Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes
Abstract
1. Introduction
2. Results
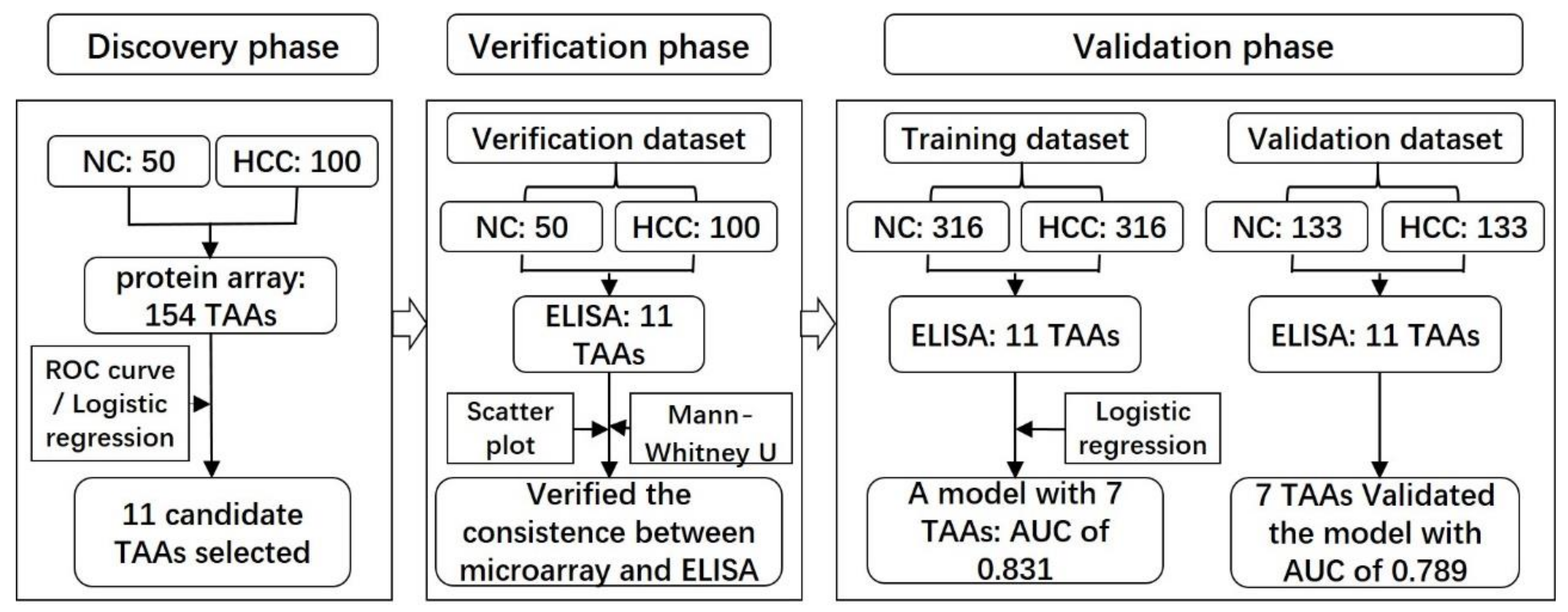
2.1. Serum Samples and Study Design
2.2. Serum Autoantibody in Discovery and Verification Phase
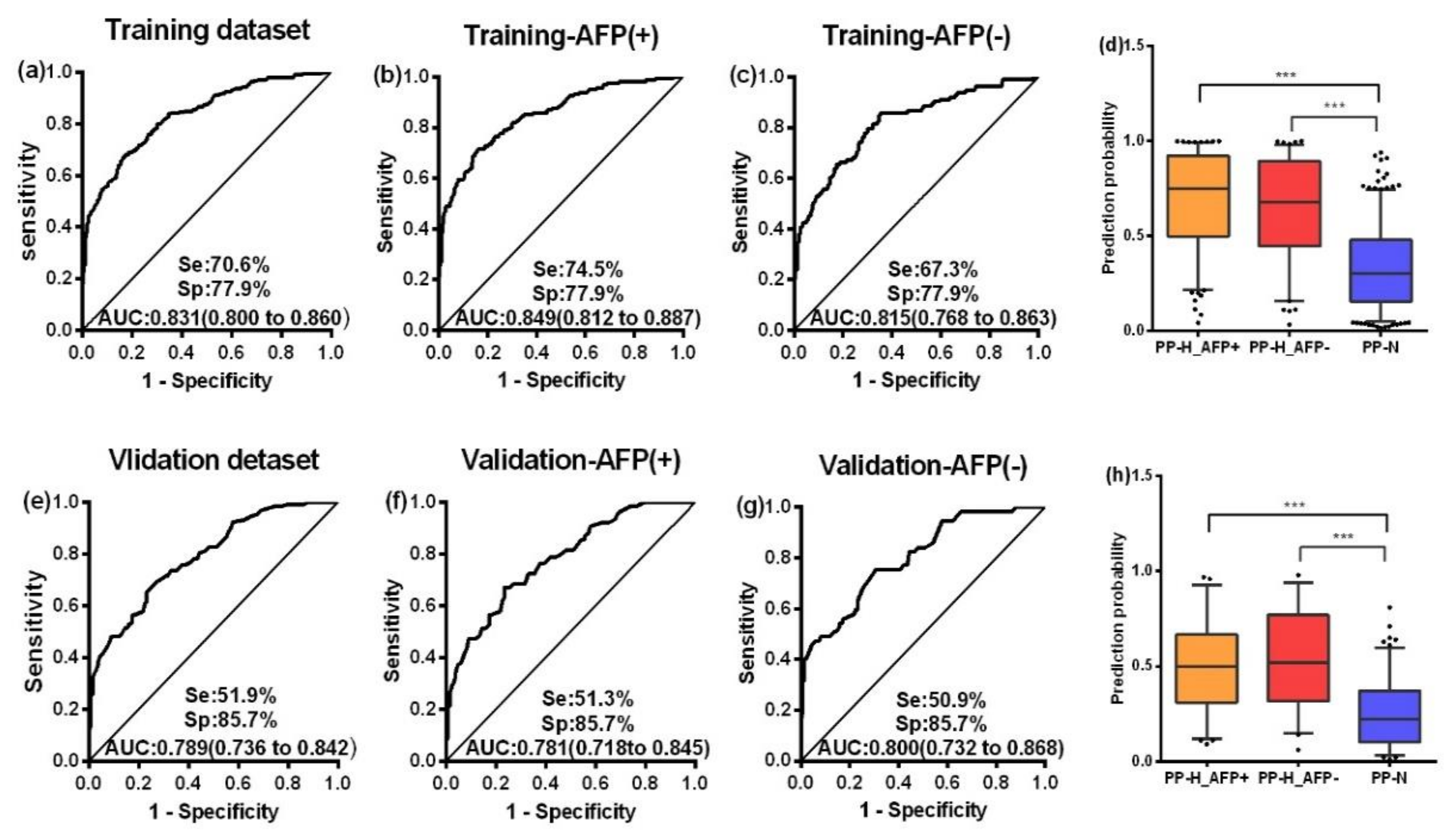
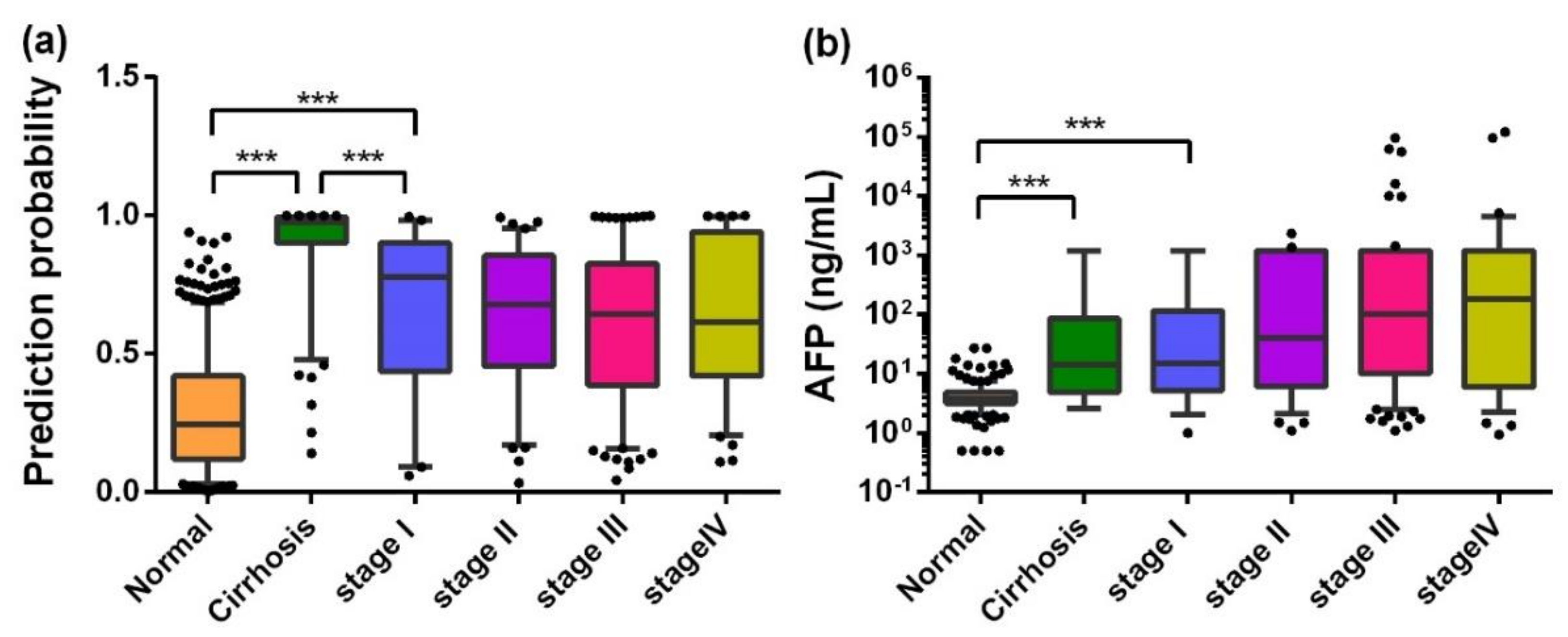
2.3. Serum Autoantibody in the Validation Phase
2.4. AFP and Autoantibodies
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of Recombinant TAAs
4.2. Human Protein Microarray Assay
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Luo, P.; Yin, P.; Hua, R.; Tan, Y.; Li, Z.; Qiu, G.; Yin, Z.; Xie, X.; Wang, X.; Chen, W.; et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology 2018, 67, 662–675. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Alpha-fetoprotein for hepatocellular carcinoma diagnosis: The demise of a brilliant star. Gastroenterology 2009, 137, 26–29. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Macdonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, M.; Dai, L.; Ying, X.; Ye, H.; Zhou, Y.; Han, S.; Zhang, J.Y. Using immunoproteomics to identify tumor-associated antigens (TAAs) as biomarkers in cancer immunodiagnosis. Autoimmun. Rev. 2013, 12, 1123–1128. [Google Scholar] [CrossRef]
- Paradis, V.; Bedossa, P. In the new area of noninvasive markers of hepatocellular carcinoma. J. Hepatol. 2007, 46, 9–11. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Zhou, J.; Zhao, H.; Zhang, H.; Wang, G. Diagnostic value of circulating cell-free DNA levels for hepatocellular carcinoma. Int. J. Infect. Dis. 2018, 67, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, X.; Huang, D.; Cui, D.; Liu, L.; Liu, J.; He, Z.; Liu, J.; Zheng, S.; Luo, Y. Plasma Heat Shock Protein 90alpha as a Biomarker for the Diagnosis of Liver Cancer: An Official, Large-scale, and Multicenter Clinical Trial. EBioMedicine 2017, 24, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Su, H.; Cao, J.; Zhang, L. CXCL13 rather than IL-31 is a potential indicator in patients with hepatocellular carcinoma. Cytokine 2017, 89, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, K.; Sartorius, B.; Kramvis, A.; Singh, E.; Turchinovich, A.; Burwinkel, B.; Madiba, T.; Winkler, C.A. Circulating microRNA’s as a diagnostic tool for hepatocellular carcinoma in a hyper endemic HIV setting, KwaZulu-Natal, South Africa: A case control study protocol focusing on viral etiology. BMC Cancer 2017, 17, 894. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Zhang, J. Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol. Rev. 2008, 222, 328–340. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Song, W.; Fang, Y.; Yu, L.; Liu, S.; Churilov, L.P.; Zhang, F. The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun. Rev. 2017, 16, 1270–1281. [Google Scholar] [CrossRef]
- Katchman, B.A.; Chowell, D.; Wallstrom, G.; Vitonis, A.F.; LaBaer, J.; Cramer, D.W.; Anderson, K.S. Autoantibody biomarkers for the detection of serous ovarian cancer. Gynecol. Oncol. 2017, 146, 129–136. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Tsay, J.J.; Yie, T.A.; Munger, J.S.; Pass, H.; Rom, W.N.; Tan, E.M.; Zhang, J.Y. Identification of autoantibodies to ECH1 and HNRNPA2B1 as potential biomarkers in the early detection of lung cancer. Oncoimmunology 2017, 6, e1310359. [Google Scholar] [CrossRef]
- Hoshino, I.; Nagata, M.; Takiguchi, N.; Nabeya, Y.; Ikeda, A.; Yokoi, S.; Kuwajima, A.; Tagawa, M.; Matsushita, K.; Satoshi, Y.; et al. Panel of autoantibodies against multiple tumor-associated antigens for detecting gastric cancer. Cancer Sci. 2017, 108, 308–315. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Wang, P.; Wang, X.; Ye, H.; Song, C.; Dai, L.; Wang, K.; Jiang, B.; Zhang, J. Autoantibody against 14-3-3 zeta: A serological marker in detection of gastric cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1253–1262. [Google Scholar] [CrossRef]
- Oshima, Y.; Shimada, H.; Yajima, S.; Nanami, T.; Matsushita, K.; Nomura, F.; Kainuma, O.; Takiguchi, N.; Soda, H.; Ueda, T.; et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: Screening in 1969 patients with various cancers. J. Gastroenterol. 