Exploring miRNA Signature and Other Potential Biomarkers for Oligometastatic Prostate Cancer Characterization: The Biological Challenge behind Clinical Practice. A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Polimetastatic versus Oligometastatic PCA
2.1. Burden of Disease
2.2. Biological Differences
3. Benefit from Metastases-Directed Therapy (MDT)
4. Emerging Biomarkers for the Identification of True Oligometastatic Patients Eligible for MDT
4.1. Liquid Biopsy and Next Generations Sequencing (NGS)
4.2. Circulating Tumor Cells (CTCs)
4.3. Circulating Cell-Free DNA (cfDNA)
4.4. Exosomes
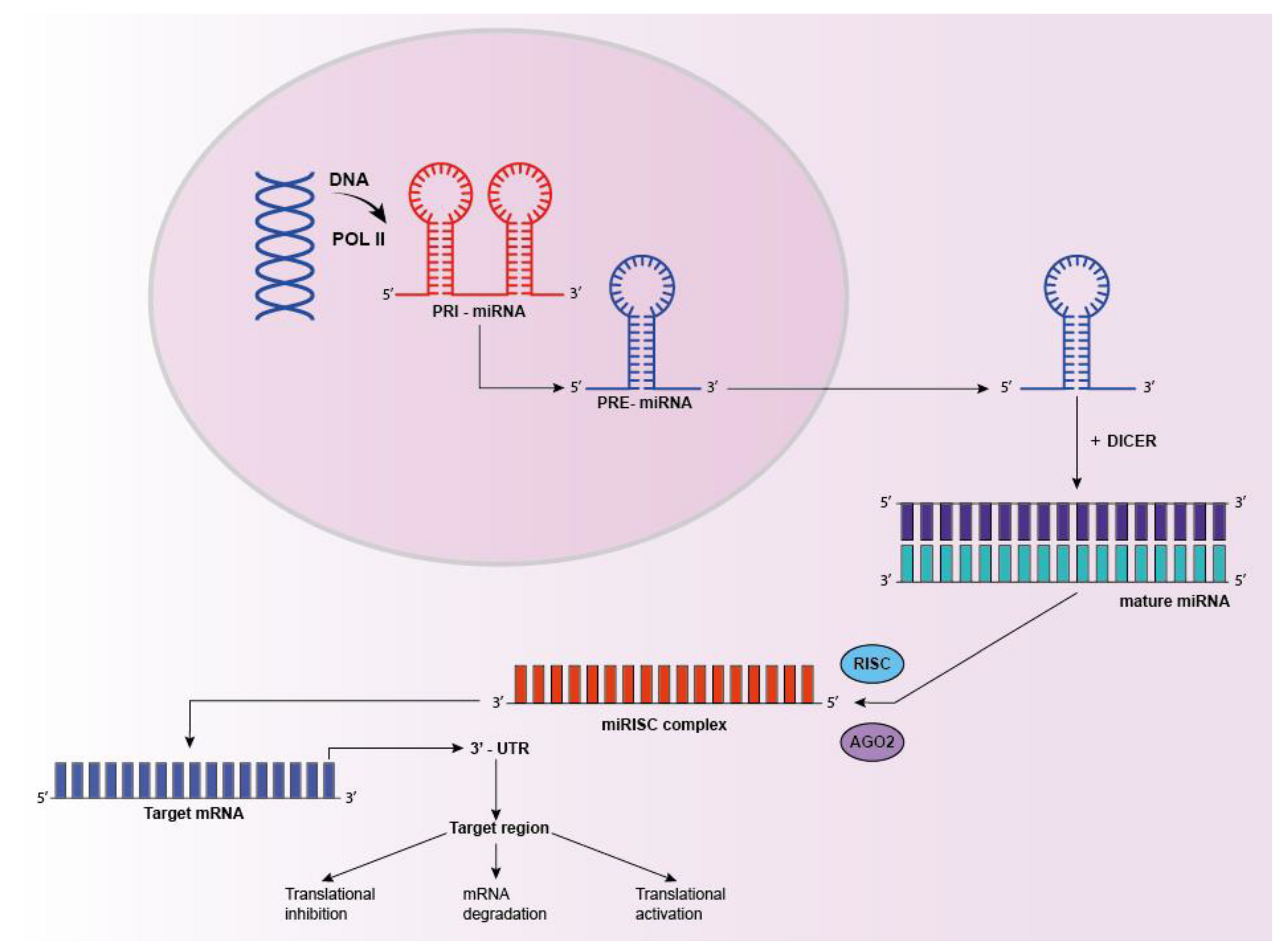
5. Micro Ribo-Nucleic Acid (miRNA)
Epigenetic (epi)-miRNAs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ADT | androgen deprivation therapy |
| ChIP-seq | chromatin immunoprecipitation sequencing |
| CT | computed tomography |
| cfDNA | circulating cell-free DNA |
| ctDNA | circulating tumor DNA |
| CTC | circulating tumor cell |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial mesenchymal transition |
| EpCAM | epithelial cellular adhesion molecule |
| hTERT | telomerase reverse transcriptase |
| miRNA | micro Ribo-Nucleic Acid |
| MDT | metastases directed treatment |
| NGS | nextgeneration sequencing |
| OCS-PCa | oligorecurrent-castration-sensitive-PCa |
| OMPC | oligometastatic prostate cancer |
| PCa | prostate cancer |
| PCR | polymerase chain reaction |
| PET | positron emission tomography |
| PMPC | polymetastatic prostate cancer |
| PSA | prostate-specific antigen |
| PSMA | prostate-specific membrane antigen |
| RISC | RNA-induced silencing complex |
| SBRT | stereotactic body radiotherapy |
| SNP | single nucleotide polymorphism |
| WB-MRI | whole-body magnetic resonance imaging |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA J. Am. Med. Assoc. 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Yi, W.S.; Brasacchio, R.A.; Muhs, A.G.; Smudzin, T.; Williams, J.P.; Messing, E.; Okunieff, P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 3–10. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, E.; Tran, P.T.; Ost, P. Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Curr. Opin. Urol. 2017, 27, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Mathieu, R.; Korn, S.M.; Bensalah, K.; Kramer, G.; Shariat, S.F. Cytoreductive radical prostatectomy in metastatic prostate cancer: Does it really make sense? World J. Urol. 2017, 35, 567–577. [Google Scholar] [CrossRef]
- Fossati, N.; Trinh, Q.D.; Sammon, J.; Sood, A.; Larcher, A.; Sun, M.; Karakiewicz, P.; Guazzoni, G.; Montorsi, F.; Briganti, A.; et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: A SEER-based Study. Eur. Urol. 2015, 67, 3–6. [Google Scholar] [CrossRef]
- Löppenberg, B.; Dalela, D.; Karabon, P.; Sood, A.; Sammon, J.D.; Meyer, C.P.; Sun, M.; Noldus, J.; Peabody, J.O.; Trinh, Q.D.; et al. The Impact of Local Treatment on Overall Survival in Patients with Metastatic Prostate Cancer on Diagnosis: A National Cancer Data Base Analysis. Eur. Urol. 2017, 72, 14–19. [Google Scholar] [CrossRef]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]
