Integrated Electrochemical and Computational Elucidation of Nitro Blue Tetrazolium Chloride as an Efficient Leveler for Copper Microvia Superfilling
Abstract
1. Introduction
2. Experimental
2.1. Microvia Electrodeplating and Electrochemical Measurements
2.2. Theory and Computation
3. Results and Discussion
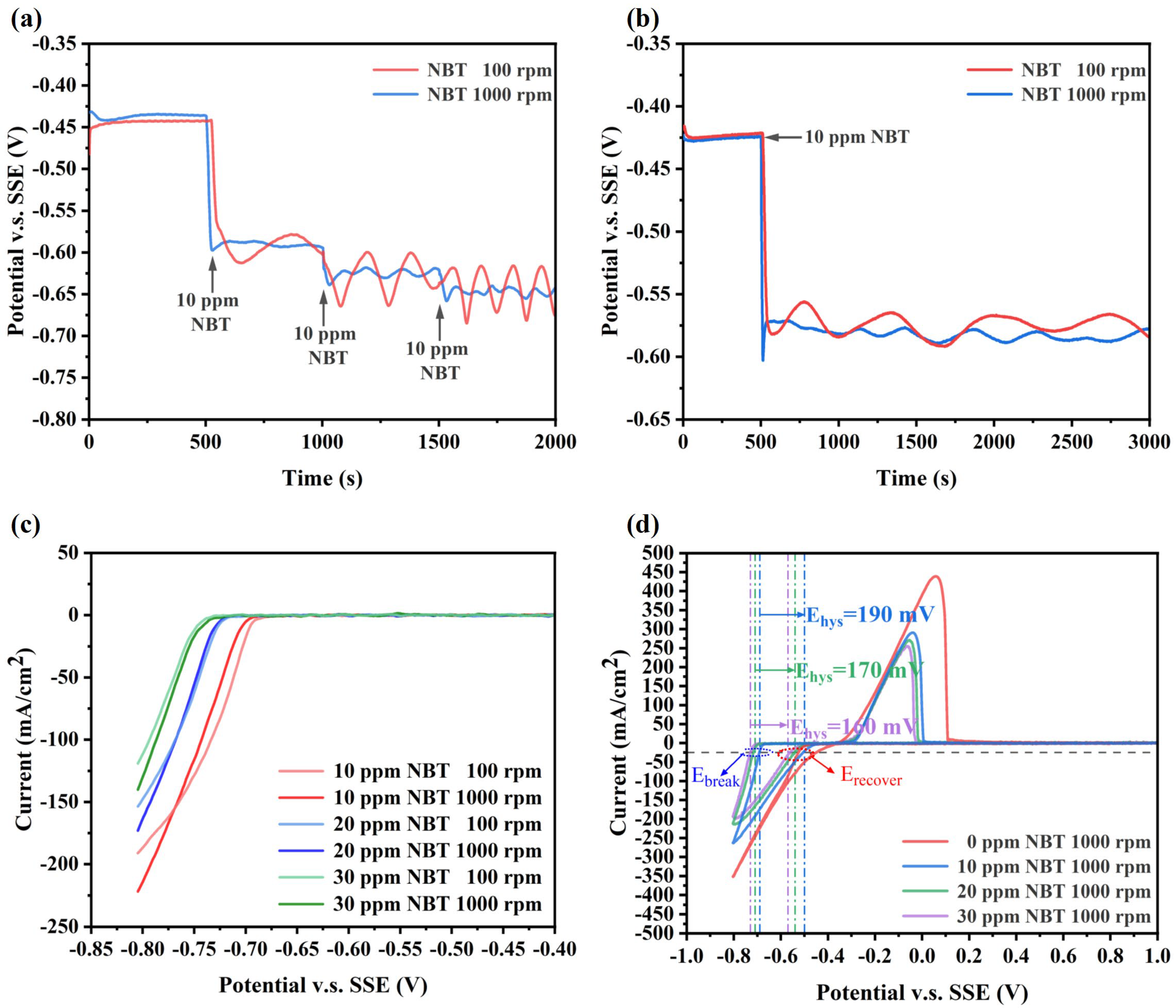
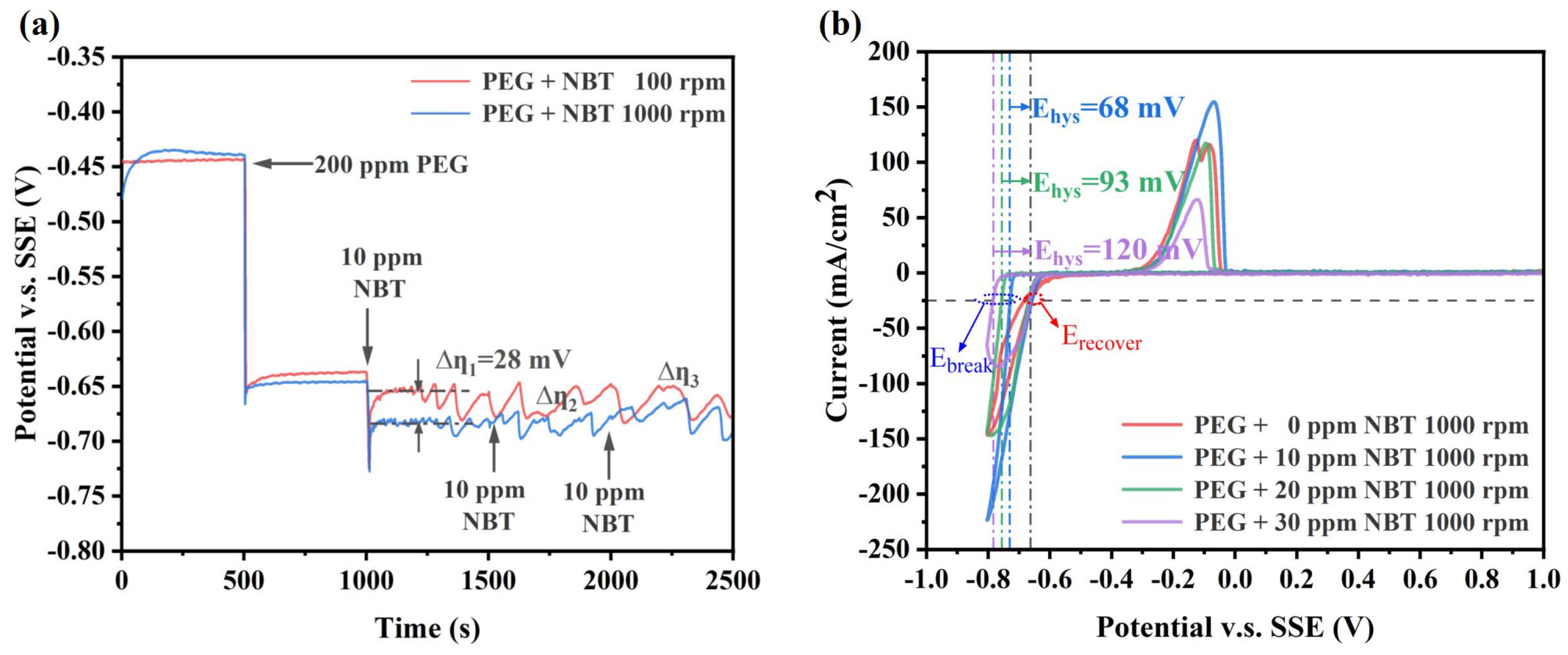
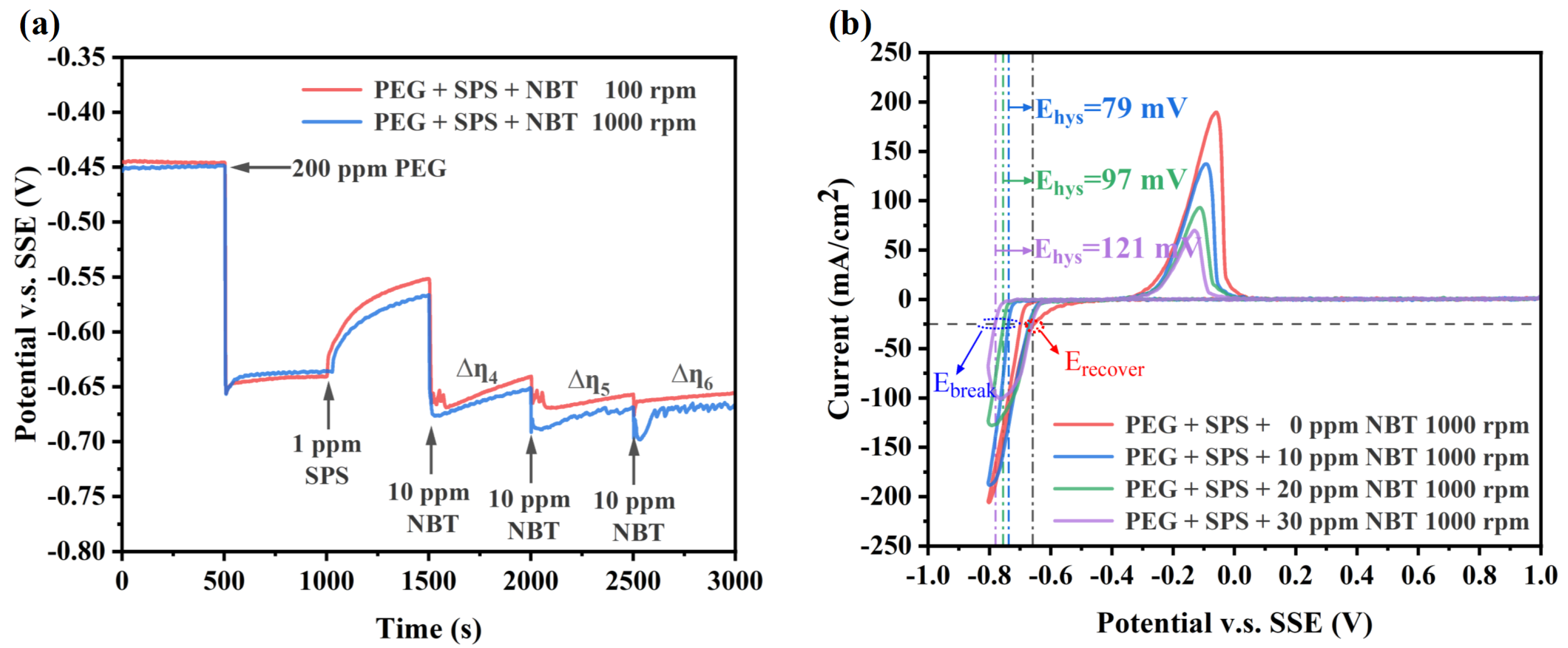
