PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells
Abstract
1. Introduction
2. Results
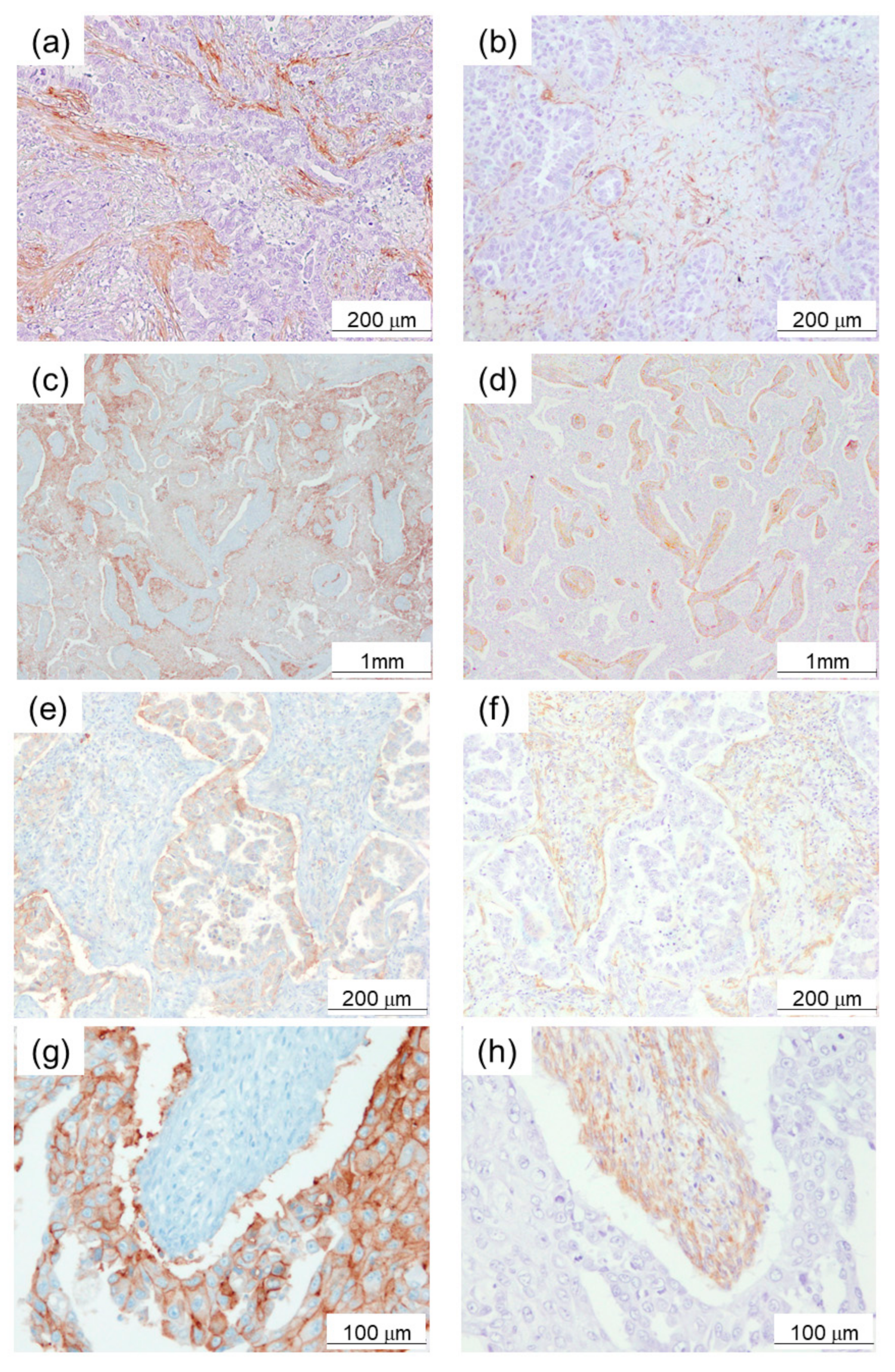
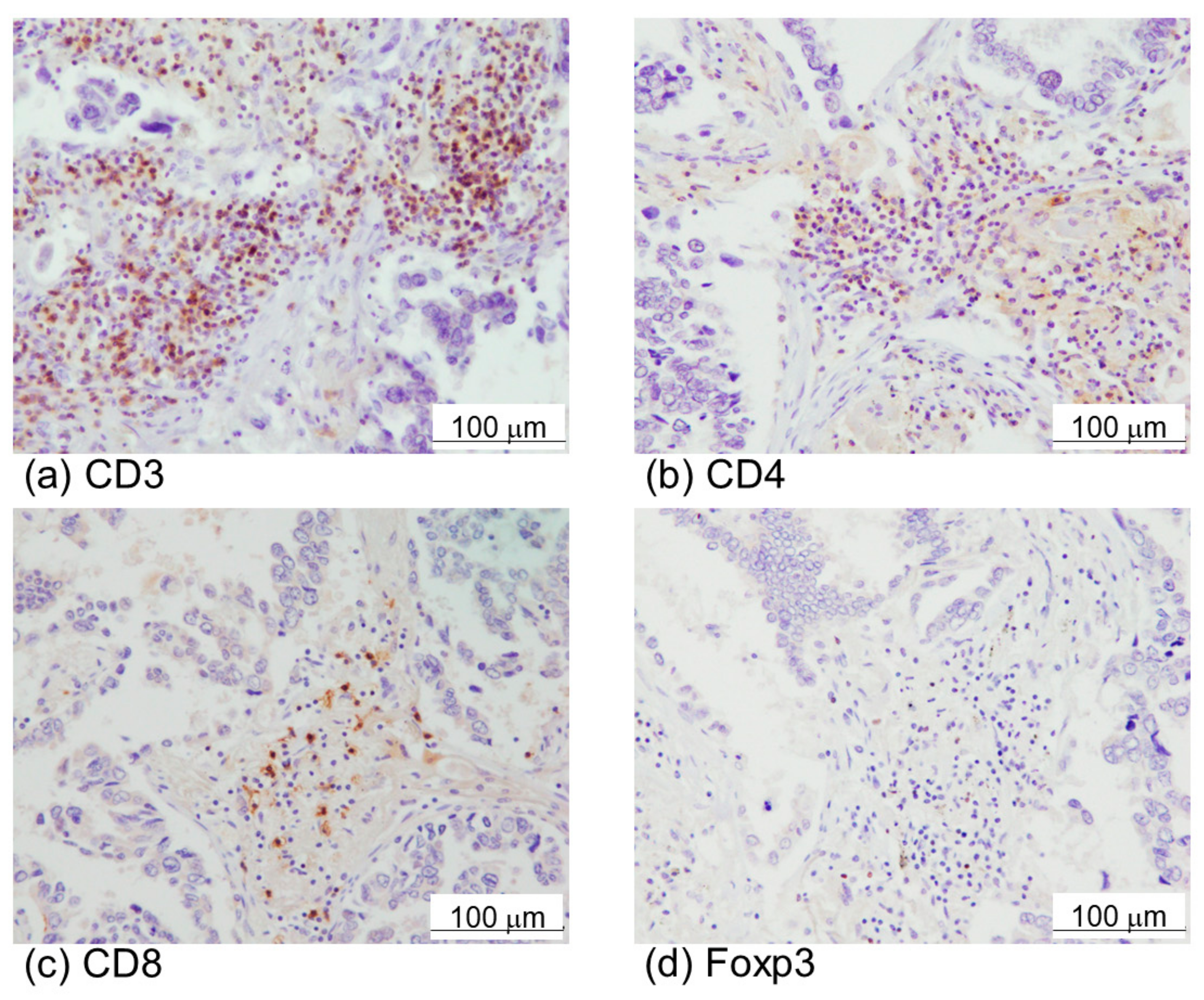
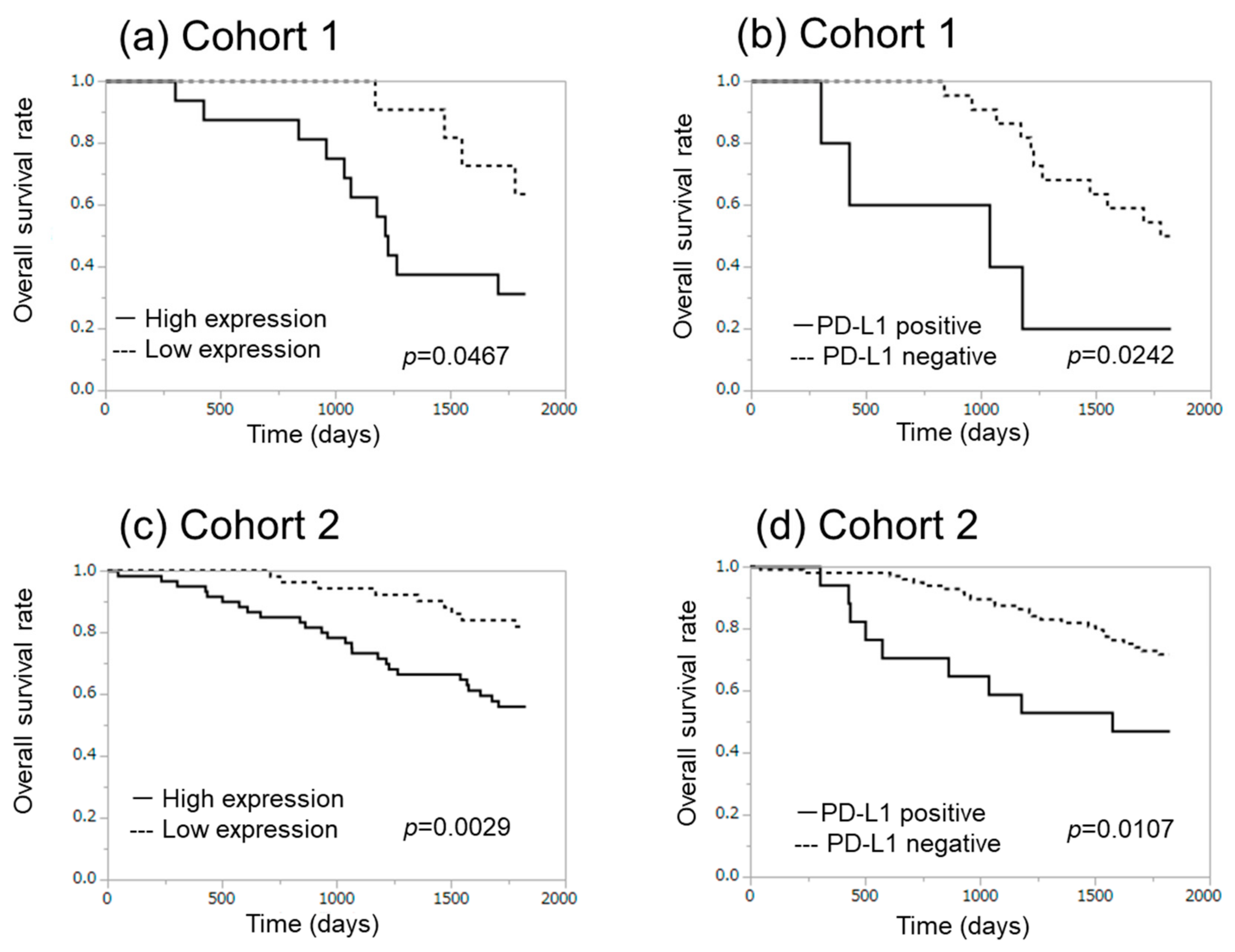
2.1. α-SMA Status in Stromal Area of Lung Cancer Was Significantly Associated with PD-L1 Status in Carcinoma Cells and Adverse Clinical Outcome of the Patients
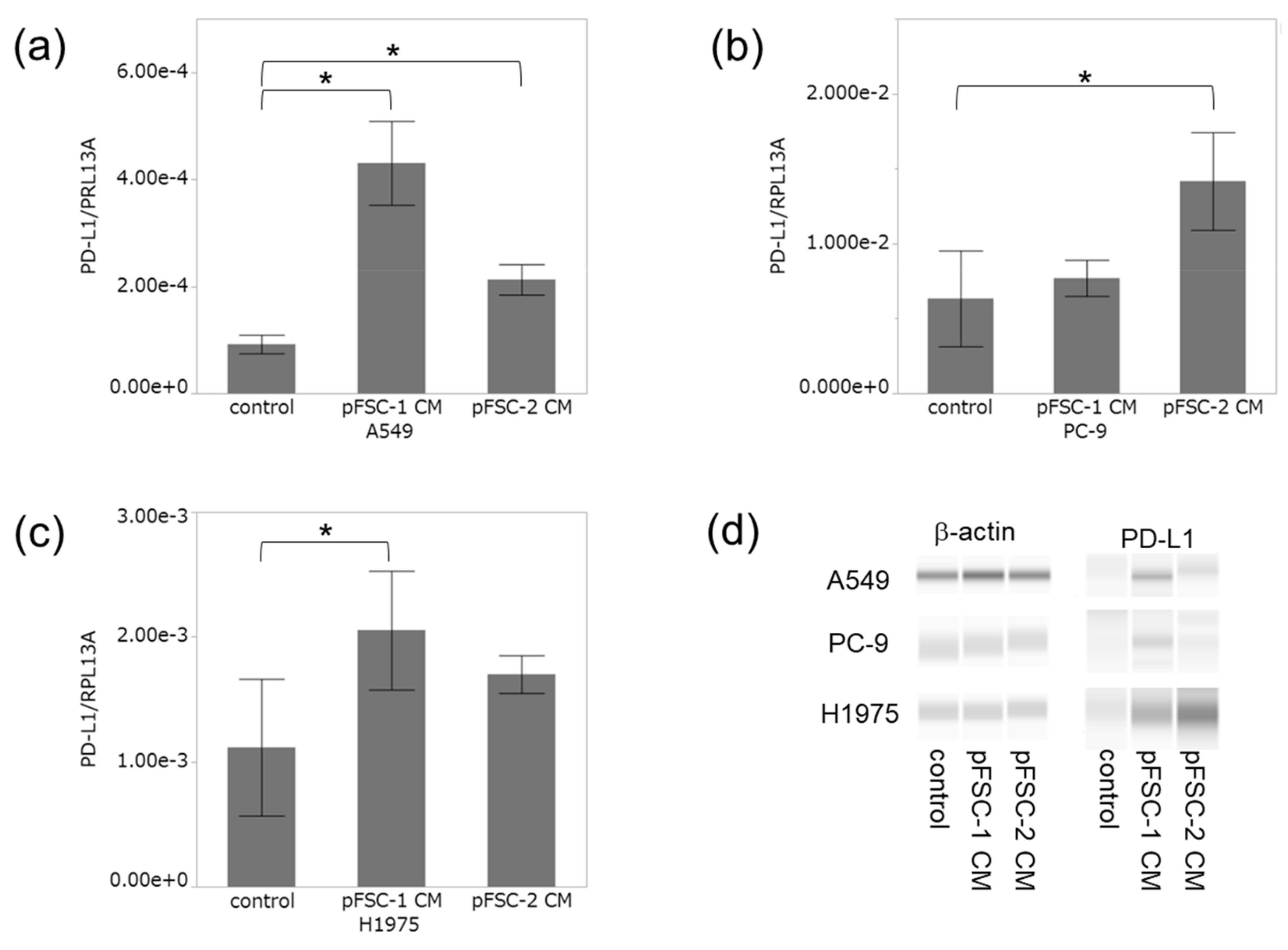
2.2. Conditioned Medium of CAFs Significantly Increased PD-L1 Expression at Both mRNA and Protein Levels
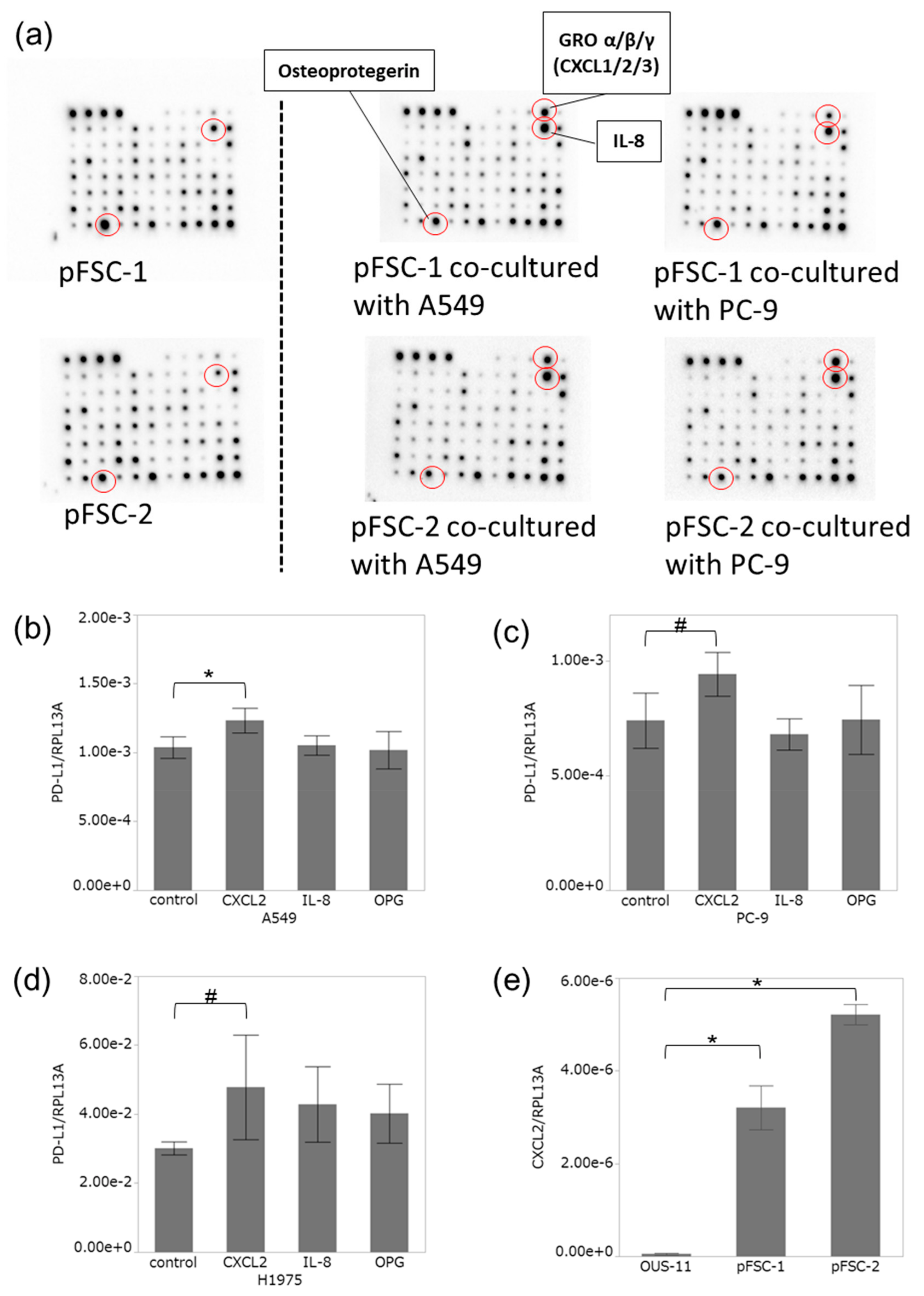
2.3. Profiles of Cytokines Secreted from CAFs Were Altered by Co-Culture with Adenocarcinoma Cells
2.4. CXCL2 Increased PD-L1 mRNA in Adenocarcinoma Cells
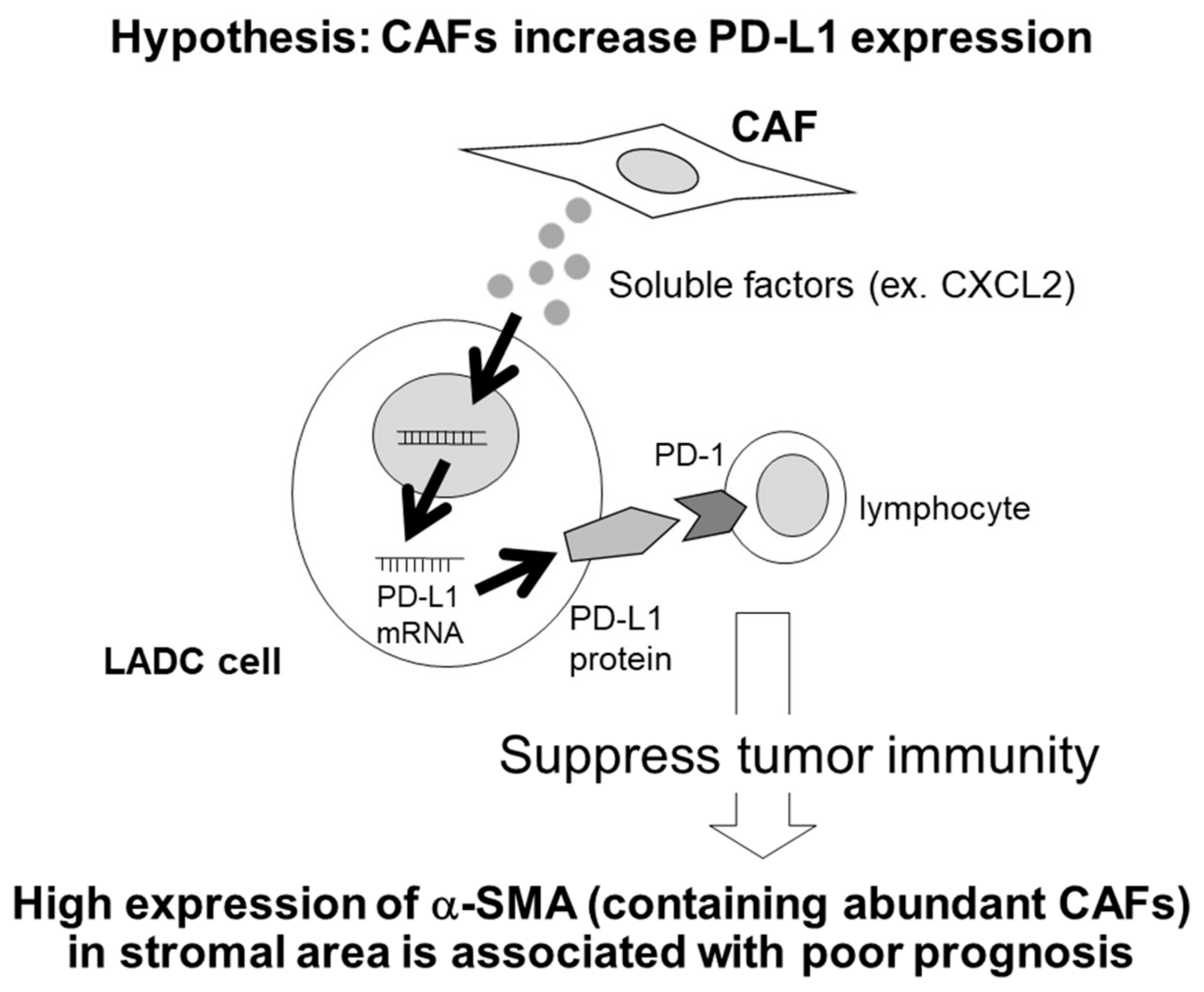
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Cell Lines
4.4. Immunocytochemistry
4.5. Conditioned Medium
4.6. Quantitative RT-PCR
4.7. Capillary Electrophoresis Immunoassay
4.8. Co-Culture System
4.9. Cytokine Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Saito, A.; Mikami, Y.; Ohshima, M.; Morishita, Y.; Nakajima, J.; Kohyama, T.; Nagase, T. Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem. Biophys. Res. Commun. 2012, 423, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Mahale, J.; Smagurauskaite, G.; Brown, K.; Thomas, A.; Howells, L.M. The role of stromal fibroblasts in lung carcinogenesis: A target for chemoprevention? Int. J. Cancer 2016, 138, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Q.; Yamada, T.; Matsumoto, K.; Matsumoto, I.; Oda, M.; Watanabe, G.; Kayano, Y.; Nishioka, Y.; Sone, S.; et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res. 2009, 15, 6630–6638. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ishii, G.; Goto, K.; Neri, S.; Hashimoto, H.; Yoh, K.; Niho, S.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin. Cancer Res. 2015, 21, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Sainson, R.C.A. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin. Cancer Biol. 2014, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J.; Yokota, S.J.; Bankert, R.B. Characterization of Human Lung Tumor-Associated Fibroblasts and Their Ability to Modulate the Activation of Tumor-Associated T Cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Luo, Y.; Markowitz, D.; Xiang, R.; Reisfeld, R.A. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE 2009, 4, e7965. [Google Scholar] [CrossRef] [PubMed]
- Viguier, M.; Lemaître, F.; Verola, O.; Cho, M.S.; Gorochov, G.; Dubertret, L.; Bachelez, H.; Kourilsky, P.; Ferradini, L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004, 173, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dong, X. Colorectal cancer-derived Foxp3+IL-17+ T cells suppress tumour-specific CD8+ T cells. Scand. J. Immunol. 2011, 74, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Shukuya, T.; Carbone, D.P. Predictive markers for the efficacy of anti-PD-1/PD-L1 antibodies in lung cancer. J. Thorac. Oncol. 2016, 11, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 expression in lung cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [PubMed]
- Ji, M.; Liu, Y.; Li, Q.; Li, X.D.; Zhao, W.Q.; Zhang, H.; Zhang, X.; Jiang, J.T.; Wu, C.P. PD–1/PD–L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J. Transl. Med. 2015, 13, 5. [Google Scholar] [PubMed]
- Igarashi, T.; Teramoto, K.; Ishida, M.; Hanaoka, J.; Daigo, Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open 2016, 1, e000083. [Google Scholar]
- Wu, S.; Shi, X.; Sun, J.; Liu, Y.; Luo, Y.; Liang, Z.; Wang, J.; Zeng, X. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget 2017, 8, 16421–16429. [Google Scholar]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar]
- Lastwika, K.J.; Wilson, W.; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007, 110, 296–304. [Google Scholar] [CrossRef]
- Li, C.; Lim, S.; Xia, W.; Lee, H.; Chan, L.; Kuo, C.; Khoo, K.; Chang, S.; Cha, J.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 1–11. [Google Scholar]
- Mezzadra, R.; Sun, C.; Jae, L.T.; Gomez-Eerland, R.; de Vries, E.; Wu, W.; Logtenberg, M.E.W.; Slagter, M.; Rozeman, E.A.; Hofland, I.; et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017, 549, 106–110. [Google Scholar] [PubMed]
- Liu, L.; Liu, L.; Yao, H.H.; Zhu, Z.Q.; Ning, Z.L.; Huang, Q. Stromal myofibroblasts are associated with poor prognosis in solid cancers: A meta-analysis of published studies. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, L.; Zhang, Y.; Chen, Y.; Xing, P.; Yang, W.; Li, F.; Ji, X.; Liu, F.; Lu, X. Transforming growth factor-β1 and α-smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I-IIIA nonsmall cell lung cancer after curative resection. Tumor. Biol. 2014, 35, 6707–6713. [Google Scholar]