- Fanetti, G.; Marvaso, G.; Ciardo, D.; Rese, A.; Ricotti, R.; Rondi, E.; Comi, S.; Cattani, F.; Zerini, D.; Fodor, C.; et al. Stereotactic body radiotherapy for castration-sensitive prostate cancer bone oligometastases. Med. Oncol. 2018, 35. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Fanetti, G.; Fodor, C.; Ciardo, D.; Santoro, L.; Francia, C.M.; Muto, M.; Surgo, A.; Zerini, D.; Marvaso, G.; et al. Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin. Genitourin. Cancer 2017, 15, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, M.; Pepa, M.; Marvaso, G.; Jereczek-Fossa, B.A. Re: Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. Eur. Urol. 2021, 79, 889–890. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; DeBruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Ciardo, D.; Corrao, G.; Gandini, S.; Fodor, C.; Zerini, D.; Rojas, D.P.; Augugliaro, M.; Bonizzi, G.; Pece, S.; et al. Radioablation +/− hormonotherapy for prostate cancer oligorecurrences (Radiosa trial): Potential of imaging and biology (AIRC IG-22159). BMC Cancer 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, E.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- McMillan, D.C.; Elahi, M.M.; Sattar, N.; Angerson, W.J.; Johnstone, J.; McArdle, C.S. Measurement of the Systemic Inflammatory Response Predicts Cancer-Specific and Non-Cancer Survival in Patients with Cancer. Nutr. Cancer 2001, 41, 64–69. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, H.; Liao, X. Trigger pSA predicting recurrence from positive choline PET/CT with prostate cancer after initial treatment. Oncotarget 2018, 9, 14630–14641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga–Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Lecouvet, F.E.; Tunariu, N.; Koh, D.-M.; De Keyzer, F.; Collins, D.; Sala, E.; Schlemmer, H.P.; Petralia, G.; Vargas, H.A.; et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur. Urol. 2017, 71, 81–92. [Google Scholar] [CrossRef]
- Tunariu, N.; Blackledge, M.; Messiou, C.; Petralia, G.; Padhani, A.; Curcean, S.; Curcean, A.; Koh, D.-M. What’s New for Clinical Whole-body MRI (WB-MRI) in the 21st Century. Br. J. Radiol. 2020, 93. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Van Leeuwen, P.J.; Stricker, P.; Hruby, G.; Kneebone, A.; Ting, F.; Thompson, B.; Nguyen, Q.; Ho, B.; Emmett, L. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016, 117, 732–739. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Zhang, Z.; Guo, Y.; Wang, R.; Wang, L.; Mao, S.; Zhang, J.; Yao, X. Controlling Nutritional Status score: A new prognostic indicator for patients with oligometastatic prostate cancer. Curr. Probl. Cancer 2019, 43, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, M.J.; Gravis, G.; Niazi, T. The Biology of Oligometastatic Prostate Cancer: A Different Beast than Polymetastatic Prostate Cancer. Eur. Urol. Focus 2019, 5, 117–118. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Dhondt, B.; De Bleser, E.; Claeys, T.; Buelens, S.; Lumen, N.; Vandesompele, J.; Beckers, A.; Fonteyne, V.; Van der Eecken, K.; De Bruycker, A.; et al. Discovery and validation of a serum microRNA signature to characterize oligo- and polymetastatic prostate cancer: Not ready for prime time. World J. Urol. 2019, 37, 2557–2564. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Xing, H.R.; Salama, J.K.; Khodarev, N.N.; Huang, Y.; Zhang, Q.; Khan, S.A.; Yang, X.; Hasselle, M.D.; Darga, T.E.; et al. MicroRNA Expression Characterizes Oligometastasis(es). PLoS ONE 2011, 6, e28650. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Khodarev, N.N.; Regan, K.; Corbin, K.; Li, H.; Ganai, S.; Khan, S.A.; Gnerlich, J.; Darga, T.E.; Fan, H.; et al. Oligo- and Polymetastatic Progression in Lung Metastasis(es) Patients Is Associated with Specific MicroRNAs. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Joice, G.A.; Rowe, S.P.; Pienta, K.J.; Gorin, M.A. Oligometastatic prostate cancer: Shaping the definition with molecular imaging and an improved understanding of tumor biology. Curr. Opin. Urol. 2017, 27, 533–541. [Google Scholar] [CrossRef]