2016, 51, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shivakumar, S.; Barker, K.; Tang, Y.; Wallstrom, G.; Park, J.G.; Tsay, J.C.; Pass, H.I.; Rom, W.N.; LaBaer, J.; et al. Comparative Study of Autoantibody Responses between Lung Adenocarcinoma and Benign Pulmonary Nodules. J. Thorac. Oncol. 2016, 11, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Megliorino, R.; Peng, X.X.; Tan, E.M.; Chen, Y.; Chan, E.K. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J. Hepatol. 2007, 46, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Wang, S.; Pang, Z.; Wang, Z.; Zhou, W.; Wu, M. Screening of autoantibodies as potential biomarkers for hepatocellular carcinoma by using T7 phase display system. Cancer Epidemiol. 2012, 36, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, X.; Nie, Y.; Dai, L.; Wang, P.; Zhang, J. Identification of tumor-associated antigens by using SEREX in hepatocellular carcinoma. Cancer Lett. 2009, 281, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Long, J.; Li, H.; Chen, S.; Liu, Q.; Zhang, B.; He, X.; Wang, Y.; Li, H.; Li, Y.; et al. An Analysis of Immunoreactive Signatures in Early Stage Hepatocellular Carcinoma. EBioMedicine 2015, 2, 438–446. [Google Scholar] [CrossRef]
- Dai, L.; Ren, P.; Liu, M.; Imai, H.; Tan, E.M.; Zhang, J.Y. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin. Immunol. 2014, 152, 127–139. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.H.; Yu, C.H.; Li, Y.M.; Wang, S.Q. Identification of hepatocellular-carcinoma-associated antigens and autoantibodies by serological proteome analysis combined with protein microarray. J. Proteome Res. 2008, 7, 611–620. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, H. Protein Array-based Approaches for Biomarker Discovery in Cancer. Genom. Proteom. Bioinform. 2017, 15, 73–81. [Google Scholar] [CrossRef]
- Ewaisha, R.; Panicker, G.; Maranian, P.; Unger, E.R.; Anderson, K.S. Serum Immune Profiling for Early Detection of Cervical Disease. Theranostics 2017, 7, 3814–3823. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Parish, A.J.; Nguyen, V.; Goodman, A.M.; Murugesan, K.; Frampton, G.M.; Kurzrock, R. GNAS, GNAQ, and GNA11 alterations in patients with diverse cancers. Cancer 2018, 124, 4080–4089. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, M.; Vazquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Chu, E.S.; Go, M.Y.; Xu, L.; Zhao, G.; Li, L.; Dai, N.; Si, J.; Tao, Q.; et al. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology 2011, 53, 843–853. [Google Scholar] [CrossRef]
- Eso, Y.; Takai, A.; Matsumoto, T.; Inuzuka, T.; Horie, T.; Ono, K.; Uemoto, S.; Lee, K.; Edelmann, W.; Chiba, T.; et al. MSH2 Dysregulation Is Triggered by Proinflammatory Cytokine Stimulation and Is Associated with Liver Cancer Development. Cancer Res. 2016, 76, 4383–4393. [Google Scholar] [CrossRef]
- Ma, G.; Yu, J.; Xiao, Y.; Chan, D.; Gao, B.; Hu, J.; He, Y.; Guo, S.; Zhou, J.; Zhang, L.; et al. Indian hedgehog mutations causing brachydactyly type A1 impair Hedgehog signal transduction at multiple levels. Cell Res. 2011, 21, 1343–1357. [Google Scholar] [CrossRef]
- Costa, H.A.; Leitner, M.G.; Sos, M.L.; Mavrantoni, A.; Rychkova, A.; Johnson, J.R.; Newton, B.W.; Yee, M.C.; De La Vega, F.M.; Ford, J.M.; et al. Discovery and functional characterization of a neomorphic PTEN mutation. Proc. Natl. Acad. Sci. USA 2015, 112, 13976–13981. [Google Scholar] [CrossRef]
- Miki, T.; Matsumoto, T.; Zhao, Z.; Lee, C.C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 2013, 4, 2444. [Google Scholar] [CrossRef]
- Liu, X.; Liu, D.; Qian, D.; Dai, J.; An, Y.; Jiang, S.; Stanley, B.; Yang, J.; Wang, B.; Liu, X.; et al. Nucleophosmin (NPM1/B23) interacts with activating transcription factor 5 (ATF5) protein and promotes proteasome- and caspase-dependent ATF5 degradation in hepatocellular carcinoma cells. J. Biol. Chem. 2012, 287, 19599–19609. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.W.; Song, E.; Feng, Z.; Sinha, A.; Chen, C.W.; Deshpande, A.J.; Cusan, M.; Farnoud, N.; Mupo, A.; Grove, C.; et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016, 6, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Knauer, S.K.; Heinrich, U.R.; Bier, C.; Habtemichael, N.; Docter, D.; Helling, K.; Mann, W.J.; Stauber, R.H. An otoprotective role for the apoptosis inhibitor protein survivin. Cell Death Dis. 2010, 1, e51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koziol, J.A.; Zhang, J.Y.; Casiano, C.A.; Peng, X.X.; Shi, F.D.; Feng, A.C.; Chan, E.K.; Tan, E.M. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin. Cancer Res. 2003, 9, 5120–5126. [Google Scholar] [PubMed]
- Wang, T.; Liu, M.; Zheng, S.J.; Bian, D.D.; Zhang, J.Y.; Yao, J.; Zheng, Q.F.; Shi, A.M.; Li, W.H.; Li, L.; et al. Tumor-associated autoantibodies are useful biomarkers in immunodiagnosis of alpha-fetoprotein-negative hepatocellular carcinoma. World J. Gastroenterol. 2017, 23, 3496–3504. [Google Scholar] [CrossRef]
- Wang, S.; Qin, J.; Ye, H.; Wang, K.; Shi, J.; Ma, Y.; Duan, Y.; Song, C.; Wang, X.; Dai, L.; et al. Using a panel of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of gastric cancer. Oncoimmunology 2018, 7, e1452582. [Google Scholar] [CrossRef]
- Koziol, J.A.; Imai, H.; Dai, L.; Zhang, J.Y.; Tan, E.M. Early detection of hepatocellular carcinoma using autoantibody profiles from a panel of tumor-associated antigens. Cancer Immunol. Immunother. 2018, 67, 835–841. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Cheng, Q.; Wang, Y.; Qian, L.; Gao, J.; Zhu, J.Y. Genetic alterations and expression of PTEN and its relationship with cancer stem cell markers to investigate pathogenesis and to evaluate prognosis in hepatocellular carcinoma. J. Clin. Pathol. 2019, 72, 588–596. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, X.; Fei, M.; Hou, W.; Greshock, J.; Bachman, K.E.; Wooster, R.; Kang, J.; Qin, C.Y. Combined phosphatase and tensin homolog (PTEN) loss and fatty acid synthase (FAS) overexpression worsens the prognosis of Chinese patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2012, 13, 9980–9991. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Qiu, S.; Wang, K.; Liu, S.; Peng, X.X.; Li, J.; Tan, E.M.; Zhang, J.Y. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010, 289, 32–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Middleton, C.H.; Irving, W.; Robertson, J.F.; Murray, A.; Parsy-Kowalska, C.B.; Macdonald, I.K.; McElveen, J.; Allen, J.; Healey, G.F.; Thomson, B.J.; et al. Serum autoantibody measurement for the detection of hepatocellular carcinoma. PLoS ONE 2014, 9, e103867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Qin, J.J.; Ren, P.F.; Shi, J.X.; Xia, J.F.; Ye, H.; Wang, P.; Song, C.H.; Wang, K.J.; Zhang, J.Y. A panel of autoantibodies against multiple tumor-associated antigens in the immunodiagnosis of esophageal squamous cell cancer. Cancer Immunol. Immunother. 2016, 65, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, S.; Shi, J.; Ma, Y.; Wang, K.; Ye, H.; Zhang, X.; Wang, P.; Wang, X.; Song, C.; et al. Using recursive partitioning approach to select tumor-associated antigens in immunodiagnosis of gastric adenocarcinoma. Cancer Sci. 2019, 110, 1829–1841. [Google Scholar] [CrossRef]
- Ma, Y. Based on Protein Microarray to Screen Ovarian Cancer Associated Antigens and the Diagnostic Value of Its Corresponding Autoantibodies. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2019. [Google Scholar]


| Characteristics | Verification Dataset | Training Dataset | Validation Dataset | LC (n = 127) | |||
|---|---|---|---|---|---|---|---|
| HCC (n = 100) | NC (n = 50) | HCC (n = 316) | NC (n = 316) | HCC (n = 133) | NC (n = 133) | ||
| Male, n (%) | 79 (81.4) | 23 (46.0) | 260 (82.3) | 245 (77.5) | 98 (73.7) | 96 (72.2) | 87 (68.5) |
| Age | |||||||
| Mean ± SD | 40.5 ± 13.0 | 56.8 ± 9.3 | 55.4 ± 12.5 | 56.7 ± 11.4 | 55.9 ± 10.0 | 56.1 ± 10.0 | 52.0 ± 11.1 |
| Age range | 37.0−77.5 | 20.0−71.0 | 24.0−87.0 | 32.5−88.0 | 26.0−79.0 | 34.0−80.0 | 24.0−78.0 |
| TNM, n (%) | |||||||
| I | 35 (36.1) | IA | 28 (8.9) | IA | 13 (9.8) | IA | IA |
| II | 19 (19.6) | IA | 63 (19.9) | IA | 21 (15.8) | IA | IA |
| III | 26 (26.8) | IA | 129 (40.8) | IA | 62 (46.6) | IA | IA |
| IV | 17 (17.5) | IA | 60 (19.0) | IA | 22 (16.5) | IA | IA |
| NA | 0 (0) | IA | 36 (11.4) | IA | 15 (11.3) | IA | IA |
| Metastasis, n (%) | |||||||
| None | 83 (85.6) | IA | 263 (83.2) | IA | 112 (84.2) | IA | IA |
| With | 14 (14.4) | IA | 53 (16.8) | IA | 21 (15.8) | IA | IA |