- Kilvaer, T.K.; Khanehkenari, M.R.; Hellevik, T.; Al-Saad, S.; Paulsen, E.E.; Bremnes, R.M.; Busund, L.T.; Donnem, T.; Martinez, I.Z. Cancer associated fibroblasts in stage I-IIIA NSCLC: Prognostic impact and their correlations with tumor molecular markers. PLoS ONE 2015, 10, 1–15. [Google Scholar]
- Cheng, J.; Deng, Y.; Yi, H.; Wang, G.; Fu, B.; Chen, W.; Liu, W.; Tai, Y.; Peng, Y.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar]
- He, J.; Hu, Y.; Hu, M.; Li, B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2015, 5, 13110. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential Roles of CXCL2 and CXCL3 and Their Receptors in Regulating Normal and Asthmatic Airway Smooth Muscle Cell Migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Belloso, S.P.; Rosete, P.G.; Alvarado-Vásquez, N.; Aquino-Jarquin, G. Role of chemokines in non-small cell lung cancer: Angiogenesis and inflammation. J. Cancer 2015, 6, 938–952. [Google Scholar]
- Song, X.; Wang, Z.; Jin, Y.; Wang, Y.; Duan, W. Loss of miR-532-5p in vitro promotes cell proliferation and metastasis by influencing CXCL2 expression in HCC. Am. J. Transl. Res. 2015, 7, 2254–2261. [Google Scholar] [PubMed]
- Milara, J.; Serrano, A.; Peiró, T.; Artigues, E.; Gavaldà, A.; Miralpeix, M.; Morcillo, E.J.; Cortijo, J. Aclidinium inhibits cigarette smoke-induced lung fibroblast-to- myofibroblast transition. Eur. Respir. J. 2013, 41, 1264–1274. [Google Scholar] [PubMed]