- Chiang, A.C.; Massagué, J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Massagué, J. Genetic determinants of cancer metastasis. Nat. Rev. Genet. 2007, 8, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, B.; Rousseau, Q.; De Wever, O.; Hendrix, A. Function of extracellular vesicle-associated miRNAs in metastasis. Cell Tissue Res. 2016, 365, 621–641. [Google Scholar] [CrossRef]
- Sonpavde, G. The biology of prostate cancer metastases: Does oligo differ from polymetastatic? Curr. Opin. Urol. 2017, 27, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Z.Y.; Ye, S.L.; Liu, Y.K.; Chen, J.; Xue, Q.; Chen, J.; Gao, D.M.; Bao, W.H. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J. Gastroenterol. 2001, 7, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Uppal, A.; Wightman, S.C.; Mallon, S.; Oshima, G.; Pitroda, S.P.; Zhang, Q.; Huang, X.; Darga, T.E.; Huang, L.; Andrade, J.; et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget 2015, 6, 3540–3552. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Högnäs, G.; Annala, M.; et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Ost, P.; Bossi, A.; Decaestecker, K.; De Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach, M.; Briganti, A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Clarebrough, E.; Duncan, C.; Christidis, D.; Lavoipierre, A.; Lawrentschuk, N. PSMA-PET guided hook-wire localization of nodal metastases in prostate cancer: A targeted approach. World J. Urol. 2019, 37, 1251–1254. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Pandey, T.; Dey, P. Liquid biopsy: A new avenue in pathology. Cytopathology 2019, 30, 138–143. [Google Scholar] [CrossRef]
- Arancio, W.; Belmonte, B.; Castiglia, M.; Di Napoli, A.; Tripodo, C. Tissue Versus Liquid Biopsy: Opposite or Complementary? In Liquid Biopsy in Cancer Patients; Humana Press: Cham, Switzerland, 2017; pp. 41–49. [Google Scholar]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 4–10. [Google Scholar] [CrossRef]
- Stelcer, E.; Konkol, M.; Głȩboka, A.; Suchorska, W.M. Liquid biopsy in oligometastatic prostate cancer—A biologist’s point of view. Front. Oncol. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines—Prostate Cancer; NCCN: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Roy-Chowdhuri, S.; Stewart, J. Preanalytic variables in cytology: Lessons learned from next-generation sequencing—The MD Anderson experience. Arch. Pathol. Lab. Med. 2016, 140, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2020, 41, 25–43. [Google Scholar] [CrossRef]
- Hynes, S.O.; Pang, B.; James, J.A.; Maxwell, P.; Salto-Tellez, M. Tissue-based next generation sequencing: Application in a universal healthcare system. Br. J. Cancer 2017. [Google Scholar] [CrossRef]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing–Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef]
- Hegemann, M.; Stenzl, A.; Bedke, J.; Chi, K.N.; Black, P.C.; Todenhöfer, T. Liquid biopsy: Ready to guide therapy in advanced prostate cancer? BJU Int. 2016, 118, 855–863. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef] [PubMed]
- Faugeroux, V.; Lefebvre, C.; Pailler, E.; Pierron, V.; Marcaillou, C.; Tourlet, S.; Billiot, F.; Dogan, S.; Oulhen, M.; Vielh, P.; et al. An Accessible and Unique Insight into Metastasis Mutational Content Through Whole-exome Sequencing of Circulating Tumor Cells in Metastatic Prostate Cancer. Eur. Urol. Oncol. 2020, 3, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.C.; Huland, H.; Tiebel, A.; Haese, A.; Salomon, G.; Budäus, L.; Tilki, D.; Chun, F.; Heinzer, H.; Graefen, M.; et al. Enumeration and Changes in Circulating Tumor Cells and Their Prognostic Value in Patients Undergoing Cytoreductive Radical Prostatectomy foeasr Oligometastatic Prostate Cancer—Translational Research Results from the Prospective ProMPT trial. Eur. Urol. Focus 2019. [Google Scholar] [CrossRef]
