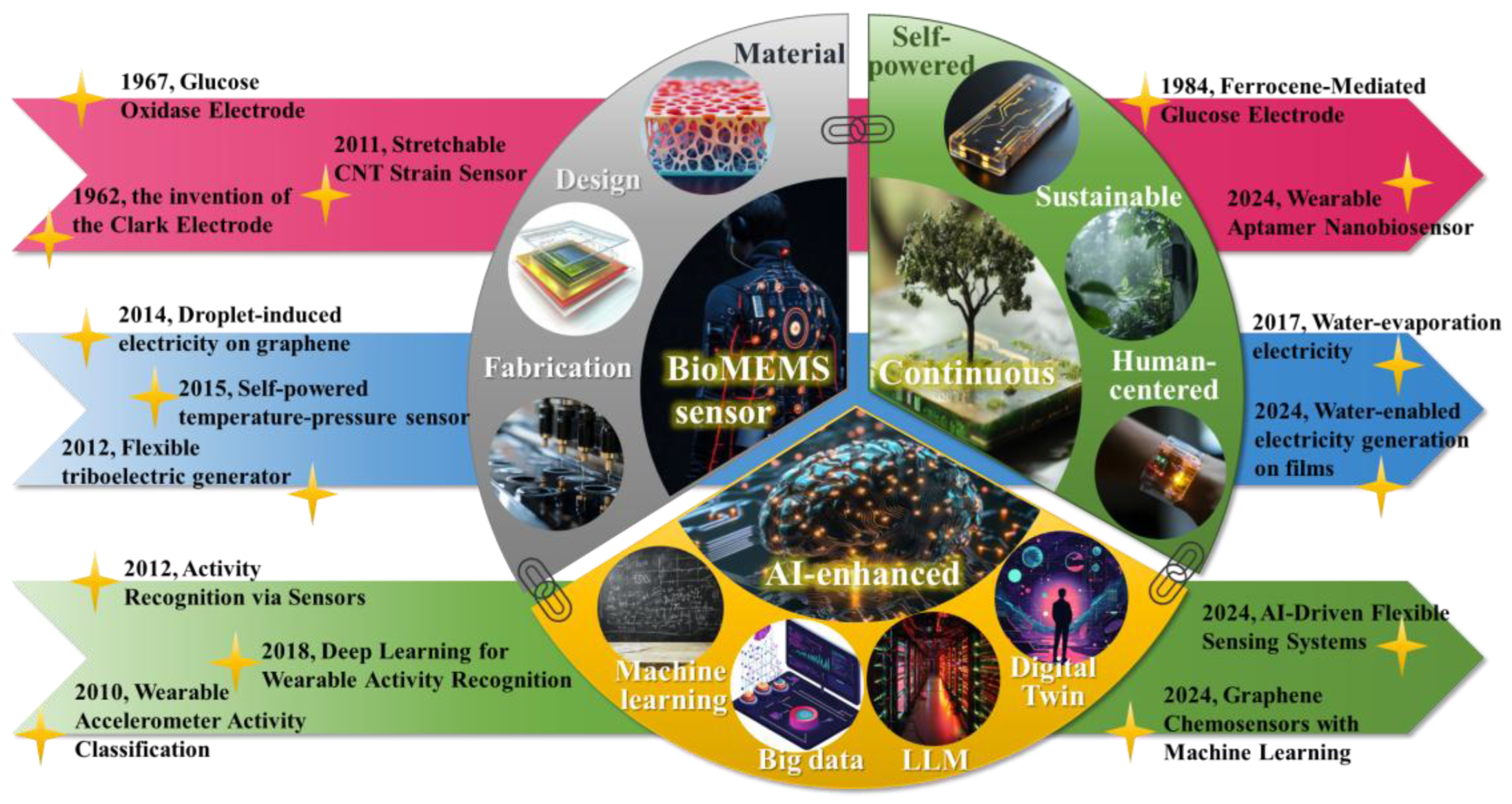
Continuous Monitoring with AI-Enhanced BioMEMS Sensors: A Focus on Sustainable Energy Harvesting and Predictive Analytics
Abstract
1. Introduction
2. BioMEMS Sensors for Continuous Monitoring
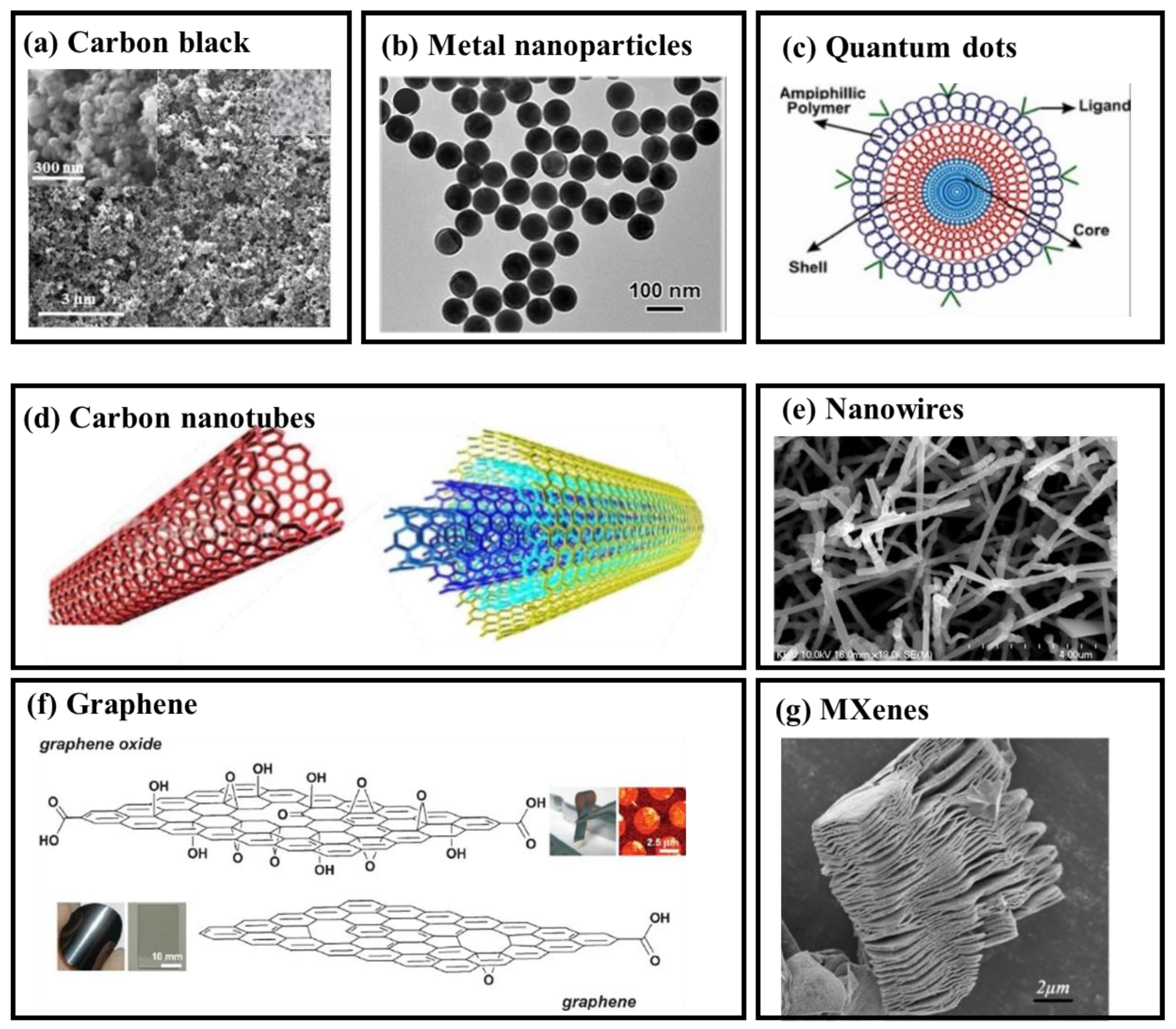
2.1. Nanomaterials
2.1.1. Particles
- Carbon Black
- Metal Nanoparticles
- Quantum Dots
2.1.2. Nanotubes, Nanowires and 2-D Materials
- Nanotubes
- Nanowires
- Graphene
- MXenes
2.2. Design and Fabrication
2.2.1. Design
2.2.2. Fabrication
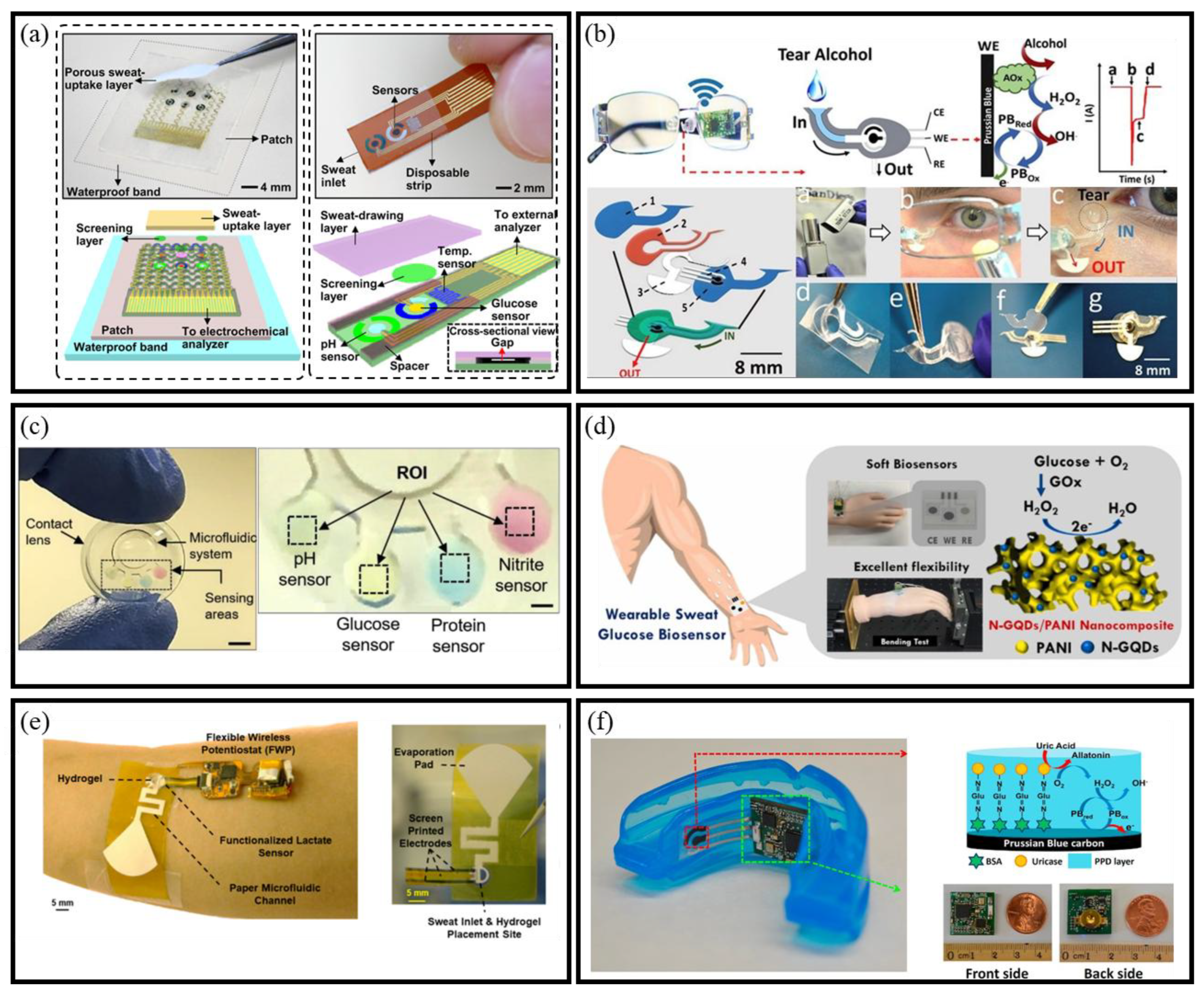
2.3. Monitoring Target
2.3.1. Glucose
2.3.2. Lactate Acid
2.3.3. Uric Acid
3. Sustainable Power Supply for BioMEMS Sensor
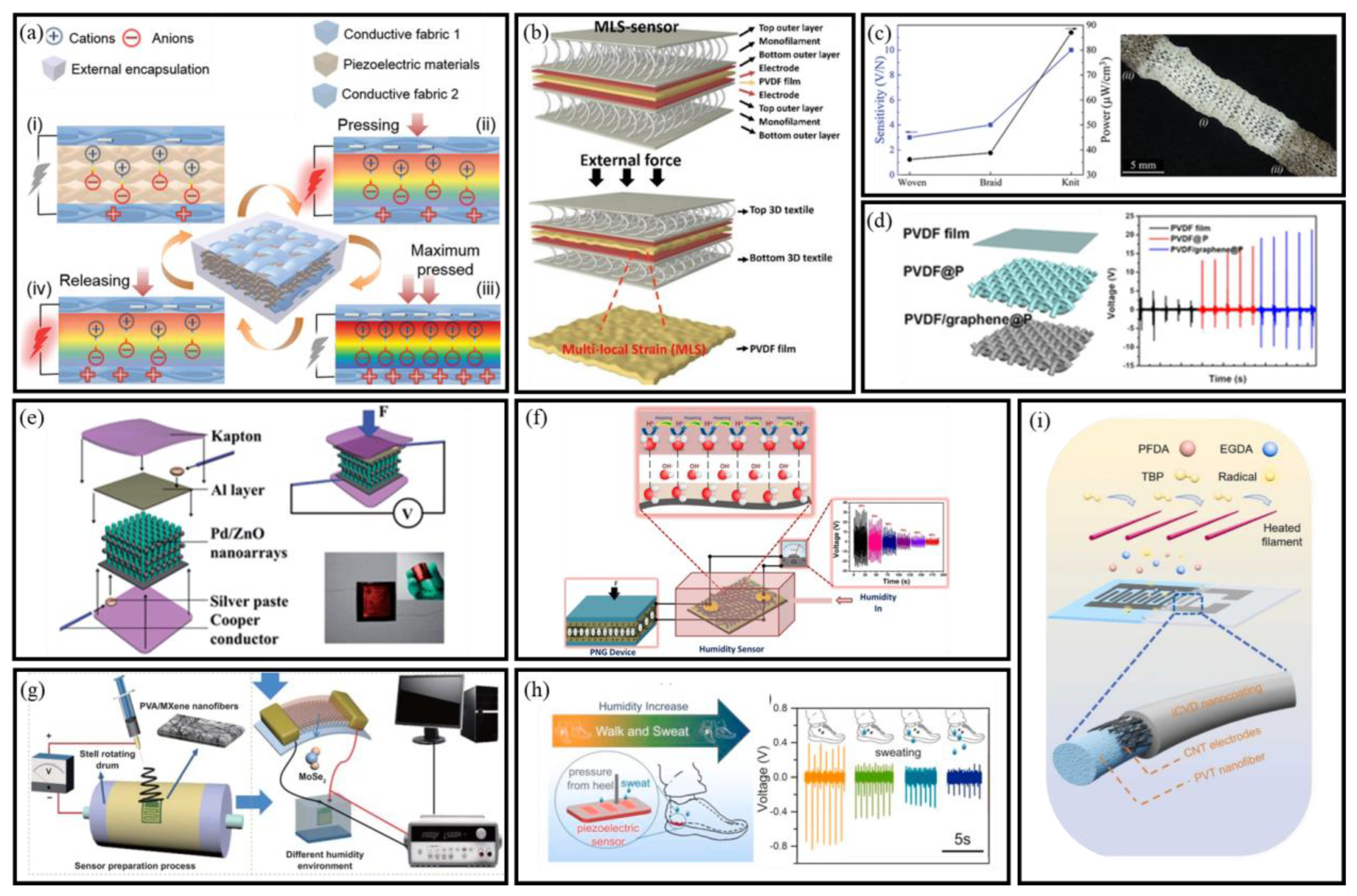
3.1. Piezoelectric Nanogenerators
3.1.1. Mechanism of PENGs
3.1.2. Architectures of PENGs
- Nanofiber Membrane-Based PENGs
- Yarn-Based Flexible PENGs
- Fabric-Based PENGs
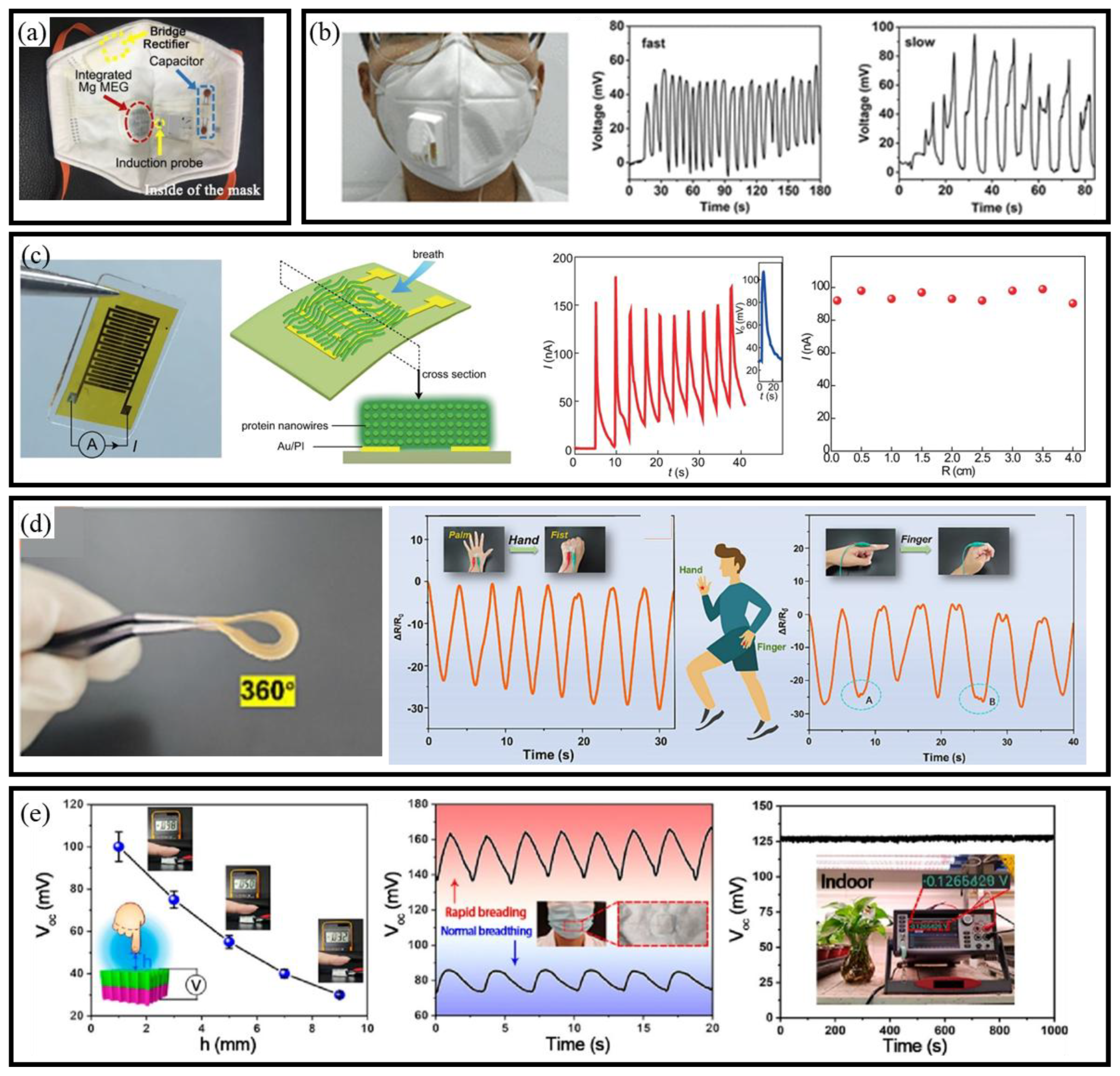
3.1.3. PENG-Based Self-Powered BioMEMS Sensors
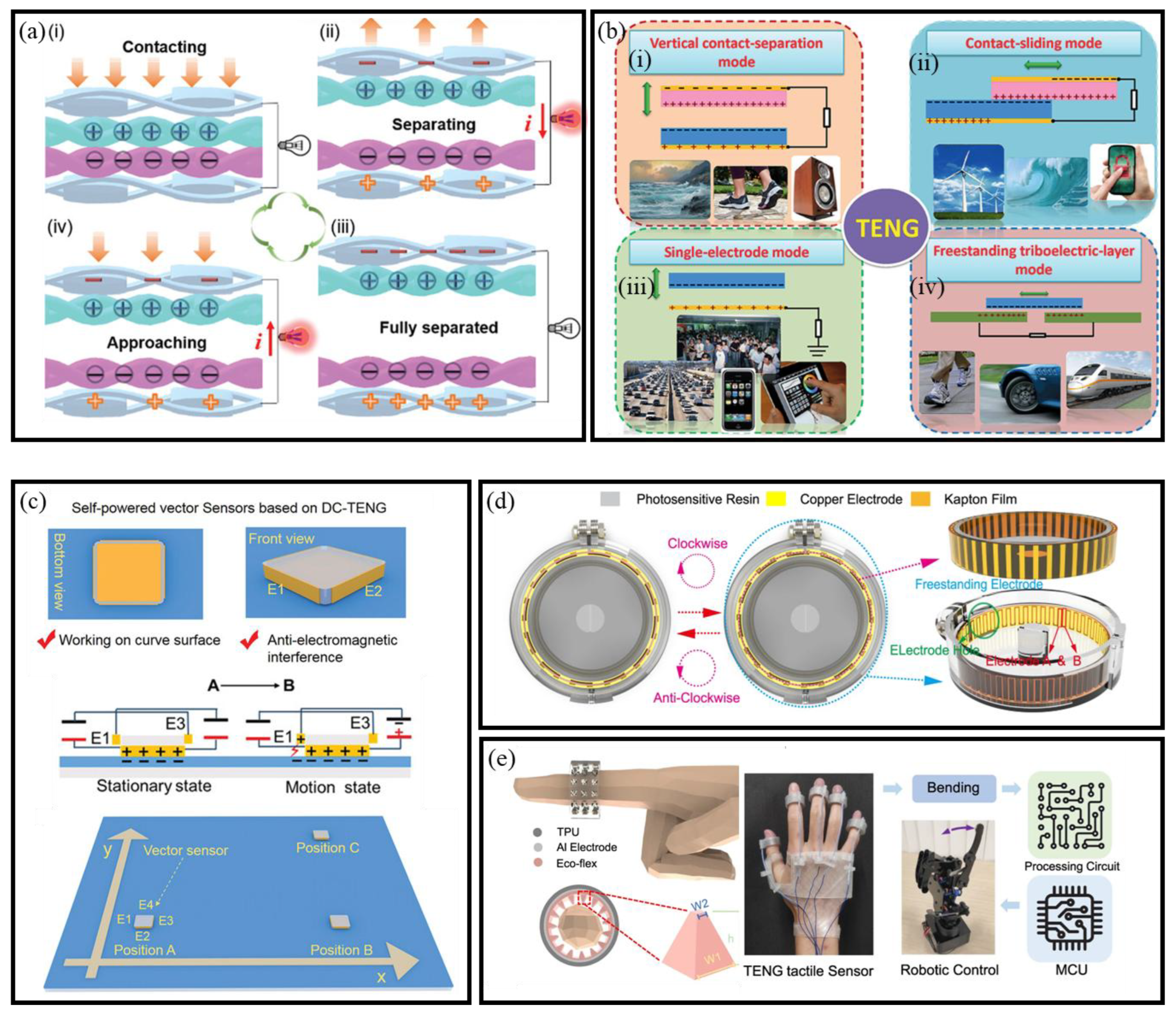
3.2. Triboelectric Nanogenerators
3.2.1. Mechanisms of TENGs
3.2.2. Working Modes of TENGs
- Vertical Contact Separation Mode
- Contact-sliding Mode
- Single-Electrode Mode
- Freestanding Triboelectric-Layer Mode
3.2.3. TENG-Based Self-Powered BioMEMS Sensors
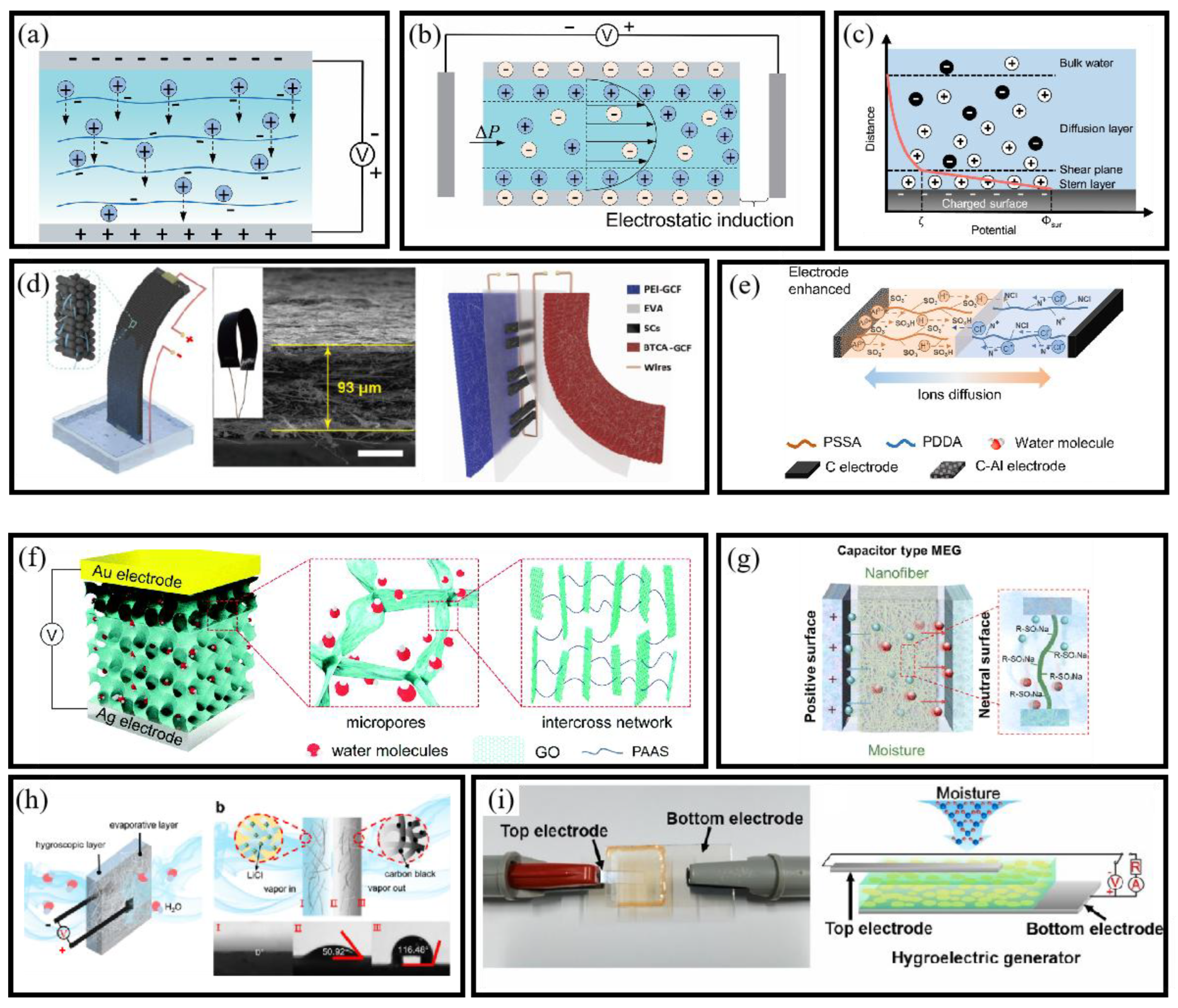
3.3. Moisture Electricity Generators
3.3.1. Mechanism of MEGs
- Ion Diffusion Mechanism
- Streaming Potential Mechanism
3.3.2. Structure of MEGs
- Planar Structure
- Sandwich Structure
- Heterogeneous Structure
- Asymmetrical Structure
3.3.3. MEG-Based Self-Powered BioMEMS Sensors
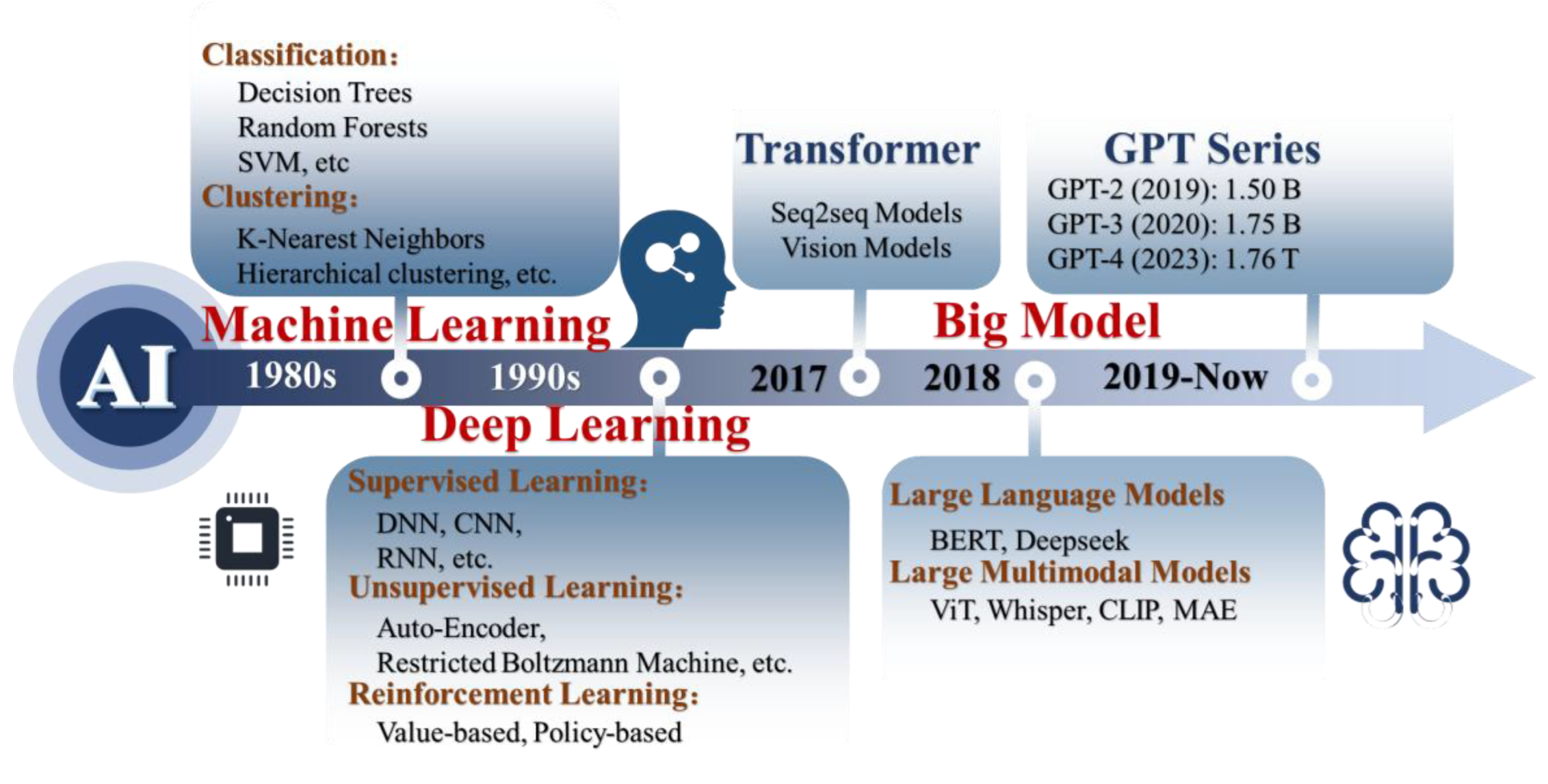
4. AI-Driven Strategies for Predictive BioMEMS Sensors
4.1. Machine Learning
4.1.1. Classic Machine Learning
- Non-Parametric Models
- Parametric Models
4.1.2. Deep Learning
- Supervised Learning
- Unsupervised Learning
- Reinforcement Learning
4.1.3. Big Model
- Large Language Models
- Large Multimodal Models
4.2. AI for BioMEMS Sensors
4.2.1. Data Interpretation
4.2.2. Predictive Analysis
4.2.3. Robustness and Interpretability in AI-Enhanced BioMEMS Sensors
4.3. Near-Sensor and In-Sensor Computing
4.3.1. Neuromorphic Devices and Near/In-Sensor Computing
- Near-sensor Computing
- In-sensor Computing
4.3.2. Near/In-Sensor Processing Applications
5. Challenges and Prospects
5.1. Energy and Computational Constraints
5.2. Material Challenges in Sensing and Harvesting Units
5.3. Integration and Deployment Barriers
5.4. Outlook: Toward Adaptive and Intelligent BioMEMS
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEGs | Moisture Electricity Generators |
| PENGs | Piezoelectric Nanogenerators |
| TENGs | Triboelectric Nanogenerators |
| BioMEMS | Bio-microelectromechanical systems |
| LLMs | Large Language Models |
| DNNs | Deep Neural Networks |
| NLP | Natural Language Processing |
| KNNs | K-Nearest Neighbors |
| LDA | Linear Discriminant Analysis |
| GO | Graphene Oxide |
| MXenes | Two-dimensional transition metal carbides, nitrides, and carbonitrides |
| CB | Carbon Black |
| MNPs | Metal Nanoparticles |
| QDs | Quantum Dots |
| CNTs | Carbon Nanotubes |
| PDMS | Polydimethylsiloxane |
| LOx | Lactate Oxidase |
| UA | Uric Acid |
| LLaMA | Large Language Model for Automatic Meta-Analysis |
| ViT | Vision Transformer |
| CLIP | Contrastive Language-Image Pre-training |
| MAE | Masked Autoencoder |
| GRU | Gated Recurrent Unit |
References
- Ferreira, L.M.C.; Silva, P.S.; Augusto, K.K.L.; Gomes, P.C.; Farra, S.O.D.; Silva, T.A.; Fatibello, O.; Vicentini, F.C. Using nanostructured carbon black-based electrochemical (bio)sensors for pharmaceutical and biomedical analyses: A comprehensive review. J. Pharm. Biomed. Anal. 2022, 221, 115032. [Google Scholar] [CrossRef] [PubMed]
- Malha, S.I.R.; Lahcen, A.A.; Arduini, F.; Ourari, A.; Amine, A. Electrochemical Characterization of Carbon Solid-like Paste Electrode Assembled Using Different Carbon Nanoparticles. Electroanalysis 2016, 28, 1044–1051. [Google Scholar] [CrossRef]
- Ibáñez-Redína, G.; Silva, T.A.; Vicentini, F.C.; Fatibello, O. Effect of carbon black functionalization on the analytical performance of a tyrosinase biosensor based on glassy carbon electrode modified with dihexadecylphosphate film. Enzym. Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef]
- Melios, N.; Tsouti, V.; Chatzandroulis, S.; Tsekenis, G. Development of an All-Carbon Electrochemical Biosensor on a Flexible Substrate for the Sensitive Detection of Glucose. Eng. Proc. 2022, 16, 17. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhou, F.; Zhang, T.; Zhang, H.; Li, X.; Duan, G.; Cai, W.; Li, Y. Rapid Synthesis of Monodisperse Au Nanospheres through a Laser Irradiation -Induced Shape Conversion, Self-Assembly and Their Electromagnetic Coupling SERS Enhancement. Sci. Rep. 2015, 5, 7686. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Ramezani, M.; Dehghani, A.; Sherif, M.M. Carbon nanotube reinforced cementitious composites: A comprehensive review. Constr. Build. Mater. 2022, 315, 125100. [Google Scholar] [CrossRef]
- Koo, J.; Kim, C. A new critical growth parameter of H2/CH4 gas flow ratio and mechanistic model for SiC nanowire synthesis via Si substrate carbonization. Sci. Rep. 2024, 14, 29629. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, S.S.; Gao, F.; Lin, J.H.; Liu, J.W.; Zheng, J.K. DNA-mediated growth of noble metal nanomaterials for biosensing applications. TrAC-Trends Anal. Chem. 2022, 148, 116533. [Google Scholar] [CrossRef]
- Thao, D.V.P.; Thuy Ngan, D.T.; Tuan, D.V.; Lan, H.; Thi Nguyet, N.; Thu, V.V.; Hung, V.-P.; Dinh Tam, P. Facile preparation of copper nanoparticles in environmentally friendly solvent for DNA sensor application. Mater. Today Commun. 2022, 33, 104161. [Google Scholar] [CrossRef]
- Castro, A.C.H.; Bezerra, I.R.S.; Pascon, A.M.; da Silva, G.H.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular Label-Free Electrochemical Biosensor Loading Nature-Inspired Peptide toward the Widespread Use of COVID-19 Antibody Tests. ACS Nano 2022, 16, 14239–14253. [Google Scholar] [CrossRef]