- Cañas, M.; Zuazo, M.; Arasanz, H.; Ibañez-Vea, M.; Lorenzo, L.; Fernandez-Hinojal, G.; Vera, R.; Smerdou, C.; Martisova, E.; Arozarena, I.; et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017, 20, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.B.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma- associated fibroblasts synergizes with anti – PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, J.; Lu, K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 239–244. [Google Scholar] [CrossRef]
- Tsujino, T.; Seshimo, I.; Yamamoto, H.; Chew, Y.N.; Ezumi, K.; Takemasa, I.; Ikeda, M.; Sekimoto, M.; Matsuura, N.; Monden, M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin. Cancer Res. 2007, 13, 2082–2090. [Google Scholar] [CrossRef]
- Yamashita, M.; Ogawa, T.; Zhang, X.; Hanamura, N.; Kashikura, Y.; Takamura, M.; Yoneda, M.; Shiraishi, T. Role of stromal myofibroblasts in invasive breast cancer: Stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer 2012, 19, 170–176. [Google Scholar]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Chan, M.; Hirakawa, H.; Suzuki, A.; Tada, H.; Watanabe, G.; Nemoto, N.; Nakagawa, S.; et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: Its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2014, 148, 525–534. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Miki, Y.; Suzuki, T.; Abe, K.; Suzuki, S.; Niikawa, H.; Iida, S.; Hata, S.; Akahira, J.I.; Mori, K.; Evans, D.B.; et al. Intratumoral localization of aromatase and interaction between stromal and parenchymal cells in the non-small cell lung carcinoma microenvironment. Cancer Res. 2010, 70, 6659–6669. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Suzuki, A.; Shitara, M.; Hikosaka, Y.; Okuda, K.; Moriyama, S.; Yano, M.; Fujii, Y. PD-L1 gene expression in Japanese lung cancer patients. Biomed. Rep. 2013, 1, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Molaeipour, Z.; Shamsasanjan, K.; Movassaghpour, A.A.; Akbarzadehlaleh, P.; Sabaghi, F.; Saleh, M. The Effect of Bone Marrow Mesenchymal Stem Cells on Vitamin D3 Induced Monocytic Differentiation of U937 Cells. Adv. Pharm. Bull. 2016, 6, 23–29. [Google Scholar] [CrossRef] [PubMed]
| Total | α-SMA | α-SMA | p-Value | ||
|---|---|---|---|---|---|
| High | Low | ||||
| Age | median | 64 | 63 | 64 | 0.9803 |
| (years) | max | 80 | 80 | 74 | |
| min | 34 | 34 | 46 | ||
| Sex | male | 8 | 6 | 2 | |
| female | 19 | 10 | 9 | ||
| Smoking | smoker | 6 | 5 | 1 | 0.3497 |
| never | 21 | 11 | 10 | ||
| Brinkman index | median | 0† | 0† | 0† | 0.1646 |
| max | 1200 | 1200 | 700 | ||
| min | 0 | 0 | 0 | ||
| Size of tumor | median | 28 | 27 | 30.5 | 0.9069 |
| (mm) | max | 80 | 80 | 40 | |
| min | 13 | 15 | 13 | ||
| EGFR | exon 19 del | 12 | 3 | 9 | 0.3172 |
| mutation | exon 21 L858R | 11 | 6 | 5 | |
| G719X, S768I | 1 | 1 | 0 | ||
| ex20 Ins | 1 | 0 | 1 | ||
| unknown | 2 | 1 | 1 | ||
| Response to | CR | 1 | 1 | 0 | 0.7354 |
| EGFR-TKI | PR | 17 | 10 | 7 | |
| SD | 8 | 4 | 4 | ||
| PD | 1 | 1 | 0 | ||