- Gabriel, M.T.; Calleja, L.R.; Chalopin, A.; Ory, B.; Heymann, D. Circulating Tumor Cells: A Review of Non–EpCAM-Based Approaches for Cell Enrichment and Isolation. Clin. Chem. 2016, 62, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, M.J.M.; Solanki, T.I.; Ordonez, A.D.; Hsiao, F.; Park, J.W. Enumeration of circulating tumor cells and disseminated tumor cells in blood and bone marrow by immunomagnetic enrichment and flow cytometry (IE/FC). In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1634, pp. 203–210. [Google Scholar]
- Barriere, G.; Fici, P.; Gallerani, G.; Fabbri, F.; Zoli, W.; Rigaud, M. Circulating tumor cells and epithelial, mesenchymal and stemness markers: Characterization of cell subpopulations. Ann. Transl. Med. 2014, 2, 5. [Google Scholar]
- Togo, S.; Katagiri, N.; Namba, Y.; Tulafu, M.; Nagahama, K.; Kadoya, K.; Takamochi, K.; Oh, S.; Suzuki, K.; Sakurai, F.; et al. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients. Oncotarget 2017, 8, 34884–34895. [Google Scholar] [CrossRef]
- Mansilla, C.; Soria, E.; Ramírez, N. The identification and isolation of CTCs: A biological Rubik’s cube. Crit. Rev. Oncol. Hematol. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Lowes, L.E.; Bratman, S.V.; Dittamore, R.; Done, S.; Kelley, S.O.; Mai, S.; Morin, R.D.; Wyatt, A.W.; Allan, A.L. Circulating tumor cells (CTC) and cell-free DNA (cfDNA)workshop 2016: Scientific opportunities and logistics for cancer clinical trial incorporation. Int. J. Mol. Sci. 2016, 17, 1505. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; Gongora, C.; Ollier, J.; Robert, B.; Ychou, M.; del Rio, M.; Molina, F. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. 2010, 38, 6159–6175. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef]
- Elshimali, Y.; Khaddour, H.; Sarkissyan, M.; Wu, Y.; Vadgama, J. The Clinical Utilization of Circulating Cell Free DNA (CCFDNA) in Blood of Cancer Patients. Int. J. Mol. Sci. 2013, 14, 18925–18958. [Google Scholar] [CrossRef]
- Salvi, S.; Gurioli, G.; De Giorgi, U.; Conteduca, V.; Tedaldi, G.; Calistri, D.; Casadio, V. Cell-free DNA as a diagnostic marker for cancer: Current insights. Oncotargets Ther. 2016, 9, 6549–6559. [Google Scholar] [CrossRef]
- Belic, J.; Koch, M.; Ulz, P.; Auer, M.; Gerhalter, T.; Mohan, S.; Fischereder, K.; Petru, E.; Bauernhofer, T.; Geigl, J.B.; et al. Rapid Identification of Plasma DNA Samples with Increased ctDNA Levels by a Modified FAST-SeqS Approach. Clin. Chem. 2015, 61, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Ulz, P.; Geigl, J.B. Circulating Tumor DNA as a Liquid Biopsy for Cancer. Clin. Chem. 2015, 61, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Torga, G.; Pienta, K.J. Patient-Paired sample congruence between 2 commercial liquid biopsy tests. JAMA Oncol. 2018, 4, 868–870. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Webber, J.P.; Gurney, M.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015, 6, 715–731. [Google Scholar] [CrossRef]
- Conti, A.; D’Elia, C.; Cheng, M.; Santoni, M.; Piva, F.; Brunelli, M.; Lopez-Beltran, A.; Giulietti, M.; Scarpelli, M.; Pycha, A.; et al. Oligometastases in Genitourinary Tumors: Recent Insights and Future Molecular Diagnostic Approach. Eur. Urol. Suppl. 2017, 16, 309–315. [Google Scholar] [CrossRef]
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin β4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int. J. Oncol. 2015, 47, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Ganguly, K.K.; Fazli, L.; Fedele, C.; Lu, H.; Dutta, A.; Liu, Q.; De Angelis, T.; Riddell, L.W.; Riobo, N.A.; et al. Trop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget 2015, 6, 14318–14328. [Google Scholar] [CrossRef]