- Medrades, J.d.P.; Maciel, C.C.; Moraes, A.d.S.; Leite, F.d.L.; Ferreira, M. Flavin adenine dinucleotide functionalized gold nanoparticles for the electrochemical detection of dopamine. Sens. Actuators Rep. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Taghavi, F.; Moeinpour, F.; Khojastehnezhad, A.; Abnous, K.; Taghdisi, S.M. Recent applications of quantum dots in optical and electrochemical aptasensing detection of Lysozyme. Anal. Biochem. 2021, 630, 114334. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, M.; Mao, H.; Zhao, N.; He, D.; Chen, Q.; Liu, D.; Zhang, W.; Song, X.-M. A sensitive cholesterol electrochemical biosensor based on biomimetic cerasome and graphene quantum dots. Anal. Bioanal. Chem. 2022, 414, 3593–3603. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Dega, N.K.; Tran, H.L.; Darmonto, W.; Doong, R.-A. Application of sulfur-doped graphene quantum dots@ gold-carbon nanosphere for electrical pulse-induced impedimetric detection of glioma cells. Biosens. Bioelectron. 2021, 181, 113151. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon nanotube (CNT)-based biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- González-Hernández, J.; Moya-Alvarado, G.; Alvarado-Gámez, A.L.; Urcuyo, R.; Barquero-Quirós, M.; Arcos-Martínez, M.J. Electrochemical biosensor for quantitative determination of fentanyl based on immobilized cytochrome c on multi-walled carbon nanotubes modified screen-printed carbon electrodes. Microchim. Acta 2022, 189, 483. [Google Scholar] [CrossRef]
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical biosensors based on in situ grown carbon nanotubes on gold microelectrode array fabricated on glass substrate for glucose determination. Microchim. Acta 2023, 190, 55. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Paguay, J.; Fernández, L.; Bolaños-Méndez, D.; González, G.; Espinoza-Montero, P.J. Evaluation of an electrochemical biosensor based on carbon nanotubes, hydroxyapatite and horseradish peroxidase for the detection of hydrogen peroxide. Sens. Bio-Sens. Res. 2022, 37, 100514. [Google Scholar] [CrossRef]
- Ramanathan, K.; Bangar, M.A.; Yun, M.; Chen, W.; Myung, N.V.; Mulchandani, A. Bioaffinity sensing using biologically functionalized conducting-polymer nanowire. J. Am. Chem. Soc. 2005, 127, 496–497. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire-based biosensors. Anal. Chem. 2006, 78, 4260–4269. [Google Scholar] [CrossRef]
- Hakim, M.M.A.; Lombardini, M.; Sun, K.; Giustiniano, F.; Roach, P.L.; Davies, D.E.; Howarth, P.H.; de Planque, M.R.R.; Morgan, H.; Ashburn, P. Thin film polycrystalline silicon nanowire biosensors. Nano Lett. 2012, 12, 1868–1872. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzo, G.; Musumeci, P.; Fazio, B.; et al. New generation of ultrasensitive label-free optical Si nanowire-based biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irrera, A.; Priolo, F. Ultrasensitive label-and PCR-free genome detection based on cooperative hybridization of silicon nanowires optical biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Parlak, O.; Tiwari, A.; Turner, A.P.F.; Tiwari, A. Template-directed hierarchical self-assembly of graphene based hybrid structure for electrochemical biosensing. Biosens. Bioelectron. 2013, 49, 53–62. [Google Scholar] [CrossRef]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B: Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in electrochemical sensors: A review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor. J. Electrochem. Soc. 2014, 162, B16. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.-S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, Y.; Jiang, C.; Shao, Y.; Barceló, D.; Ying, Y.; Ping, J. Self-reduction bimetallic nanoparticles on ultrathin MXene nanosheets as functional platform for pesticide sensing. J. Hazard. Mater. 2020, 384, 121358. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; Garcia-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.; Li, X.; Sun, D.; Hu, T.; Xiang, N.; Ni, Z. Paper-based graphene oxide biosensor coupled with smartphone for the quantification of glucose in oral fluid. Biomed. Microdevices 2018, 20, 89. [Google Scholar] [CrossRef]
- Moreddu, R.; Wolffsohn, J.S.; Vigolo, D.; Yetisen, A.K. Laser-inscribed contact lens sensors for the detection of analytes in the tear fluid. Sens. Actuators B-Chem. 2020, 317, 128183. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Rinawati, M.; Chang, L.-Y.; Wang, Y.-X.; Wu, Y.-T.; Yen, Y.-H.; Chen, K.-J.; Ho, K.-C.; Yeh, M.-H. A non-invasive wearable sweat biosensor with a flexible N-GQDs/PANI nanocomposite layer for glucose monitoring. Sens. Actuators B: Chem. 2023, 383, 133617. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero- Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Wan, J.-j.; Qin, Z.; Wang, P.-y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Karpova, E.V.; Laptev, A.I.; Andreev, E.A.; Karyakina, E.E.; Karyakin, A.A. Relationship Between Sweat and Blood Lactate Levels During Exhaustive Physical Exercise. Chemelectrochem 2020, 7, 191–194. [Google Scholar] [CrossRef]
- Piedras, J.; Dominguez, R.B.; Gutierrez, J.M. Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode. Chemosensors 2021, 9, 73. [Google Scholar] [CrossRef]
- Moran, M.E. Uric acid stone disease. Front. Biosci.-Landmark 2003, 8, 1339–1355. [Google Scholar] [CrossRef]
- Falasca, G.F. Metabolic diseases: Gout. Clin. Dermatol. 2006, 24, 498–508. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Wu, J.; Wei, Q.; Chen, C.; Bao, N.; Yu, C.; Gu, H. An electrochemical biosensor based on multi-wall carbon nanotube-modified screen-printed electrode immobilized by uricase for the detection of salivary uric acid. Anal. Bioanal. Chem. 2020, 412, 7275–7283. [Google Scholar] [CrossRef]
- Wang, A.C.; Wu, C.; Pisignano, D.; Wang, Z.L.; Persano, L. Polymer nanogenerators: Opportunities and challenges for large-scale applications. J. Appl. Polym. Sci. 2018, 135, 45674. [Google Scholar] [CrossRef]
