 2021).
- Rossmann, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Erdmann, B.; Seebass, M. Impact of nonlinear heat transfer on temperature control in regional hyperthermia. IEEE Trans. Biomed. Eng. 1999, 46, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Takook, P.; Persson, M.; Gellermann, J.; Trefná, H.D. Compact self-grounded Bow-Tie antenna design for an UWB phased-array hyperthermia applicator. Int. J. Hyperth. 2017, 33, 387–400. [Google Scholar] [CrossRef]
- Canters, R.; Franckena, M.; van der Zee, J.; Van Rhoon, G. Optimizing deep hyperthermia treatments: Are locations of patient pain complaints correlated with modelled SAR peak locations? Phys. Med. Biol. 2010, 56, 439. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, M.; Trefná, H.D. Optimization of microwave hyperthermia array applicators using field interpolation. In Proceedings of the 2019 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 537–538. [Google Scholar]
- Kennedy, J.; Eberhart, R. Particle swarm optimization. In Proceedings of the ICNN’95-International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; Volume 4, pp. 1942–1948. [Google Scholar]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, C.; Paddick, I. The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Oper. Neurosurg. 2007, 61, ONS-130–ONS-141. [Google Scholar] [CrossRef]
- Torrens, M.; Chung, C.; Chung, H.T.; Hanssens, P.; Jaffray, D.; Kemeny, A.; Larson, D.; Levivier, M.; Lindquist, C.; Lippitz, B.; et al. Standardization of terminology in stereotactic radiosurgery: Report from the Standardization Committee of the International Leksell Gamma Knife Society: Special topic. J. Neurosurg. 2014, 121, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Dewey, W.; Hopwood, L.; Sapareto, S.; Gerweck, L. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef]
- Fowler, J.F. Sensitivity analysis of parameters in linear-quadratic radiobiologic modeling. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1532–1537. [Google Scholar] [CrossRef]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Toma-Dasu, I.; Dasu, A. Modelling tumour oxygenation, reoxygenation and implications on treatment outcome. Comput. Math. Methods Med. 2013, 2013, 141087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alper, T. Cellular Radiobiology; CUP Archive: Cambridge, UK, 1979. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Wolters Kluwer Health: Philadelphia, PA, USA, 2006; Volume 6. [Google Scholar]
- Vaupel, P.; Multhoff, G. Accomplices of the hypoxic tumor microenvironment compromising antitumor immunity: Adenosine, lactate, acidosis, vascular endothelial growth factor, potassium ions, and phosphatidylserine. Front. Immunol. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daşu, A.; Toma-Daşu, I. Treatment modelling: The influence of micro-environmental conditions. Acta Oncol. 2008, 47, 896–905. [Google Scholar] [CrossRef]
- Brahme, A. Optimized radiation therapy based on radiobiological objectives. In Seminars in Radiation Oncology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 9, pp. 35–47. [Google Scholar]
- Dewey, W.C. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperth. 1994, 10, 457–483. [Google Scholar] [CrossRef] [PubMed]
- van Rhoon, G.C. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.; Vujaskovic, Z.; Kondo, M.; Sugahara, T. The Kadota fund international forum 2004–Clinical group consensus. Int. J. Hyperth. 2008, 24, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Haveman, J.; Sminia, P.; Wondergem, J.; van der Zee, J.; Hulshof, M. Effects of hyperthermia on the central nervous system: What was learnt from animal studies? Int. J. Hyperth. 2005, 21, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lindblom, E.; Toma-Dasu, I.; Dasu, A. Accounting for two forms of hypoxia for predicting tumour control probability in radiotherapy: An in silico study. In Oxygen Transport to Tissue XL; Springer: Berlin, Germany, 2018; pp. 183–187. [Google Scholar]
- Horsman, M.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef]
- Oei, A.; Kok, H.; Oei, S.; Horsman, M.; Stalpers, L.; Franken, N.; Crezee, J. Molecular and biological rationale of hyperthermia as radio-and chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163, 84–97. [Google Scholar] [CrossRef]
- Kok, H.P.; Crezee, J.; Franken, N.A.; Stalpers, L.J.; Barendsen, G.W.; Bel, A. Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F. 21 years of biologically effective dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Crezee, J.; Oei, A.; Franken, N.; Stalpers, L.; Bel, A.; Kok, H. 3D radiobiological evaluation of combined radiotherapy and hyperthermia treatments. Int. J. Hyperth. 2017, 33, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Prescription Dose | Coverage | Selectivity | Gradient Index | Beam-on Time (min) @ 3 Gy/min | Number of Shots |
|---|---|---|---|---|---|
| 15 Gy | 0.994 | 0.850 | 2.63 | 54.8 | 110 |
| Parameters | Tumour | Healthy Tissue |
|---|---|---|
| (Gy) | 0.35 | 0.35 |
| (Gy) | 10 | 3 |
| 2.36 | 2.36 | |
| 0.53 | 0.53 | |
| (h) | 0.047 | 1 |
| (cal/°C/mol) | 423.14 | 423.14 |
| (cal/mol) | 157,312.3 | 157,312.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaderi Aram, M.; Zanoli, M.; Nordström, H.; Toma-Dasu, I.; Blomgren, K.; Dobšíček Trefná, H. Radiobiological Evaluation of Combined Gamma Knife Radiosurgery and Hyperthermia for Pediatric Neuro-Oncology. Cancers 2021, 13, 3277. https://doi.org/10.3390/cancers13133277
Ghaderi Aram M, Zanoli M, Nordström H, Toma-Dasu I, Blomgren K, Dobšíček Trefná H. Radiobiological Evaluation of Combined Gamma Knife Radiosurgery and Hyperthermia for Pediatric Neuro-Oncology. Cancers. 2021; 13(13):3277. https://doi.org/10.3390/cancers13133277
Chicago/Turabian StyleGhaderi Aram, Morteza, Massimiliano Zanoli, Håkan Nordström, Iuliana Toma-Dasu, Klas Blomgren, and Hana Dobšíček Trefná. 2021. "Radiobiological Evaluation of Combined Gamma Knife Radiosurgery and Hyperthermia for Pediatric Neuro-Oncology" Cancers 13, no. 13: 3277. https://doi.org/10.3390/cancers13133277
APA StyleGhaderi Aram, M., Zanoli, M., Nordström, H., Toma-Dasu, I., Blomgren, K., & Dobšíček Trefná, H. (2021). Radiobiological Evaluation of Combined Gamma Knife Radiosurgery and Hyperthermia for Pediatric Neuro-Oncology. Cancers, 13(13), 3277. https://doi.org/10.3390/cancers13133277