| Ki-67 LI of carcinoma cells (%) | median | 12.3 | 13.1 | 10.9 | 0.5053 |
| max | 58.8 | 58.8 | 32 | ||
| min | 3.3 | 3.3 | 4.7 | ||
| pStage | I | 9 | 2 | 7 | 0.0263* |
| II | 5 | 4 | 1 | ||
| III | 10 | 8 | 2 | ||
| IV | 3 | 2 | 1 | ||
| pT | 1 | 13 | 7 | 6 | 0.6146 |
| 2 | 11 | 7 | 4 | ||
| 3 | 1 | 1 | 0 | ||
| 4 | 2 | 1 | 1 | ||
| pN | 0 | 13 | 5 | 8 | 0.0514# |
| 1 | 5 | 4 | 1 | ||
| 2 | 9 | 7 | 2 | ||
| 3 | 0 | 0 | 0 | ||
| cM | 0 | 24 | 14 | 10 | 1.000 |
| 1 | 3 | 2 | 1 | ||
| PD-L1 | positive | 5 | 5 | 0 | 0.0598# |
| negative | 22 | 11 | 11 | ||
| CD3 | median | 766 | 758 | 767 | 0.941 |
| max | 1656 | 1656 | 1022 | ||
| min | 404 | 404 | 462 | ||
| CD4 | median | 697 | 693.5 | 697 | 0.2669 |
| max | 1486 | 1486 | 806 | ||
| min | 263 | 303 | 263 | ||
| CD8 | median | 416 | 432.5 | 399 | 0.7484 |
| max | 611 | 599 | 611 | ||
| min | 177 | 209 | 177 | ||
| Foxp3 | median | 518 | 110.5 | 111 | 0.9214 |
| max | 111 | 518 | 249 | ||
| min | 15 | 32 | 15 | ||
| Foxp3/CD8 | median | 0.3 | 0.28 | 0.31 | 0.941 |
| max | 1 | 1 | 0.89 | ||
| min | 0.03 | 0.12 | 0.03 | ||
| CD8/CD4 | median | 0.67 | 0.61 | 0.7 | 0.4443 |
| max | 1.3 | 1.03 | 1.3 | ||
| min | 0.26 | 0.33 | 0.26 |
| Total | α-SMA | α-SMA | p-Value | ||
|---|---|---|---|---|---|
| High | Low | ||||
| Age | median | 66 | 64.5 | 70 | 0.0872 |
| (years) | max | 82 | 80 | 82 | |
| min | 30 | 30 | 46 | ||
| Sex | male | 58 | 37 | 21 | 0.0241* |
| female | 55 | 23 | 32 | ||
| Smoking | smoker | 61 | 38 | 23 | 0.0391* |
| never | 52 | 22 | 30 | ||
| Brinkman index | median | 240 | 520 | 0† | 0.0131* |