- Fan, F.R.; Tang, W.; Wang, Z.L. Flexible Nanogenerators for Energy Harvesting and Self-Powered Electronics. Adv. Mater. 2016, 28, 4283–4305. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Peng, X.; Wang, Z.L. Fiber/Fabric-Based Piezoelectric and Triboelectric Nanogenerators for Flexible/Stretchable and Wearable Electronics and Artificial Intelligence. Adv. Mater. 2020, 32, e1902549. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, W.; Xu, L.; Chen, W.; Zhu, Y.; Xu, C.; Kotov, N. Nanoparticle-based environmental sensors. Mater. Sci. Eng. R-Rep. 2010, 70, 265–274. [Google Scholar] [CrossRef]
- Ahn, S.; Cho, Y.; Park, S.; Kim, J.; Sun, J.; Ahn, D.; Lee, M.; Kim, D.; Kim, T.; Shin, H.; et al. Wearable multimode sensors with amplified piezoelectricity due to the multi local strain using 3D textile structure for detecting human body signals. Nano Energy 2020, 74, 104932. [Google Scholar] [CrossRef]
- Mokhtari, F.; Spinks, G.M.; Fay, C.; Cheng, Z.; Raad, R.; Xi, J.; Foroughi, J. Wearable Electronic Textiles from Nanostructured Piezoelectric Fibers. Adv. Mater. Technol. 2020, 5, 1900900. [Google Scholar] [CrossRef]
- Huang, T.; Yang, S.; He, P.; Sun, J.; Zhang, S.; Li, D.; Meng, Y.; Zhou, J.; Tang, H.; Liang, J.; et al. Phase-Separation-Induced PVDF/Graphene Coating on Fabrics toward Flexible Piezoelectric Sensors. ACS Appl. Mater. Interfaces 2018, 10, 30732–30740. [Google Scholar] [CrossRef]
- Lin, Y.; Deng, P.; Nie, Y.; Hu, Y.; Xing, L.; Zhang, Y.; Xue, X. Room-temperature self-powered ethanol sensing of a Pd/ZnO nanoarray nanogenerator driven by human finger movement. Nanoscale 2014, 6, 4604–4610. [Google Scholar] [CrossRef]
- Vivekananthan, V.; Alluri, N.R.; Purusothaman, Y.; Chandrasekhar, A.; Selvarajan, S.; Kim, S.-J. Biocompatible Collagen Nanofibrils: An Approach for Sustainable Energy Harvesting and Battery-Free Humidity Sensor Applications. ACS Appl. Mater. Interfaces 2018, 10, 18650–18656. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Li, P.; Yang, Z.; Mi, Q.; Yu, L. Electrospinning of Flexible Poly(vinyl alcohol)/MXene Nanofiber-Based Humidity Sensor Self-Powered by Monolayer Molybdenum Diselenide Piezoelectric Nanogenerator. Nano-Micro Lett. 2021, 13, 57. [Google Scholar] [CrossRef]
- Wang, S.; Shao, H.-Q.; Liu, Y.; Tang, C.-Y.; Zhao, X.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. Boosting piezoelectric response of PVDF-TrFE via MXene for self-powered linear pressure sensor. Compos. Sci. Technol. 2021, 202, 108600. [Google Scholar] [CrossRef]
- Su, C.; Huang, X.; Zhang, L.; Zhang, Y.; Yu, Z.; Chen, C.; Ye, Y.; Guo, S. Robust superhydrophobic wearable piezoelectric nanogenerators for self-powered body motion sensors. Nano Energy 2023, 107, 108095. [Google Scholar] [CrossRef]
- Yang, T.; Pan, H.; Tian, G.; Zhang, B.; Xiong, D.; Gao, Y.; Yan, C.; Chu, X.; Chen, N.; Zhong, S.; et al. Hierarchically structured PVDF/ZnO core-shell nanofibers for self-powered physiological monitoring electronics. Nano Energy 2020, 72, 104706. [Google Scholar] [CrossRef]
- Shahzad, A.; Chen, Z.; Haidary, A.A.; Mehmood, A.; Khan, Z.M. Piezoelectric pressure sensors based on GO-modified P(VDF-TrFE) fibers for vacuum applications. J. Mater. Sci. Mater. Electron. 2020, 31, 18627–18639. [Google Scholar] [CrossRef]
- Qu, Z.; Fu, Y.; Yu, B.; Deng, P.; Xing, L.; Xue, X. High and fast H2S response of NiO/ZnO nanowire nanogenerator as a self-powered gas sensor. Sens. Actuators B Chem. 2016, 222, 78–86. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Wee, M.G.V.; Chinnappan, A.; Ramakrishna, S. A Self-Powered Piezoelectric Nanofibrous Membrane as Wearable Tactile Sensor for Human Body Motion Monitoring and Recognition. Adv. Fiber Mater. 2023, 5, 1417–1430. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator! Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z. Triboelectric nanogenerators as new energy technology and self-powered sensors—Principles, problems and perspectives. Faraday Discuss. 2014, 176, 447–458. [Google Scholar] [CrossRef]
- Yin, X.; Liu, D.; Zhou, L.; Li, X.; Xu, G.; Liu, L.; Li, S.; Zhang, C.; Wang, J.; Wang, Z.L. A Motion Vector Sensor via Direct-Current Triboelectric Nanogenerator. Adv. Funct. Mater. 2020, 30, 2002547. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Xu, C.; Wang, Z.; Shu, S.; Sun, Z.; Tang, W.; Wang, Z.L. Sensing of joint and spinal bending or stretching via a retractable and wearable badge reel. Nat. Commun. 2021, 12, 2950. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, M.; Shan, X.; Lee, C. Augmented tactile-perception and haptic-feedback rings as human-machine interfaces aiming for immersive interactions. Nat. Commun. 2022, 13, 5224. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, L.; Wang, Z.L. Nanoscale Triboelectric-Effect-Enabled Energy Conversion for Sustainably Powering Portable Electronics. Nano Lett. 2012, 12, 6339–6346. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, L.; Xie, Y.; Jing, Q.; Niu, S.; Wang, Z.L. Sliding-Triboelectric Nanogenerators Based on In-Plane Charge-Separation Mechanism. Nano Lett. 2013, 13, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, J.; Jing, Q.; Niu, S.; Wu, W.; Wang, Z.L. Triboelectric Nanogenerator Built on Suspended 3D Spiral Structure as Vibration and Positioning Sensor and Wave Energy Harvester. ACS Nano 2013, 7, 10424–10432. [Google Scholar] [CrossRef]
- Lin, L.; Wang, S.; Xie, Y.; Jing, Q.; Niu, S.; Hu, Y.; Wang, Z.L. Segmentally Structured Disk Triboelectric Nanogenerator for Harvesting Rotational Mechanical Energy. Nano Lett. 2013, 13, 2916–2923. [Google Scholar] [CrossRef]
- Niu, S.; Liu, Y.; Wang, S.; Lin, L.; Zhou, Y.S.; Hu, Y.; Wang, Z.L. Theory of Sliding-Mode Triboelectric Nanogenerators. Adv. Mater. 2013, 25, 6184–6193. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Pan, L.; Wu, C.; Yu, H.; Yang, L.; Liao, R.; Wang, Z.L. Self-powered wind sensor system for detecting wind speed and direction based on a triboelectric nanogenerator. ACS Nano 2018, 12, 3954–3963. [Google Scholar] [CrossRef]
- Jiang, T.; Tang, W.; Chen, X.; Bao Han, C.; Lin, L.; Zi, Y.; Wang, Z.L. Figures-of-merit for rolling-friction-based triboelectric nanogenerators. Adv. Mater. Technol. 2016, 1, 1600017. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Niu, S.; Lin, L.; Wang, Z.L. Freestanding triboelectric-layer-based nanogenerators for harvesting energy from a moving object or human motion in contact and non-contact modes. Adv. Mater. 2014, 26, 2818–2824. [Google Scholar] [CrossRef]