- Pan, J.; Ding, M.; Xu, K.; Yang, C.; Mao, L.J. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget 2017, 8, 97693–97700. [Google Scholar] [CrossRef]
- Peter, M.E. Targeting of mRNAs by multiple miRNAs: The next step. Oncogene 2010, 29, 2161–2164. [Google Scholar] [CrossRef]
- miRbase. Available online: http://www.mirbase.org/ (accessed on 16 November 2020).
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—MicroRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Cheng, H.H.; Plets, M.; Li, H.; Higano, C.S.; Tangen, C.M.; Agarwal, N.; Vogelzang, N.J.; Hussain, M.; Thompson, I.M.; Tewari, M.; et al. Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer. Prostate 2018, 78, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal miRNAs as biomarkers for prostate cancer. Front. Genet. 2013, 4, 36. [Google Scholar] [CrossRef]
- Mulrane, L.; McGee, S.F.; Gallagher, W.M.; O’Connor, D.P. miRNA dysregulation in breast cancer. Cancer Res. 2013, 73, 6554–6562. [Google Scholar] [CrossRef] [PubMed]
- Zabolotneva, A.A.; Zhavoronkov, A.; Garazha, A.V.; Roumiantsev, S.A.; Buzdin, A.A. Characteristic patterns of microrna expression in human bladder cancer. Front. Genet. 2013, 3, 310. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Edbauer, D. Good guy or bad guy: The opposing roles of microRNA 125b in cancer. Cell Commun. Signal. 2014, 12, 1–13. [Google Scholar] [CrossRef]
- Sorrentino, A.; Liu, C.G.; Addario, A.; Peschle, C.; Scambia, G.; Ferlini, C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol. Oncol. 2008, 111, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, P.T.; Sanford, T.; Choudhary, A.; Mydlarz, W.W.; Brown, D.; Adai, A.T.; Ochs, M.F.; Ahrendt, S.A.; Mambo, E.; Califano, J.A. Serum microrna biomarkers for detection of non-small cell lung cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Kotaki, R.; Koyama-Nasu, R.; Yamakawa, N.; Kotani, A. MiRNAs in normal and malignant hematopoiesis. Int. J. Mol. Sci. 2017, 18, 1495. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Said, H.M.; Alam, M.A.; Al Naieb, Z.T. Blood-based microRNAs as diagnostic biomarkers to discriminate localized prostate cancer from benign prostatic hyperplasia and allow cancer-risk stratification. Oncol. Lett. 2018, 16, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Peng, Q.; Shen, Y.; Hong, Y.; Zhu, J.; Feng, Z.; Zhou, P.; Fan, S.; Zhu, Y.; Zhang, Y. Identification of biomarker microRNA-mRNA regulatory pairs for predicting the docetaxel resistance in prostate cancer. J. Cancer 2019, 10, 5469–5482. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, D.; Yang, T.L.; Bucay, N.; Sekhon, K.; Majid, S.; Shahryari, V.; Dahiya, R.; Tanaka, Y.; Saini, S.; Affairs, V.; et al. MicroRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2019, 78, 1833–1844. [Google Scholar] [CrossRef]
- Paziewska, A.; Mikula, M.; Dabrowska, M.; Kulecka, M.; Goryca, K.; Antoniewicz, A.; Dobruch, J.; Borowka, A.; Rutkowski, P.; Ostrowski, J. Candidate diagnostic miRNAs that can detect cancer in prostate biopsy. Prostate 2018, 78, 178–185. [Google Scholar] [CrossRef]
- Bryant, R.J.; Pawlowski, T.; Catto, J.W.F.; Marsden, G.; Vessella, R.L.; Rhees, B.; Kuslich, C.; Visakorpi, T.; Hamdy, F.C. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 2012, 106, 768–774. [Google Scholar] [CrossRef]