3.1. Electrochemical Analysis
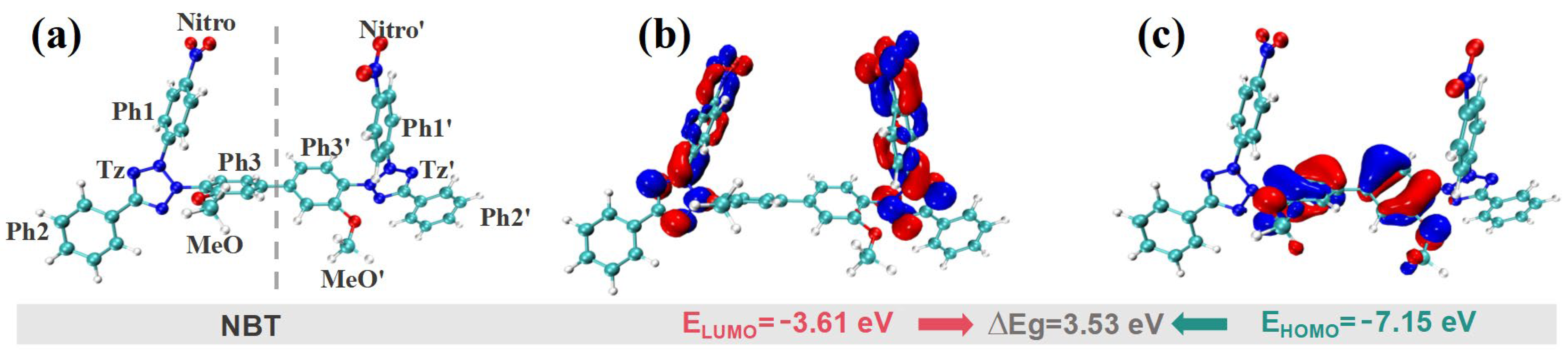
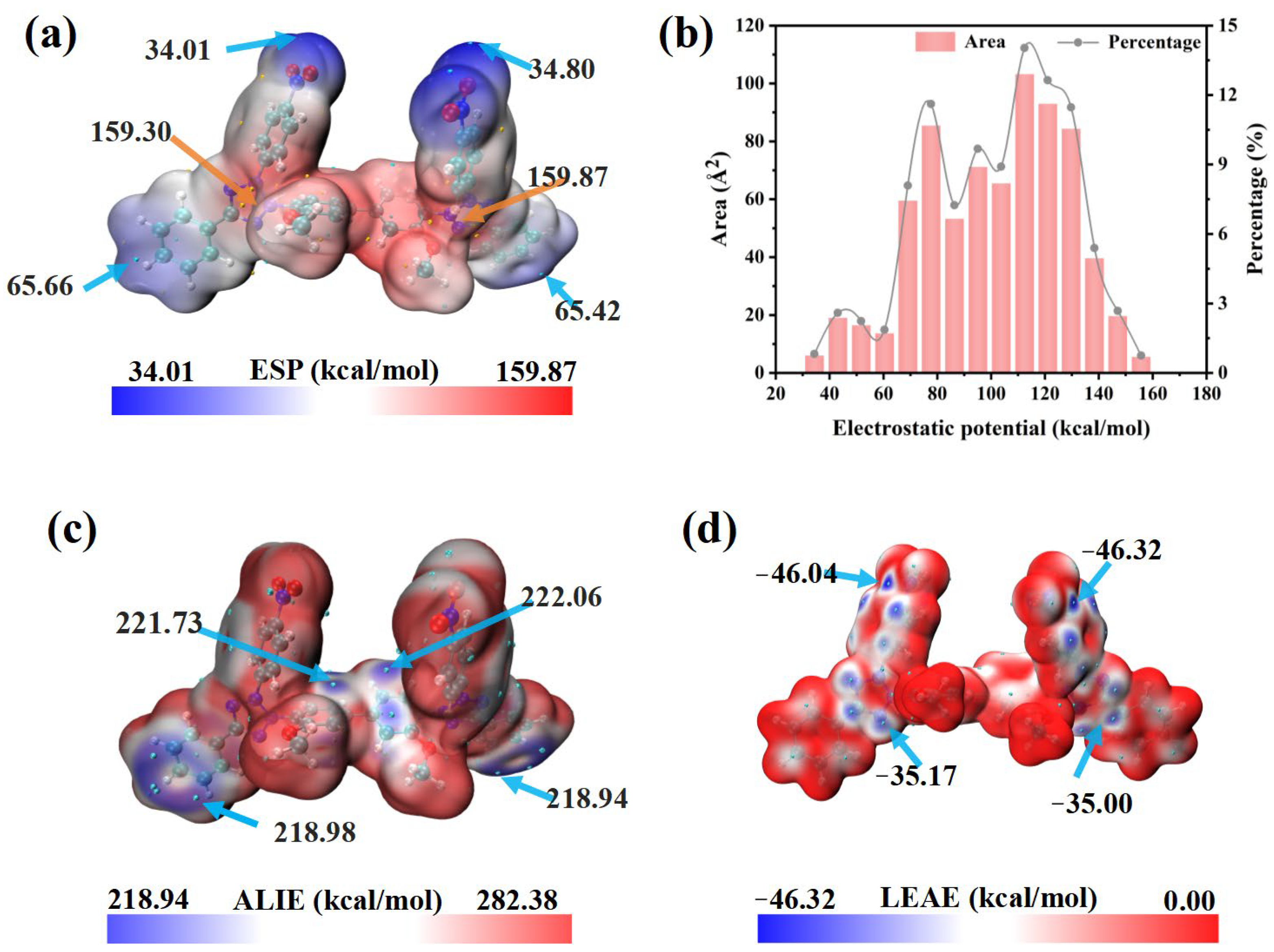
3.2. Theoretical Calculations