- Deng, H.-T.; Xia, Y.-X.; Liu, Y.-C.; Kim, B.; Zhang, X.-S. Stretchable nanogenerator with micro-nano hierarchical interfaces for self-powered biometric authentication. npj Flex. Electron. 2024, 8, 81. [Google Scholar] [CrossRef]
- Gao, A.; Zhou, Q.; Cao, Z.; Xu, W.; Zhou, K.; Wang, B.; Pan, J.; Pan, C.; Xia, F. A Self-Powered Biochemical Sensor for Intelligent Agriculture Enabled by Signal Enhanced Triboelectric Nanogenerator. Adv. Sci. 2024, 11, 2309824. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Saha, S.; Barman, S.R.; Khan, I.; Pao, Y.-P.; Lee, S.; Choi, D.; Lin, Z.-H. Enhanced sensing performance of triboelectric nanosensors by solid-liquid contact electrification. Nano Energy 2020, 77, 105093. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, J.; Shi, R.; Liu, H.; Zhang, M.; Guo, Z.; Bai, Y.; Li, X.; Gao, X.; Guo, J.; et al. Triboelectric nanogenerator sensors-based trans-media motion monitoring system for Gannet-inspired vehicle aiming at digital twin applications. Nano Energy 2025, 136, 110763. [Google Scholar] [CrossRef]
- Stephens, G.L.; Li, J.; Wild, M.; Clayson, C.A.; Loeb, N.; Kato, S.; L’Ecuyer, T.; Stackhouse, P.W., Jr.; Lebsock, M.; Andrews, T. An update on Earth’s energy balance in light of the latest global observations. Nat. Geosci. 2012, 5, 691–696. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, Y.; Ren, G.; Zhang, B.; Zhou, S. Water evaporation-induced electricity with Geobacter sulfurreducens biofilms. Sci. Adv. 2022, 8, eabm8047. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, X.; Fu, C.; Zhang, Y.; Fang, J.; Qin, J.; He, Q.-C.; Yang, T. Patterned Coating of Ionic Diode Arrays Toward Flexible Moist-Electric Generators to Power Wireless Sensor Nodes. Adv. Funct. Mater. 2024, 34, 2311465. [Google Scholar] [CrossRef]
- Wei, Q.; Ge, W.; Yuan, Z.; Wang, S.; Lu, C.; Feng, S.; Zhao, L.; Liu, Y. Moisture electricity generation: Mechanisms, structures, and applications. Nano Res. 2023, 16, 7496–7510. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Wang, R.; Li, T. Emerging self-sustained electricity generation enabled by moisture. Cell Rep. Phys. Sci. 2023, 4, 101517. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Ding, T.; Yang, P.; Duan, J.; Zhou, J. Surface functional modification boosts the output of an evaporation-driven water flow nanogenerator. Nano Energy 2019, 58, 797–802. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Lu, B.; Guang, T.; Yang, C.; Huang, Y.; Cheng, H.; Qu, L. Fully printed planar moisture-enabled electric generator arrays for scalable function integration. Joule 2023, 7, 935–951. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, H.; Yang, C.; Yao, H.; Li, C.; Qu, L. All-region-applicable, continuous power supply of graphene oxide composite. Energy Environ. Sci. 2019, 12, 1848–1856. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, X.; Wang, L.; Yu, J.; Qin, X. Capacitor-inspired high-performance and durable moist-electric generator. Energy Environ. Sci. 2022, 15, 4584–4591. [Google Scholar] [CrossRef]
- Tan, J.; Fang, S.; Zhang, Z.; Yin, J.; Li, L.; Wang, X.; Guo, W. Self-sustained electricity generator driven by the compatible integration of ambient moisture adsorption and evaporation. Nat. Commun. 2022, 13, 3643. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wang, Z.; Zhang, B.; Liu, X.; Ye, J.; Hu, Q.; Zhou, S. A facile and sustainable hygroelectric generator using whole-cell Geobacter sulfurreducens. Nano Energy 2021, 89, 106361. [Google Scholar] [CrossRef]
- Fruchtman, A. Electric field in a double layer and the imparted momentum. Phys. Rev. Lett. 2006, 96, 065002. [Google Scholar] [CrossRef]
- Ma, Q.; He, Q.; Yin, P.; Cheng, H.; Cui, X.; Yun, Q.; Zhang, H. Rational Design of MOF-Based Hybrid Nanomaterials for Directly Harvesting Electric Energy from Water Evaporation. Adv. Mater. 2020, 32, 2003720. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, H.; Zhang, Z.; Jiang, L.; Qu, L. Direct Power Generation from a Graphene Oxide Film under Moisture. Adv. Mater. 2015, 27, 4351–4357. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; He, T.; Huang, Y.; Cheng, H.; Li, C.; Xie, D.; Yang, P.; Zhang, Y.; Qu, L. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1,000 V output. Nat. Nanotechnol. 2021, 16, 811–819. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, A.; Li, Y.; Chen, X.; Chen, Y.; Xu, Y.; Li, Y.; Ge, H.; Ning, X. A sandwich-like flexible nanofiber device boosts moisture induced electricity generation for power supply and multiple sensing applications. Nano Energy 2023, 113, 108529. [Google Scholar] [CrossRef]
- Nandakumar, D.K.; Vaghasiya, J.V.; Yang, L.; Zhang, Y.; Tan, S.C. A solar cell that breathes in moisture for energy generation. Nano Energy 2020, 68, 104263. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, H.; Yang, C.; Zhang, P.; Liao, Q.; Yao, H.; Shi, G.; Qu, L. Interface-mediated hygroelectric generator with an output voltage approaching 1.5 volts. Nat. Commun. 2018, 9, 4166. [Google Scholar] [CrossRef]
- Liu, X.; Gao, H.; Ward, J.E.; Liu, X.; Yin, B.; Fu, T.; Chen, J.; Lovley, D.R.; Yao, J. Power generation from ambient humidity using protein nanowires. Nature 2020, 578, 550–554. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Dai, L.; Sun, X.; An, M.; Duan, C.; Li, J.; Ni, Y. Asymmetrically Patterned Cellulose Nanofibers/Graphene Oxide Composite Film for Humidity Sensing and Moist-Induced Electricity Generation. ACS Appl. Mater. Interfaces 2020, 12, 55205–55214. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Q.; Liu, C.; Zhang, G.; Jing, J.; Shao, C.; Han, Y.; Qu, L. MEG actualized by high-valent metal carrier transport. Nano Energy 2019, 65, 104047. [Google Scholar] [CrossRef]
- Lyu, Q.; Peng, B.; Xie, Z.; Du, S.; Zhang, L.; Zhu, J. Moist-Induced Electricity Generation by Electrospun Cellulose Acetate Membranes with Optimized Porous Structures. ACS Appl. Mater. Interfaces 2020, 12, 57373–57381. [Google Scholar] [CrossRef]