- Bidarra, D.; Constâncio, V.; Barros-Silva, D.; Ramalho-Carvalho, J.; Moreira-Barbosa, C.; Antunes, L.; Maurício, J.; Oliveira, J.; Henrique, R.; Jerónimo, C. Circulating MicroRNAs as Biomarkers for Prostate Cancer Detection and Metastasis Development Prediction. Front. Oncol. 2019, 9, 900. [Google Scholar] [CrossRef]
- Osipov, I.D.; Zaporozhchenko, I.A.; Bondar, A.A.; Zaripov, M.M.; Voytsitskiy, V.E.; Vlassov, V.V.; Laktionov, P.P.; Morozkin, E.S. Cell-free miRNA-141 and miRNA-205 as prostate cancer biomarkers. Adv. Exp. Med. Biol. 2016, 924, 9–12. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.Y.; Wang, J.; Zeng, X.F.; Li, R.; Kang, W.; Hao, X.K. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Oncotargets Ther. 2015, 9, 139–148. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, L.; Xiang, Q.; Zhu, Z.; Wang, J.; Liu, Y.; Deng, Y.; Luo, J.; Kang, R. Cancer-Derived Exosomal miR-199b-5p Inhibits Distant Metastases of Prostate Cancer by Counteracting the DDR1-MAPK/ERK-EMT Pathway. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Hudson, R.S.; Yi, M.; Esposito, D.; Glynn, S.A.; Starks, A.M.; Yang, Y.; Schetter, A.J.; Watkins, S.K.; Hurwitz, A.A.; Dorsey, T.H.; et al. MicroRNA-106b-25 cluster expression is associated with early disease recurrence and targets caspase-7 and focal adhesion in human prostate cancer. Oncogene 2013, 32, 4139–4147. [Google Scholar] [CrossRef]
- Xu, B.; Wang, N.; Wang, X.; Tong, N.; Shao, N.; Tao, J.; Li, P.; Niu, X.; Feng, N.; Zhang, L.; et al. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate 2012, 72, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- El Bezawy, R.; Cominetti, D.; Fenderico, N.; Zuco, V.; Beretta, G.L.; Dugo, M.; Arrighetti, N.; Stucchi, C.; Rancati, T.; Valdagni, R.; et al. miR-875-5p counteracts epithelial-to-mesenchymal transition and enhances radiation response in prostate cancer through repression of the EGFR-ZEB1 axis. Cancer Lett. 2017, 395, 53–62. [Google Scholar] [CrossRef]
- Tao, J.; Wu, D.; Xu, B.; Qian, W.; Li, P.; Lu, Q.; Yin, C.; Zhang, W. microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol. Rep. 2012, 27, 1967–1975. [Google Scholar] [CrossRef]
- Bhagirath, D.; Yang, T.L.; Tabatabai, Z.L.; Shahryari, V.; Majid, S.; Dahiya, R.; Tanaka, Y.; Saini, S. Role of a novel race-related tumor suppressor microRNA located in frequently deleted chromosomal locus 8p21 in prostate cancer progression. Carcinogenesis 2019, 40, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Abdellateif, M.S.; Kassem, S.H.; Abd El Salam, M.A.; El Gammal, M.M. Diagnostic significance of miR-21, miR-141, miR-18a and miR-221 as novel biomarkers in prostate cancer among Egyptian patients. Andrologia 2019, 51, e13384. [Google Scholar] [CrossRef] [PubMed]
- Uppal, A.; Ferguson, M.K.; Posner, M.C.; Hellman, S.; Khodarev, N.N.; Weichselbaum, R.R. Towards a molecular basis of oligometastatic disease: Potential role of micro-RNAs. Clin. Exp. Metastasis 2014, 31, 735–748. [Google Scholar] [CrossRef]
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498. [Google Scholar] [CrossRef]
- Mongroo, P.S.; Rustgi, A.K. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol. Ther. 2010, 10, 219–222. [Google Scholar] [CrossRef] [PubMed]
- McLerran, D.; Grizzle, W.E.; Feng, Z.; Bigbee, W.L.; Banez, L.L.; Cazares, L.H.; Chan, D.W.; Diaz, J.; Izbicka, E.; Kagan, J.; et al. Analytical validation of serum proteomic profiling for diagnosis of prostate cancer: Sources of sample bias. Clin. Chem. 2008, 54, 44–52. [Google Scholar] [CrossRef]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA spectrum between serum and plasma. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, N.; Abdi, J.; Hou, J.; Chang, H. Role of epigenetics-microRNA axis in drug resistance of multiple myeloma. J. Hematol. Oncol. 2017, 10, 121. [Google Scholar] [CrossRef]