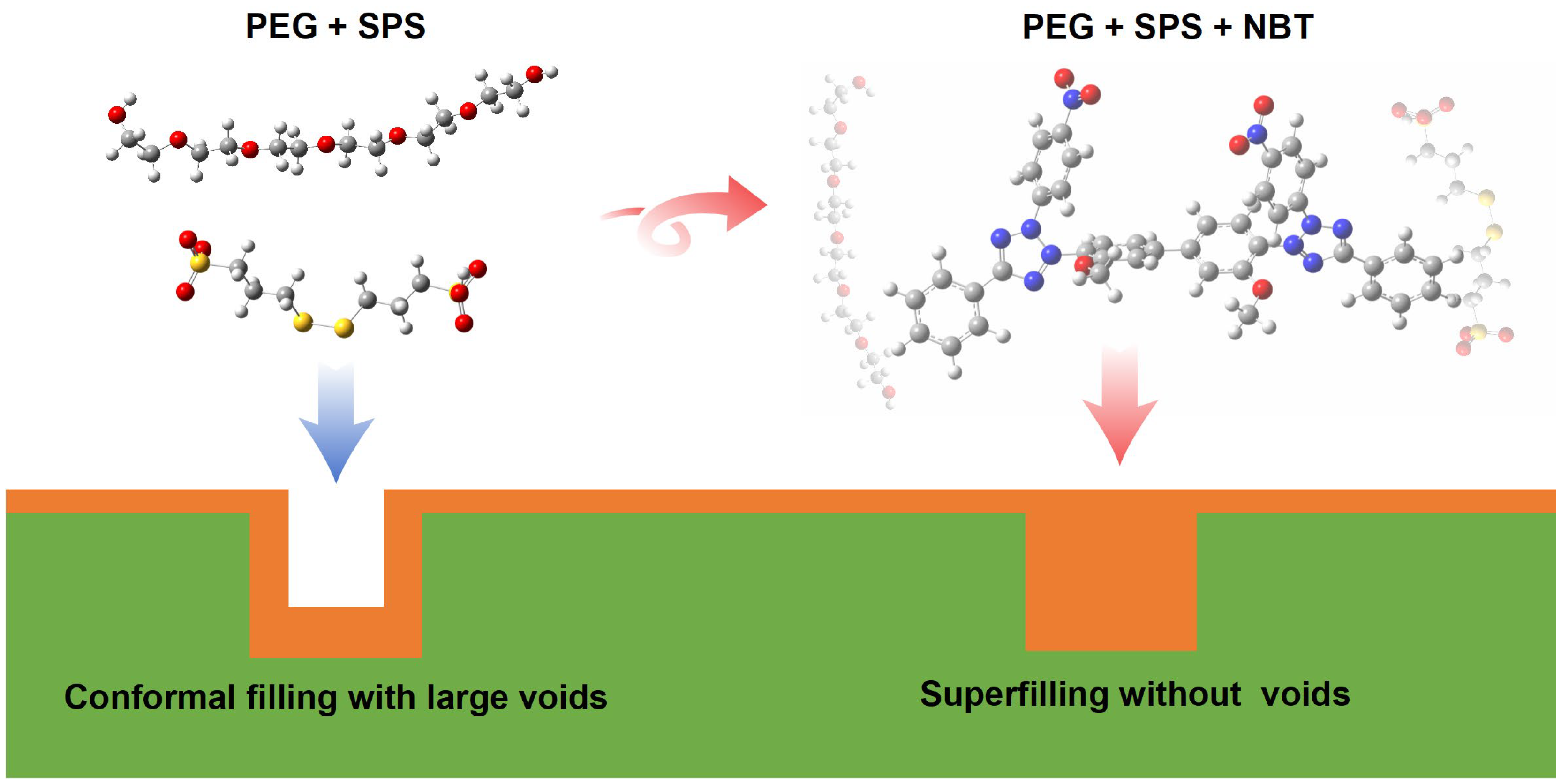
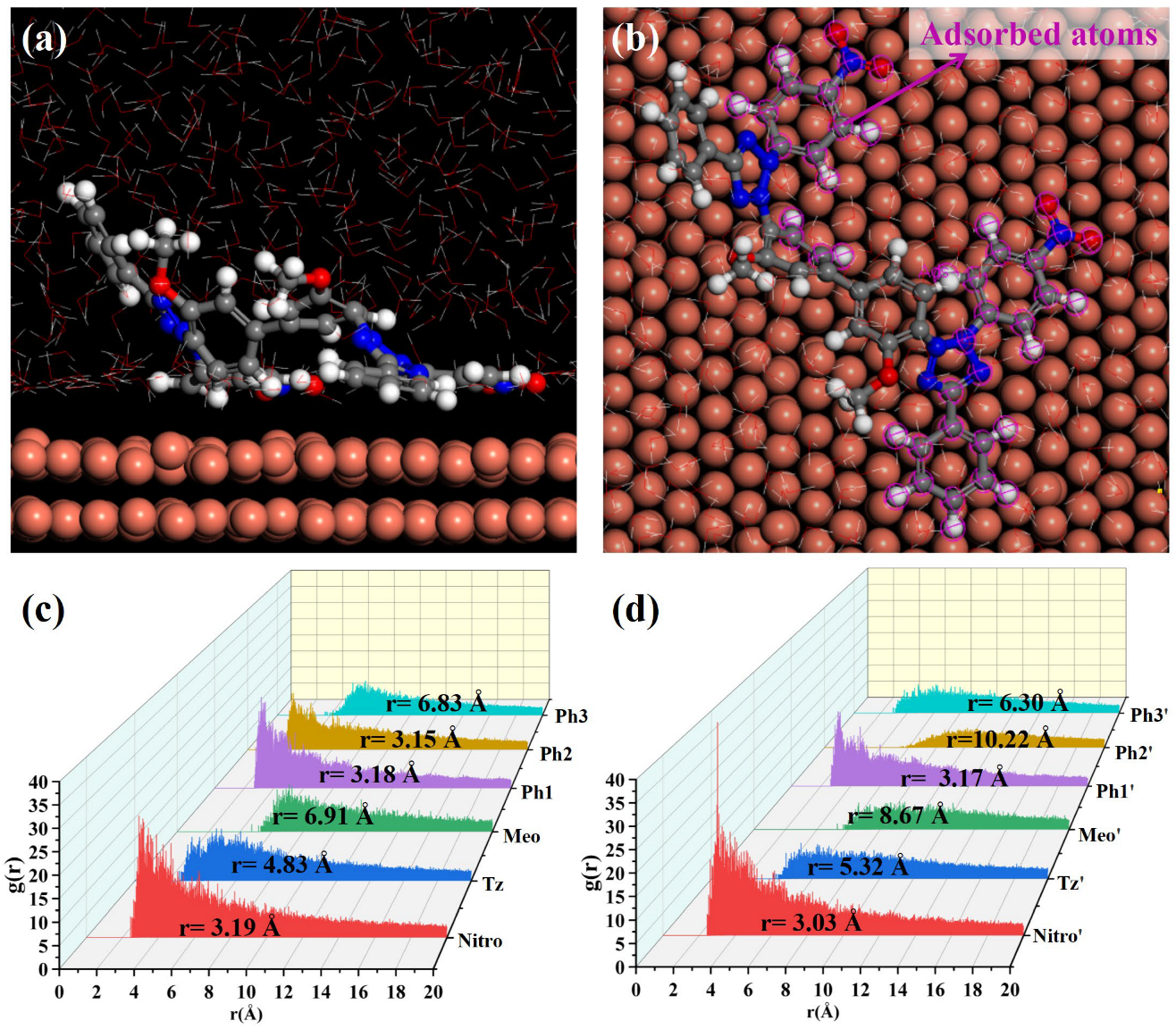
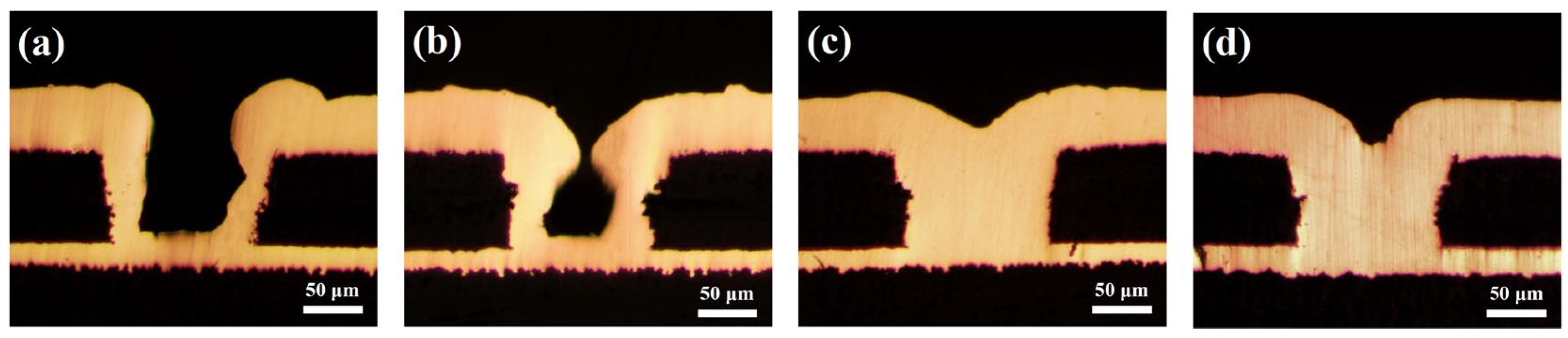
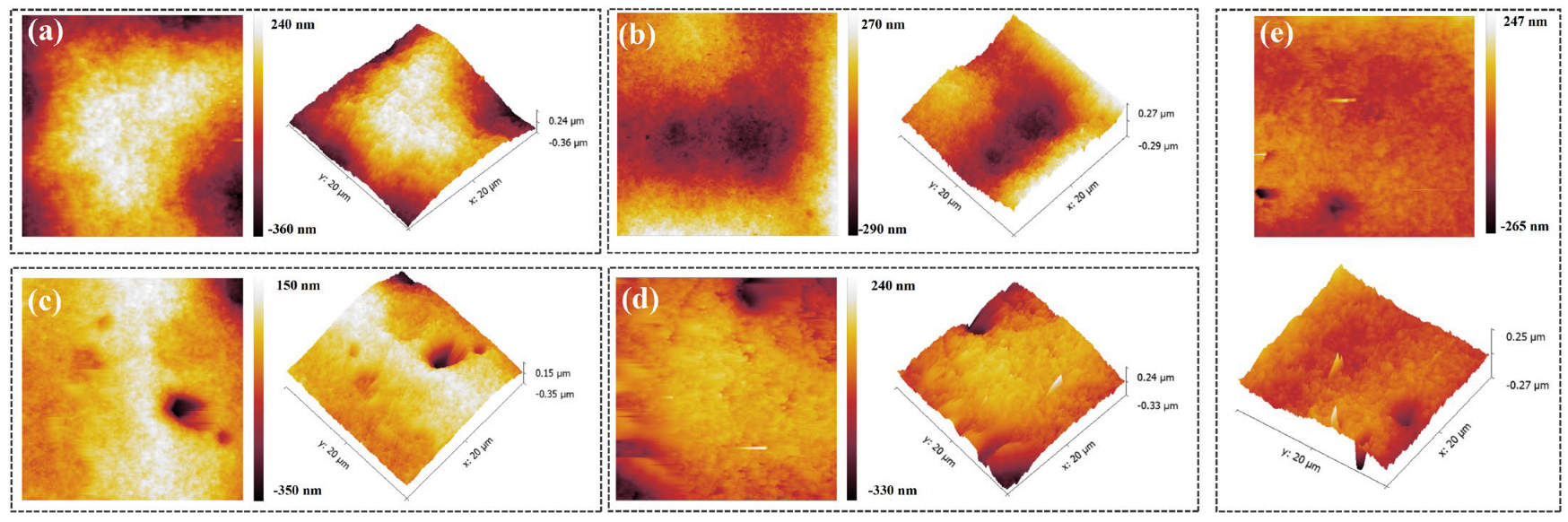