- Fu, T.; Liu, X.; Fu, S.; Woodard, T.; Gao, H.; Lovley, D.R.; Yao, J. Self-sustained green neuromorphic interfaces. Nat. Commun. 2021, 12, 3351. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, D.; Tian, X.; Qiang, S.; Sun, C.; Dai, L.; Zhang, M.; Ni, Y.; Jiang, X. Highly ordered asymmetric cellulose-based honeycomb membrane for moisture-electricity generation and humidity sensing. Carbohydr. Polym. 2022, 294, 119809. [Google Scholar] [CrossRef]
- Cai, T.; Lan, L.; Peng, B.; Zhang, C.; Dai, S.; Zhang, C.; Ping, J.; Ying, Y. Bilayer Wood Membrane with Aligned Ion Nanochannels for Spontaneous Moist-Electric Generation. Nano Lett. 2022, 22, 6476–6483. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Dong, H.; Cui, S.; Dong, W.; Sun, H. Fluid Classification via the Dual Functionality of Moisture-Enabled Electricity Generation Enhanced by Deep Learning. ACS Appl. Mater. Interfaces 2024, 16, 63723–63734. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhao, L.; Wang, K.; Zheng, Y.; Yuan, Z.; Wang, D.; Fu, X.; Shen, G.; Han, W. Flexible Self-Powered Integrated Sensing System with 3D Periodic Ordered Black Phosphorus@MXene Thin-Films. Adv. Mater. 2021, 33, 2007890. [Google Scholar] [CrossRef]
- Azimi, S.; Golabchi, A.; Nekookar, A.; Rabbani, S.; Amiri, M.H.; Asadi, K.; Abolhasani, M.M. Self-powered cardiac pacemaker by piezoelectric polymer nanogenerator implant. Nano Energy 2021, 83, 105781. [Google Scholar] [CrossRef]
- Rana, M.M.; Khan, A.A.; Huang, G.; Mei, N.; Saritas, R.; Wen, B.; Zhang, S.; Voss, P.; Abdel-Rahman, E.; Leonenko, Z.; et al. Porosity Modulated High-Performance Piezoelectric Nanogenerator Based on Organic/Inorganic Nanomaterials for Self-Powered Structural Health Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 47503–47512. [Google Scholar] [CrossRef]
- Li, N.; Yi, Z.; Ma, Y.; Xie, F.; Huang, Y.; Tian, Y.; Dong, X.; Liu, Y.; Shao, X.; Li, Y.; et al. Direct Powering a Real Cardiac Pacemaker by Natural Energy of a Heartbeat. ACS Nano 2019, 13, 2822–2830. [Google Scholar] [CrossRef]
- Yi, Z.; Xie, F.; Tian, Y.; Li, N.; Dong, X.; Ma, Y.; Huang, Y.; Hu, Y.; Xu, X.; Qu, D.; et al. A Battery- and Leadless Heart-Worn Pacemaker Strategy. Adv. Funct. Mater. 2020, 30, 2000477. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, J.; Long, Y.; Wei, H.; Ferreira, C.A.; Jeffery, J.J.; Lin, Y.; Cai, W.; Wang, X. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 2018, 9, 5349. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xu, B.; Guan, X.; Chen, Y.; Li, S.; Feng, J. Towards truly wearable energy harvesters with full structural integrity of fiber materials. Nano Energy 2019, 58, 365–374. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Sha, Z.; Boyer, C.; Wang, C.-H.; Zhang, J. Enhancing output performance of PVDF-HFP fiber-based nanogenerator by hybridizing silver nanowires and perovskite oxide nanocrystals. Nano Energy 2022, 98, 107343. [Google Scholar] [CrossRef]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Qu, X.; Liu, Y.; Cheng, S.; Zhang, Z.; Shan, Y.; Luo, R.; Weng, S.; Li, H.; et al. A self-powered intracardiac pacemaker in swine model. Nat. Commun. 2024, 15, 507. [Google Scholar] [CrossRef]
- Zhao, K.; Lee, J.W.; Yu, Z.G.; Jiang, W.; Oh, J.W.; Kim, G.; Han, H.; Kim, Y.; Lee, K.; Lee, S.; et al. Humidity-Tolerant Moisture-Driven Energy Generator with MXene Aerogel–Organohydrogel Bilayer. ACS Nano 2023, 17, 5472–5485. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, Y.; Li, N.; Wang, Y.; Yu, J.; Hu, Z. Integrated Extraction of Electrical Energy and Freshwater from Seawater via Asymmetric Evaporator. Adv. Funct. Mater. 2025, 35, 2422725. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, S.; Wang, Z.; Jiang, J. Asymmetric self-powered cellulose-based aerogel for moisture-electricity generation and humidity sensing. Chem. Eng. J. 2024, 486, 150203. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, T.; Zhang, X. Empowerment of AI algorithms in biochemical sensors. Trac-Trends Anal. Chem. 2024, 173, 117613. [Google Scholar] [CrossRef]
- Dong, H.; Lin, J.; Tao, Y.; Jia, Y.; Sun, L.; Li, W.J.; Sun, H. AI-enhanced biomedical micro/nanorobots in microfluidics. Lab Chip 2024, 24, 1419–1440. [Google Scholar] [CrossRef]
- De Ville, B. Decision trees. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 448–455. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- John, G.H.; Langley, P. Estimating continuous distributions in Bayesian classifiers. arXiv 2013, arXiv:1302.4964. [Google Scholar]
- Aha, D.W.; Kibler, D.; Albert, M.K. Instance-based learning algorithms. Mach. Learn. 1991, 6, 37–66. [Google Scholar] [CrossRef]
- Keerthi, S.S.; Shevade, S.K.; Bhattacharyya, C.; Murthy, K.R.K. Improvements to Platt’s SMO algorithm for SVM classifier design. Neural Comput. 2001, 13, 637–649. [Google Scholar] [CrossRef]
- Chalichalamala, S.; Govindan, N.; Kasarapu, R. Logistic Regression Ensemble Classifier for Intrusion Detection System in Internet of Things. Sensors 2023, 23, 9583. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R.; Taylor, J. Linear regression. In An Introduction to Statistical Learning: With Applications in Python; Springer: Berlin/Heidelberg, Germany, 2023; pp. 69–134. [Google Scholar]
- Werbos, P. New Tools for Prediction and Analysis in the Behavioral Science. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, January 1974. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning internal representations by error propagation. In Parallel Distributed Processing, Explorations in the Microstructure of Cognition; Rumelhart, D.E., Mcclelland, J., Eds.; MIT Press: Cambridge, MA, USA, 1987; Volume 1, pp. 318–362. [Google Scholar]
- Kattenborn, T.; Leitloff, J.; Schiefer, F.; Hinz, S. Review on Convolutional Neural Networks (CNN) in vegetation remote sensing. ISPRS J. Photogramm. Remote Sens. 2021, 173, 24–49. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, X.; Zeng, N.; Liu, Y.; Alsaadi, F.E. A survey of deep neural network architectures and their applications. Neurocomputing 2017, 234, 11–26. [Google Scholar] [CrossRef]