- Memari, F.; Joneidi, Z.; Taheri, B.; Aval, S.F.; Roointan, A.; Zarghami, N. Epigenetics and Epi-miRNAs: Potential markers/therapeutics in leukemia. Biomed. Pharmacother. 2018, 106, 1668–1677. [Google Scholar] [CrossRef]
- Amodio, N.; Rossi, M.; Raimondi, L.; Pitari, M.R.; Botta, C.; Tagliaferri, P.; Tassone, P. miR-29s: A family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget 2015, 6, 12837–12861. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Varambally, S.; Cao, Q.; Mani, R.S.; Shankar, S.; Wang, X.; Ateeq, B.; Laxman, B.; Cao, X.; Jing, X.; Ramnarayanan, K.; et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 2008, 322, 1695–1699. [Google Scholar] [CrossRef]
- Duursma, A.M.; Kedde, M.; Schrier, M.; Le Sage, C.; Agami, R. miR-148 targets human DNMT3b protein coding region. RNA 2008, 14, 872–877. [Google Scholar] [CrossRef]
- Reale, E.; Taverna, D.; Cantini, L.; Martignetti, L.; Osella, M.; De Pittà, C.; Virga, F.; Orso, F.; Caselle, M. Investigating the epi-miRNome: Identification of epi-miRNAs using transfection experiments. Epigenomics 2019, 11, 1581–1599. [Google Scholar] [CrossRef]
- Gurbuz, V.; Kiliccioglu, I.; Dikmen, A.U.; Bilen, C.Y.; Sozen, S.; Konac, E. Comparative analysis of epi-miRNA expression levels in local/locally advanced and metastatic prostate cancer patients. Gene 2020, 758, 144963. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, M.; Lee, Y.; Bang, L.; Garg, T.; Sohn, K.A.; Kim, D. Identification of epigenetic interactions between miRNA and DNA methylation associated with gene expression as potential prognostic markers in bladder cancer. BMC Med. Genomics 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 28 April 2021).
| Author and Year | Samples Analyzed | Significant miRNAs Analyzed | miRNA Modulation | Clinical Value |
|---|---|---|---|---|
| Cheng et al. 2018 [81] | mPCa (50 pts) | miRNAs miR-141, miR-200a, miR-200c and miR-375 | Baseline vs. end of treatment | miR-375 and miR-200b were significantly associated with 28 weeks PSA response miR-141, miR-200a, miR-200c and miR-375 levels were significantly correlated with CTCs levels |
| Bryant et al. 2012 [93] | Pca (78 pts, including mPCa and lPCa pts) and normal control (28 pts) | miR-375 and miR-141 | Metastatic PCa vs. localized PCa | ↑ miR-375 and miR-141 expression significantly increased in metastatic Pca |
| Li et al. 2016 [96] | PCa (20 pts), BPH (20 pts), Healthy individuals (20 pts) | miR-141 | PCa vs. BPH vs. healthy | ↑ Elevated levels of serum exosomal miR-141 were considerably correlated with cancer metastasis |
| Osipov et al. 2016 [95] | PCa (48 pts) and Healthy donors (48 pts) | miR-141, miR-205 | PCa vs. healthy | ↑ The two miRNAs were significantly upregulated in PCa pts. miR-141 expression level efficiently discriminates early-stage prostate cancer patients and correlates with the Gleason score miRNA-205 expression showed no dependence on the stage of PCa |
| Zhao et al. 2019 [97] | localized PCa (25 pts) mPCa (35 pts) with bone or lymph node metastases metastases | miR-199b-5p | lPCa vs. mPCa | ↓ Exosomal miR-199b-5p serves as a tumor suppressor with prognostic impact in human PCa. Down-regulating miR-199b-5p might confer a proliferative advantage, accelerate migration, and promote metastasis in PCa cells |
| Bidarra et al. 2019 [94] | lPCa and mPCa (350 pts) and Healthy individuals (52 pts) | miR-182-5p and miR-375-3p | PCa vs. lPCa vs. healthy | ↑ miR-182-5p and miR-375-3p were associated with more advanced pathological stages. Higher circulating miR-375-3p levels in pts more prone to develop the metastatic disease with 71.43% accuracy. |