| max | 1920 | 1840 | 1920 | ||
| min | 0 | 0 | 0 | ||
| Size of tumor | median | 25 | 24.5 | 27 | 0.2570 |
| (mm) | max | 80 | 80 | 70 | |
| min | 10 | 10 | 10 | ||
| pStage | I | 68 | 29 | 39 | 0.0022* |
| II | 13 | 7 | 6 | ||
| III | 24 | 17 | 7 | ||
| IV | 8 | 7 | 1 | ||
| pT | 1 | 65 | 34 | 31 | 0.7978 |
| 2 | 36 | 19 | 17 | ||
| 3 | 3 | 2 | 1 | ||
| 4 | 9 | 5 | 4 | ||
| pN | 0 | 85 | 38 | 47 | 0.0015* |
| 1 | 8 | 6 | 2 | ||
| 2 | 19 | 15 | 4 | ||
| 3 | 1 | 1 | 0 | ||
| cM | 0 | 105 | 53 | 52 | 0.0648# |
| 1 | 8 | 7 | 1 | ||
| PD-L1 | positive | 17 | 17 | 0 | <0.0001* |
| negative | 96 | 43 | 53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, C.; Miki, Y.; Saito, R.; Hata, S.; Abe, J.; Sato, I.; Okada, Y.; Sasano, H. PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers 2019, 11, 1257. https://doi.org/10.3390/cancers11091257
Inoue C, Miki Y, Saito R, Hata S, Abe J, Sato I, Okada Y, Sasano H. PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers. 2019; 11(9):1257. https://doi.org/10.3390/cancers11091257
Chicago/Turabian StyleInoue, Chihiro, Yasuhiro Miki, Ryoko Saito, Shuko Hata, Jiro Abe, Ikuro Sato, Yoshinori Okada, and Hironobu Sasano. 2019. "PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells" Cancers 11, no. 9: 1257. https://doi.org/10.3390/cancers11091257
APA StyleInoue, C., Miki, Y., Saito, R., Hata, S., Abe, J., Sato, I., Okada, Y., & Sasano, H. (2019). PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers, 11(9), 1257. https://doi.org/10.3390/cancers11091257