- Yadav, S.P.; Zaidi, S.; Mishra, A.; Yadav, V. Survey on machine learning in speech emotion recognition and vision systems using a recurrent neural network (RNN). Arch. Comput. Methods Eng. 2022, 29, 1753–1770. [Google Scholar] [CrossRef]
- Srinidhi, C.; Ciga, O.; Martel, A. Deep neural network models for computational histopathology: A survey. Med. Image Anal. 2021, 67, 101813. [Google Scholar] [CrossRef]
- Mousavi, S.; Beroza, G. Deep-learning seismology. Science 2022, 377, eabm4470. [Google Scholar] [CrossRef]
- Wang, J.; Biljecki, F. Unsupervised machine learning in urban studies: A systematic review of applications. Cities 2022, 129, 103925. [Google Scholar] [CrossRef]
- Fu, G.; Jin, Y.; Sun, S.; Yuan, Z.; Butler, D. The role of deep learning in urban water management: A critical review. Water Res. 2022, 223, 118973. [Google Scholar] [CrossRef]
- Khojaste-Sarakhsi, M.; Haghighi, S.; Ghomi, S.; Marchiori, E. Deep learning for Alzheimer’s disease diagnosis: A survey. Artif. Intell. Med. 2022, 130, 102332. [Google Scholar] [CrossRef]
- Tao, X.; Gong, X.; Zhang, X.; Yan, S.; Adak, C. Deep Learning for Unsupervised Anomaly Localization in Industrial Images: A Survey. IEEE Trans. Instrum. Meas. 2022, 71, 5018021. [Google Scholar] [CrossRef]
- Celard, P.; Iglesias, E.; Sorribes-Fdez, J.; Romero, R.; Vieira, A.; Borrajo, L. A survey on deep learning applied to medical images: From simple artificial neural networks to generative models. Neural Comput. Appl. 2023, 35, 2291–2323. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, L.; Chen, L.; Jiang, Z.; Zhou, F.; Zhang, Q.; Zhang, X.; Jin, Y.; Zhou, H. Deep learning based brain tumor segmentation: A survey. Complex Intell. Syst. 2023, 9, 1001–1026. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, Z.; Xiao, A. Deep learning in economics: A systematic and critical review. Artif. Intell. Rev. 2023, 56, 9497–9539. [Google Scholar] [CrossRef] [PubMed]
- Newbury, R.; Gu, M.; Chumbley, L.; Mousavian, A.; Eppner, C.; Leitner, J.; Bohg, J.; Morales, A.; Asfour, T.; Kragic, D.; et al. Deep Learning Approaches to Grasp Synthesis: A Review. IEEE Trans. Robot. 2023, 39, 3994–4015. [Google Scholar] [CrossRef]
- Devlin, J.; Chang, M.; Lee, K.; Toutanova, K.; Linguist, A.C. BERT: Pre-training of Deep Bidirectional Transformers for Language Understanding. In Proceedings of the Conference of the North-American-Chapter of the Association-for-Computational-Linguistics—Human Language Technologies (NAACL-HLT), Minneapolis, MN, USA, 2–7 June 2019; pp. 4171–4186. [Google Scholar]
- Radford, A.; Wu, J.; Child, R.; Luan, D.; Amodei, D.; Sutskever, I. Language models are unsupervised multitask learners. OpenAI Blog 2019, 1, 9. [Google Scholar]
- Brown, T.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language Models are Few-Shot Learners. In Proceedings of the 34th Conference on Neural Information Processing Systems (NeurIPS), Online, 6–12 December 2020. [Google Scholar]
- Achiam, J.; Adler, S.; Agarwal, S.; Ahmad, L.; Akkaya, I.; Aleman, F.L.; Almeida, D.; Altenschmidt, J.; Altman, S.; Anadkat, S. Gpt-4 technical report. arXiv 2023, arXiv:2303.08774. [Google Scholar]
- Anil, R.; Dai, A.M.; Firat, O.; Johnson, M.; Lepikhin, D.; Passos, A.; Shakeri, S.; Taropa, E.; Bailey, P.; Chen, Z. Palm 2 technical report. arXiv 2023, arXiv:2305.10403. [Google Scholar]
- Chowdhery, A.; Narang, S.; Devlin, J.; Bosma, M.; Mishra, G.; Roberts, A.; Barham, P.; Chung, H.; Sutton, C.; Gehrmann, S.; et al. PaLM: Scaling Language Modeling with Pathways. J. Mach. Learn. Res. 2023, 24, 4–6. [Google Scholar]
- Touvron, H.; Lavril, T.; Izacard, G.; Martinet, X.; Lachaux, M.-A.; Lacroix, T.; Rozière, B.; Goyal, N.; Hambro, E.; Azhar, F. Llama: Open and efficient foundation language models. arXiv 2023, arXiv:2302.13971. [Google Scholar]
- Touvron, H.; Martin, L.; Stone, K.; Albert, P.; Almahairi, A.; Babaei, Y.; Bashlykov, N.; Batra, S.; Bhargava, P.; Bhosale, S. Llama 2: Open foundation and fine-tuned chat models. arXiv 2023, arXiv:2307.09288. [Google Scholar]
- Ren, X.; Zhou, P.; Meng, X.; Huang, X.; Wang, Y.; Wang, W.; Li, P.; Zhang, X.; Podolskiy, A.; Arshinov, G. Pangu-∑: Towards trillion parameter language model with sparse heterogeneous computing. arXiv 2023, arXiv:2303.10845. [Google Scholar]
- Zeng, W.; Ren, X.; Su, T.; Wang, H.; Liao, Y.; Wang, Z.; Jiang, X.; Yang, Z.; Wang, K.; Zhang, X. Pangu: Large-scale autoregressive pretrained Chinese language models with auto-parallel computation. arXiv 2021, arXiv:2104.12369. [Google Scholar]
- Bi, X.; Chen, D.; Chen, G.; Chen, S.; Dai, D.; Deng, C.; Ding, H.; Dong, K.; Du, Q.; Fu, Z. Deepseek llm: Scaling open-source language models with longtermism. arXiv 2024, arXiv:2401.02954. [Google Scholar]
- Guo, D.; Yang, D.; Zhang, H.; Song, J.; Zhang, R.; Xu, R.; Zhu, Q.; Ma, S.; Wang, P.; Bi, X. Deepseek-r1: Incentivizing reasoning capability in llms via reinforcement learning. arXiv 2025, arXiv:2501.12948. [Google Scholar]
- Dirgová Luptáková, I.; Kubovčík, M.; Pospíchal, J. Wearable Sensor-Based Human Activity Recognition with Transformer Model. Sensors 2022, 22, 1911. [Google Scholar] [CrossRef]
- Dosovitskiy, A. An image is worth 16x16 words: Transformers for image recognition at scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Radford, A.; Kim, J.W.; Xu, T.; Brockman, G.; McLeavey, C.; Sutskever, I. Robust speech recognition via large-scale weak supervision. In Proceedings of the International Conference on Machine Learning, Honolulu, HI, USA, 23–29 July 2023; pp. 28492–28518. [Google Scholar]
- Wu, Y.; Chen, K.; Zhang, T.; Hui, Y.; Berg-Kirkpatrick, T.; Dubnov, S. Large-scale contrastive language-audio pretraining with feature fusion and keyword-to-caption augmentation. In Proceedings of the ICASSP 2023-2023 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Rhodes Island, Greece, 4–10 June 2023; pp. 1–5. [Google Scholar]
- He, K.; Chen, X.; Xie, S.; Li, Y.; Dollár, P.; Girshick, R.; SOC, I.C. Masked Autoencoders Are Scalable Vision Learners. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), New Orleans, LA, USA, 18–24 June 2022; pp. 15979–15988. [Google Scholar]
- Xiao, X.; Yin, J.; Xu, J.; Tat, T.; Chen, J. Advances in machine learning for wearable sensors. ACS Nano 2024, 18, 22734–22751. [Google Scholar] [CrossRef]