| Hudson et al. [98] | 28 non-cancerous tissues, 99 primary tumors and 14 distant metastases | miR-106b-25 cluster | Tumor tissues vs. metastatic tissue vs. non-cancerous tissues | ↑ miR-106-25 increased expression associated with PCa progression and disease prognosis, and caspase-7 is identified as a target of this cluster. |
| Trial ID | Trial Description | Study Type | Conditions | Interventions | Outcomes Measures | Estimated Primary Completion Date |
|---|---|---|---|---|---|---|
| NCT04324983 | BioPoP, Identification of Predictive Biomarkers | Interventional | Prostate Cancer Recurrent | Blood sample | -Rate of complete biochemical response -Prostate cancer-specific treatment-free survival after salvage surgery -Questionnaire Quality of life | December 2021 |
| NCT03902951 | Antiandrogen Therapy and SBRT in Treating Patients With Recurrent, Metastatic Prostate Cancer | Interventional, Phase II | -Metastatic Prostate Adenocarcinoma -Recurrent Prostate Carcinoma | -Drugs: Abiraterone Acetate/Apalutamide/Leuprolide Acetate -Stereotactic Body Radiation Therapy | -Percent of patients achieving a PSA < 0.05 ng/mL -Time to biochemical/ radiographic progression -Time to initiation of alternative antineoplastic therapy -Prostate cancer-specific Survival -Health-related quality of life -Biomarker analysis | July 2021 |
| NCT03421015 | Genetic Analysis of Prostate Cancer to Identify PredictiveMarkers of Disease Relapse or Metastatic Evolution | Observational Retrospective | Prostate Cancer | - | -The genetic alteration frequencies of TMPRSS2- ERG gene fusion •Frequency of amplification of proto-oncogenes (MYC, AR, PIK3CA) •Frequency of mutations or deletions of tumor suppressor genes (PTEN, TP53, NKX3-1), •Frequency of point mutations modifying protein function | July 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, G.; Zaffaroni, M.; Bergamaschi, L.; Augugliaro, M.; Volpe, S.; Pepa, M.; Bonizzi, G.; Pece, S.; Amodio, N.; Mistretta, F.A.; et al. Exploring miRNA Signature and Other Potential Biomarkers for Oligometastatic Prostate Cancer Characterization: The Biological Challenge behind Clinical Practice. A Narrative Review. Cancers 2021, 13, 3278. https://doi.org/10.3390/cancers13133278
Corrao G, Zaffaroni M, Bergamaschi L, Augugliaro M, Volpe S, Pepa M, Bonizzi G, Pece S, Amodio N, Mistretta FA, et al. Exploring miRNA Signature and Other Potential Biomarkers for Oligometastatic Prostate Cancer Characterization: The Biological Challenge behind Clinical Practice. A Narrative Review. Cancers. 2021; 13(13):3278. https://doi.org/10.3390/cancers13133278
Chicago/Turabian StyleCorrao, Giulia, Mattia Zaffaroni, Luca Bergamaschi, Matteo Augugliaro, Stefania Volpe, Matteo Pepa, Giuseppina Bonizzi, Salvatore Pece, Nicola Amodio, Francesco Alessandro Mistretta, and et al. 2021. "Exploring miRNA Signature and Other Potential Biomarkers for Oligometastatic Prostate Cancer Characterization: The Biological Challenge behind Clinical Practice. A Narrative Review" Cancers 13, no. 13: 3278. https://doi.org/10.3390/cancers13133278
APA StyleCorrao, G., Zaffaroni, M., Bergamaschi, L., Augugliaro, M., Volpe, S., Pepa, M., Bonizzi, G., Pece, S., Amodio, N., Mistretta, F. A., Luzzago, S., Musi, G., Alessi, S., La Fauci, F. M., Tordonato, C., Tosoni, D., Cattani, F., Gandini, S., Petralia, G., ... Jereczek-Fossa, B. A. (2021). Exploring miRNA Signature and Other Potential Biomarkers for Oligometastatic Prostate Cancer Characterization: The Biological Challenge behind Clinical Practice. A Narrative Review. Cancers, 13(13), 3278. https://doi.org/10.3390/cancers13133278