| AFP, n (%) | |||||||
| ≥20 ng/mL | 57 (58.8) | 0 (0) | 161 (50.9) | 2 (0.6) | 76 (57.1) | 0 (0) | 52 (40.9) |
| <20 ng/mL | 40 (41.2) | 50 (100) | 113 (35.8) | 259 (82.0) | 57 (42.9) | 73 (54.9) | 75 (59.1) |
| NA | 0 (0) | 0 (0) | 42 (13.3) | 55 (17.4) | 0 (0) | 60 (45.1) | 0 (0) |
| Markers | AUC (95%CI) | Se (%) | Sp (%) | Youden’s Index | PLR | NLR | PPV (%) | NPV (%) | Accuracy (%) | Kappa |
|---|---|---|---|---|---|---|---|---|---|---|
| Training dataset | ||||||||||
| PP | 0.831 (0.800, 0.860) | 70.6 | 77.8 | 0.484 | 3.186 | 0.378 | 76.1 | 72.6 | 74.2 | 0.484 |
| AFP | 0.860 (0.826, 0.894) | 58.8 | 99.2 | 0.580 | 76.681 | 0.416 | 98.8 | 69.6 | 78.5 | 0.574 |
| PP + AFP | 0.929 (0.908, 0.951) | 81.0 | 92.3 | 0.734 | 10.573 | 0.206 | 91.7 | 82.3 | 86.5 | 0.731 |
| Validation dataset | ||||||||||
| PP | 0.789 (0.736, 0.842) | 51.9 | 85.7 | 0.376 | 3.632 | 0.561 | 78.4 | 64.0 | 68.8 | 0.376 |
| AFP | 0.800 (0.738, 0.861) | 57.1 | 100 | 0.571 | + ∞ | 0.429 | 100 | 56.2 | 72.3 | 0.486 |
| PP + AFP | 0.891 (0.847, 0.934) | 74.4 | 97.3 | 0.717 | 27.169 | 0.263 | 98.0 | 67.6 | 82.5 | 0.652 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, M.; Qin, J.; Sun, G.; Dai, L.; Wang, P.; Ye, H.; Shi, J.; Cheng, L.; Yang, Q.; et al. Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes. Cancers 2020, 12, 1271. https://doi.org/10.3390/cancers12051271
Wang K, Li M, Qin J, Sun G, Dai L, Wang P, Ye H, Shi J, Cheng L, Yang Q, et al. Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes. Cancers. 2020; 12(5):1271. https://doi.org/10.3390/cancers12051271
Chicago/Turabian StyleWang, Keyan, Miao Li, Jiejie Qin, Guiying Sun, Liping Dai, Peng Wang, Hua Ye, Jianxiang Shi, Lin Cheng, Qian Yang, and et al. 2020. "Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes" Cancers 12, no. 5: 1271. https://doi.org/10.3390/cancers12051271
APA StyleWang, K., Li, M., Qin, J., Sun, G., Dai, L., Wang, P., Ye, H., Shi, J., Cheng, L., Yang, Q., Qiu, C., Jiang, D., Wang, X., & Zhang, J. (2020). Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes. Cancers, 12(5), 1271. https://doi.org/10.3390/cancers12051271