- Saad, H.S.; Zaki, J.F.; Abdelsalam, M.M. Employing of machine learning and wearable devices in healthcare system: Tasks and challenges. Neural Comput. Appl. 2024, 36, 17829–17849. [Google Scholar] [CrossRef]
- Babu, A.; Mandal, D. Roadmap to human–machine interaction through triboelectric nanogenerator and machine learning convergence. ACS Appl. Energy Mater. 2024, 7, 822–833. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Liu, L.; Wu, H.; Hu, X.; Xie, M.; Zhu, Y.; Chen, X.; Ou-Yang, W. Machine learning-assisted self-powered intelligent sensing systems based on triboelectricity. Nano Energy 2023, 113, 108559. [Google Scholar] [CrossRef]
- Jin, X.F.; Liu, C.H.; Xu, T.L.; Su, L.; Zhang, X.J. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Hoshyar, A.N.; Al-Jumaily, A.; Hoshyar, A.N. The Beneficial Techniques in Preprocessing Step of Skin Cancer Detection System Comparing. Procedia Comput. Sci. 2014, 42, 25–31. [Google Scholar] [CrossRef]
- Thrift, W.J.; Ragan, R. Quantification of Analyte Concentration in the Single Molecule Regime Using Convolutional Neural Networks. Anal. Chem. 2019, 91, 13337–13342. [Google Scholar] [CrossRef]
- Chen, R.J.; Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Mahmood, F. Synthetic data in machine learning for medicine and healthcare. Nat. Biomed. Eng. 2021, 5, 493–497. [Google Scholar] [CrossRef]
- Tucker, A.; Wang, Z.C.; Rotalinti, Y.; Myles, P. Generating high-fidelity synthetic patient data for assessing machine learning healthcare software. npj Digit. Med. 2020, 3, 147. [Google Scholar] [CrossRef]
- Sandfort, V.; Yan, K.; Pickhardt, P.J.; Summers, R.M. Data augmentation using generative adversarial networks (CycleGAN) to improve generalizability in CT segmentation tasks. Sci. Rep. 2019, 9, 16884. [Google Scholar] [CrossRef]
- Haick, H.; Tang, N. Artificial Intelligence in Medical Sensors for Clinical Decisions. ACS Nano 2021, 15, 3557–3567. [Google Scholar] [CrossRef]
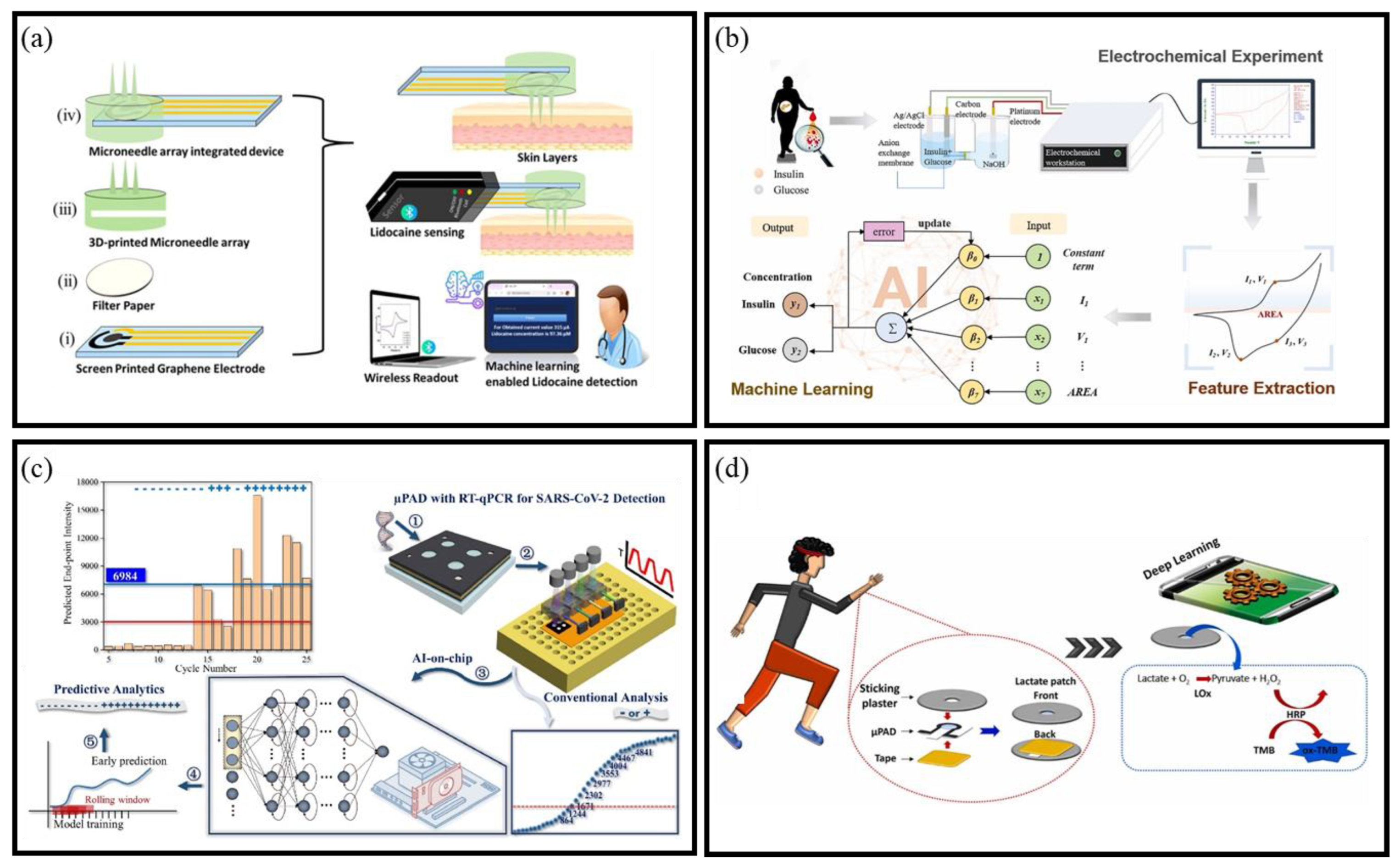
- Kadian, S.; Sahoo, S.S.; Kumari, P.; Narayan, R.J. Machine learning enabled onsite electrochemical detection of lidocaine using a microneedle array integrated screen printed electrode. Electrochim. Acta 2024, 475, 143664. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, H.Y.; Li, Y.; Yu, X.D.; Cai, Y.; Sha, X.P.; Wang, S.Y.; Zhan, Z.K.; Xu, J.H.; Liu, L.Q. AI powered electrochemical multi-component detection of insulin and glucose in serum. Biosens. Bioelectron. 2021, 186, 113291. [Google Scholar] [CrossRef]
- Sun, H.; Xiong, L.H.; Huang, Y.; Chen, X.K.; Yu, Y.J.; Ye, S.Z.; Dong, H.; Jia, Y.; Zhang, W.W. AI-aided on-chip nucleic acid assay for smart diagnosis of infectious disease. Fundam. Res. 2022, 2, 476–486. [Google Scholar] [CrossRef]
- Yüzer, E.; Doğan, V.; Kılıç, V.; Şen, M. Smartphone embedded deep learning approach for highly accurate and automated colorimetric lactate analysis in sweat. Sens. Actuators B Chem. 2022, 371, 132489. [Google Scholar] [CrossRef]
- Sun, H.; Pan, Y.H.; Dong, H.; Liu, C.F.; Yang, J.T.; Tao, Y.H.; Jia, Y. Generalized predictive analysis of reactions in paper devices via graph neural networks. Sens. Actuators B-Chem. 2024, 417, 136085. [Google Scholar] [CrossRef]
- Veeralingam, S.; Khandelwal, S.; Badhulika, S. AI/ML-Enabled 2-D-RuS2 Nanomaterial-Based Multifunctional, Low Cost, Wearable Sensor Platform for Non-Invasive Point of Care Diagnostics. IEEE Sens. J. 2020, 20, 8437–8444. [Google Scholar] [CrossRef]
- Antonijevic, M.; Zivkovic, M.; Arsic, S.; Jevremovic, A. Using AI-Based Classification Techniques to Process EEG Data Collected during the Visual Short-Term Memory Assessment. J. Sens. 2020, 2020, 8767865. [Google Scholar] [CrossRef]
- Brunmair, J.; Gotsmy, M.; Niederstaetter, L.; Neuditschko, B.; Bileck, A.; Slany, A.; Feuerstein, M.L.; Langbauer, C.; Janker, L.; Zanghellini, J.; et al. Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun. 2021, 12, 5993. [Google Scholar] [CrossRef]
- Simonyan, K.; Vedaldi, A.; Zisserman, A. Deep inside convolutional networks: Visualising image classification models and saliency maps. arXiv 2013, arXiv:1312.6034. [Google Scholar]
- Karagoz, M.A.; Breton, M.D.; El Fathi, A. A Comparative Study of Transformer-Based Models for Multi-Horizon Blood Glucose Prediction. IFAC-PapersOnLine 2025, 59, 155–160. [Google Scholar] [CrossRef]
- Barki, H.; Chung, W.-Y. Mental stress detection using a wearable in-ear plethysmography. Biosensors 2023, 13, 397. [Google Scholar] [CrossRef]
- Kong, L.; Li, W.; Zhang, T.; Ma, H.; Cao, Y.; Wang, K.; Zhou, Y.; Shamim, A.; Zheng, L.; Wang, X.; et al. Wireless technologies in flexible and wearable sensing: From materials design, system integration to applications. Adv. Mater. 2024, 36, e2400333. [Google Scholar] [CrossRef]
- Babu, A.; Thuau, D.; Mandal, D. AI-enabled wearable sensor for real-time monitored personalized training of sportsperson. MRS Commun. 2023, 13, 1071–1075. [Google Scholar] [CrossRef]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Hassan, A.R.; Subasi, A. A decision support system for automated identification of sleep stages from single-channel EEG signals. Knowl.-Based Syst. 2017, 128, 115–124. [Google Scholar] [CrossRef]
- Um, T.T.; Pfister, F.M.; Pichler, D.; Endo, S.; Lang, M.; Hirche, S.; Fietzek, U.; Kulić, D. Data augmentation of wearable sensor data for parkinson’s disease monitoring using convolutional neural networks. In Proceedings of the 19th ACM International Conference on Multimodal Interaction, Glasgow, Scotland, 13–17 November 2017; pp. 216–220. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4765–4774. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 30, 5998–6008. [Google Scholar]
- Zhong, X.; Gallagher, B.; Liu, S.; Kailkhura, B.; Hiszpanski, A.; Han, T.Y.-J. Explainable machine learning in materials science. npj Comput. Mater. 2022, 8, 204. [Google Scholar] [CrossRef]
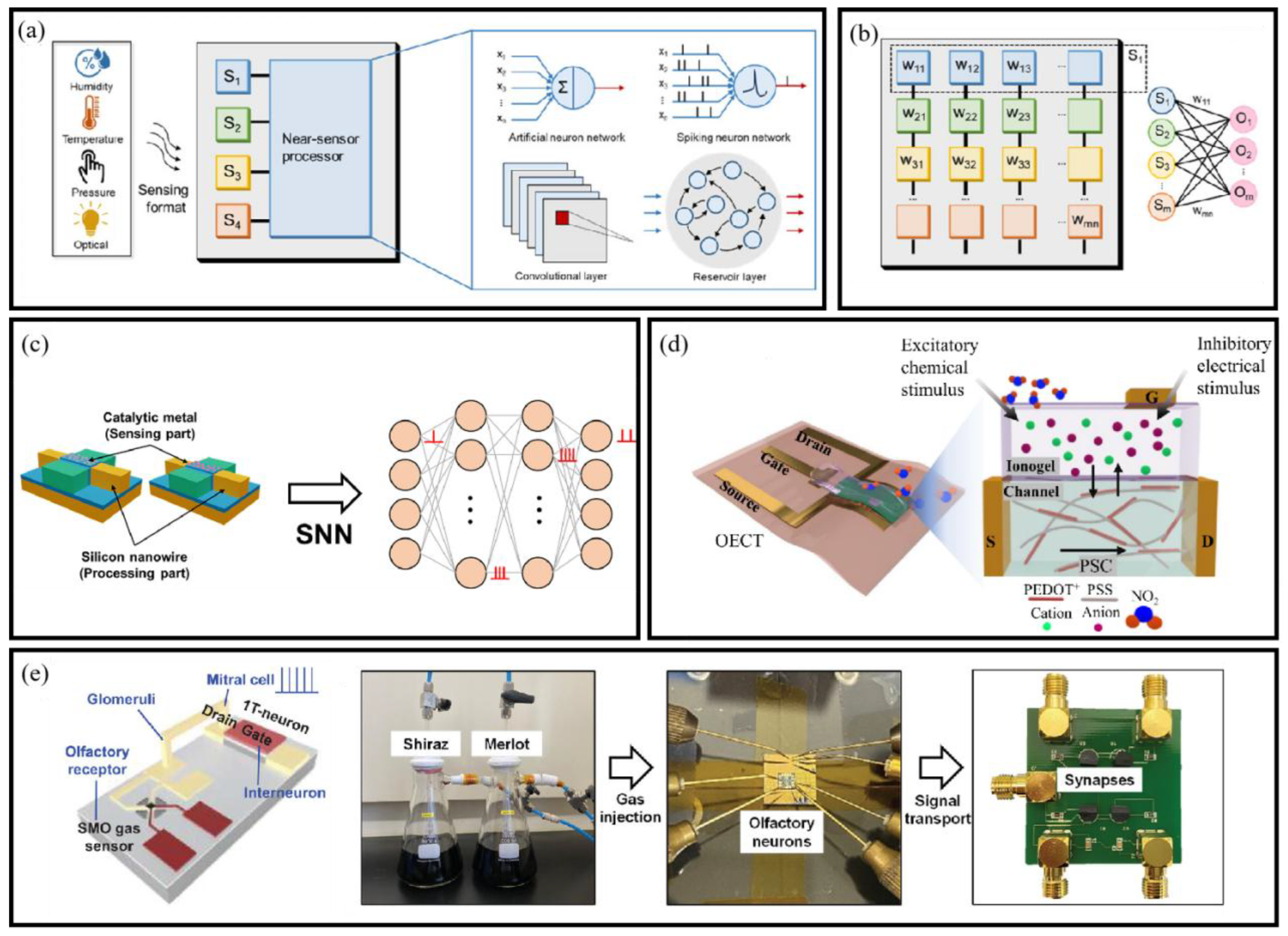
- Zhou, F.; Chai, Y. Near-sensor and in-sensor computing. Nat. Electron. 2020, 3, 664–671. [Google Scholar] [CrossRef]
- Wang, M.; Tu, J.; Huang, Z.; Wang, T.; Liu, Z.; Zhang, F.; Li, W.; He, K.; Pan, L.; Zhang, X.; et al. Tactile Near-Sensor Analogue Computing for Ultrafast Responsive Artificial Skin. Adv. Mater. 2022, 34, 2201962. [Google Scholar] [CrossRef]
- Cho, S.W.; Jo, C.; Kim, Y.-H.; Park, S.K. Progress of Materials and Devices for Neuromorphic Vision Sensors. Nano-Micro Lett. 2022, 14, 203. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhou, H.; Xu, S.; Lee, C. Advances in Machine-Learning Enhanced Nanosensors: From Cloud Artificial Intelligence Toward Future Edge Computing at Chip Level. Small Struct. 2024, 5, 2300325. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kang, M.; Han, J.-K.; Yun, S.-Y.; Park, I.; Choi, Y.-K. An artificial olfactory sensory neuron for selective gas detection with in-sensor computing. Device 2023, 1, 100063. [Google Scholar] [CrossRef]
- Chouhdry, H.H.; Lee, D.H.; Bag, A.; Lee, N.-E. A flexible artificial chemosensory neuronal synapse based on chemoreceptive ionogel-gated electrochemical transistor. Nat. Commun. 2023, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-K.; Kang, M.; Jeong, J.; Cho, I.; Yu, J.-M.; Yoon, K.-J.; Park, I.; Choi, Y.-K. Artificial Olfactory Neuron for an In-Sensor Neuromorphic Nose. Adv. Sci. 2022, 9, 2106017. [Google Scholar] [CrossRef]
- Liu, D.; Yu, H.; Chai, Y. Low-Power Computing with Neuromorphic Engineering. Adv. Intell. Syst. 2021, 3, 2000150. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, X.; Chen, M.; Zhang, M.; Chen, Y.; Niu, X.; Sha, X.; Zhan, Z.; Li, W.J. Continuous Monitoring of Train Parameters Using IoT Sensor and Edge Computing. IEEE Sens. J. 2021, 21, 15458–15468. [Google Scholar] [CrossRef]
- Alagumalai, A.; Shou, W.; Mahian, O.; Aghbashlo, M.; Tabatabaei, M.; Wongwises, S.; Liu, Y.; Zhan, J.; Torralba, A.; Chen, J.; et al. Self-powered sensing systems with learning capability. Joule 2022, 6, 1475–1500. [Google Scholar] [CrossRef]
| Material Type | Sensing Performance | Stability | Biocompatibility | Electrical Conductivity | Processability | Representative Applications |
|---|---|---|---|---|---|---|
| 0D Metal Nanoparticles | High sensitivity due to large surface area; excellent for molecular recognition | Prone to aggregation; requires stabilizers | Good (especially AuNPs) | Moderate to high | Easy surface functionalization; scalable | Antibody/antigen biosensors, dopamine sensing [13,14] |
| 0D Quantum Dots | Tunable photoluminescence; excellent for optical sensors | Sensitive to oxidation and photobleaching | Generally good (especially carbon-based) | Low to moderate | Requires encapsulation or passivation | Cholesterol and glioma sensing [17,18] |
| 1D Nanotubes | Excellent electrochemical response; fast electron transfer | Chemically stable, but long-term biocompatibility varies | Moderate to good (depends on functionalization) | High | Complex alignment and purification | Glucose sensors, drug detection [20,21] |
| 1D Nanowires | High aspect ratio; excellent for label-free sensing | Good structural stability | High (especially silicon-based) | Moderate | Compatible with top-down and bottom-up fabrication | C-reactive protein and DNA detection [25,27] |
| 2D Graphene | High carrier mobility; ultrathin interface enhances sensitivity | Chemically stable, but functionalization affects performance | Good (especially GO) | High | Excellent lithographic compatibility | H2O2, cholesterol, sweat glucose sensors [30,31,32] |
| 2D MXenes | Strong signal amplification, fast response | Sensitive to oxidation in ambient air | Excellent; hydrophilic surface supports immobilization | Very high | Requires etching and passivation | Paraoxon, phenol, H2O2 detection [34,35,36] |
| Working Mechanism | Materials | Open-Circuit Voltage | Output Power | Current Density | Power Density | Refs. |
|---|---|---|---|---|---|---|
| PENG | Mxene/black phosphorus | 6.94 mA cm−2 | 2.22 mW cm−2 | [110] | ||
| PENG | PVDF/ZnO/rGO | 138 ± 2.82 μW/cm3 | [111] | |||
| PENG | PVDF/BT | 4 V | 87 μW cm−3 | [56] | ||
| PENG | PVDF/ZnO | 84.5 V | 0.46 mW | 41.02 μW/cm2 | [112] | |
| PENG | PVDF-TrFE/MXene | 1.5 N (at 20 N) | 3.64 mW/m2 (at 20 N) | [61] | ||
| PENG | PMN-PT | 20 V (series); 12 V (parallel) | [113] | |||
| PENG | PMN-PT | 8.1 V | 6.9 μW | [114] | ||
| TENG | PTFE/Cu | 40 μW | [115] | |||
| TENG | PDMS/Cu | 1768.2 mW m−2 (at 1200 N) | [116] | |||
| TENG | PVDF-HFP/AgNWs/Mn-BNT-BT | 2170 V | 47 W/m2 | [117] | ||
| TENG | PTFE/Al | 65.2 V | 110 mW m−2 (at 100 MΩ) | [118] | ||
| TENG | POM/PTFE | 6.0 V | 2200 mW/m3 (at 100 MΩ) | [119] | ||
| MEG | Mxene/PAM | 600 mV | 1160 μA cm−2 | 24.8 μW cm−2 | [120] | |
| MEG | BPF | 0.95 V (at 25% RH and 25 °C) | 5.52 μW cm−2 (at 85% RH) | [98] | ||
| MEG | ANF/MXene/CNT | 0.42 V | 1.577 µW cm−2 (at 100 KΩ.) | [121] | ||
| MEG | Nonwoven fabrics/HCNTs/PVA | 0.56 V (at 42% RH and 21.5 °C) | 105.7 μW cm−3 | [109] | ||
| MEG | Nanowires/Mg/Al | 37 mV (Mg; at 25% RH); 51 mV (Al; at 25% RH) | 20.8 mW cm−3 (Mg), 39.3 mW cm−3 (Al) | [104] | ||
| MEG | SMEG/Q-CNF/CMC/SWCNT | 668 mV | 6.4 μA | 0.871 μW cm−2 (at 90% RH) | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Sun, H.; Yang, T.; Hu, H.; Li, X.; Jia, Y. Continuous Monitoring with AI-Enhanced BioMEMS Sensors: A Focus on Sustainable Energy Harvesting and Predictive Analytics. Micromachines 2025, 16, 902. https://doi.org/10.3390/mi16080902
Cai M, Sun H, Yang T, Hu H, Li X, Jia Y. Continuous Monitoring with AI-Enhanced BioMEMS Sensors: A Focus on Sustainable Energy Harvesting and Predictive Analytics. Micromachines. 2025; 16(8):902. https://doi.org/10.3390/mi16080902
Chicago/Turabian StyleCai, Mingchen, Hao Sun, Tianyue Yang, Hongxin Hu, Xubing Li, and Yuan Jia. 2025. "Continuous Monitoring with AI-Enhanced BioMEMS Sensors: A Focus on Sustainable Energy Harvesting and Predictive Analytics" Micromachines 16, no. 8: 902. https://doi.org/10.3390/mi16080902
APA StyleCai, M., Sun, H., Yang, T., Hu, H., Li, X., & Jia, Y. (2025). Continuous Monitoring with AI-Enhanced BioMEMS Sensors: A Focus on Sustainable Energy Harvesting and Predictive Analytics. Micromachines, 16(8), 902. https://doi.org/10.3390/mi16080902