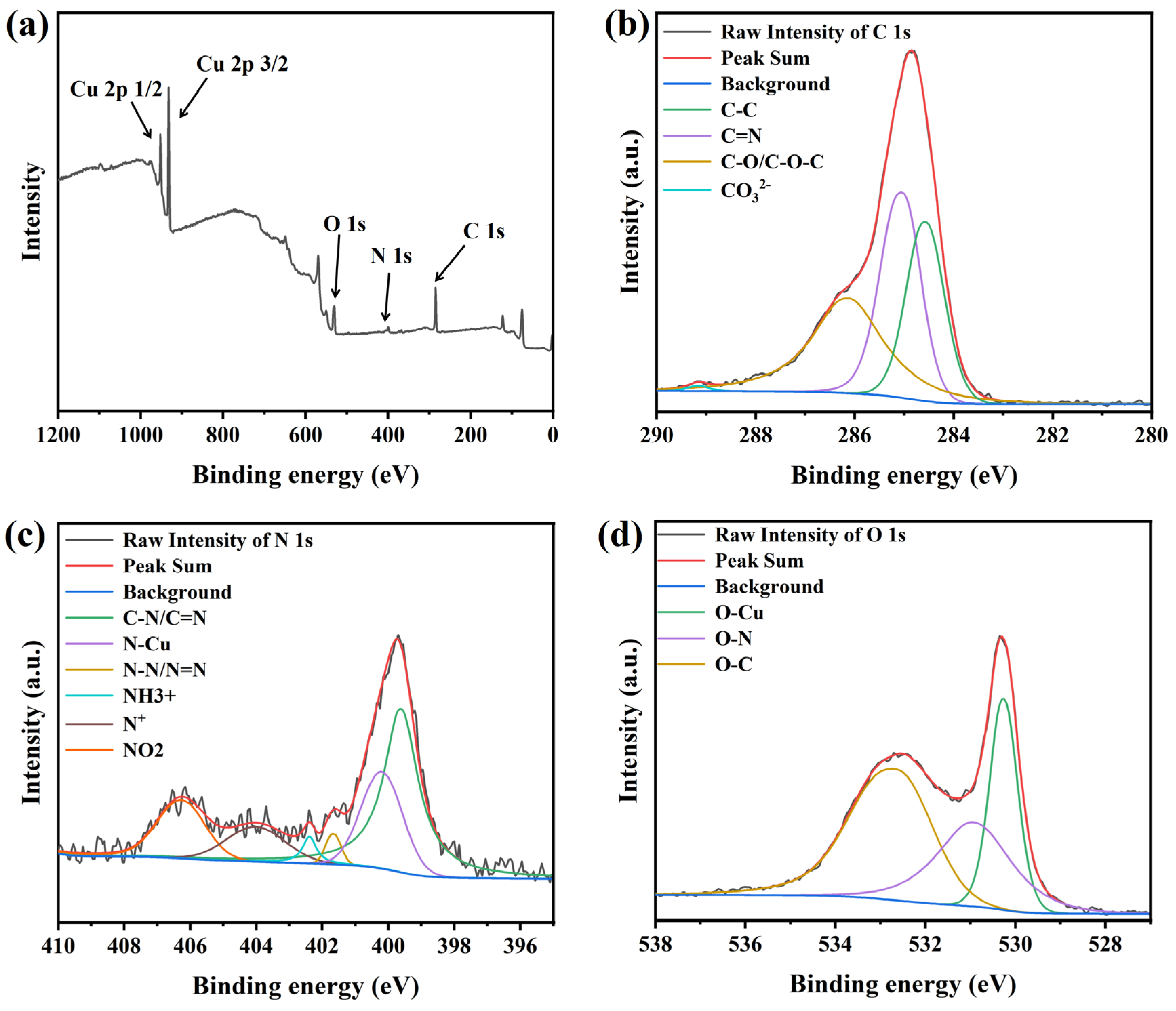
3.3. Electrodeposition and Characterization Analysis
4. Conclusions
- (1)
- Electrochemical tests show that NBT alone suppresses copper deposition and shows strong synergism with PEG, forming a dense suppressor film that resists displacement by SPS. The NBT–PEG synergy is convection-sensitive and potential-sensitive, and its incorporation markedly improves the plating performance of the PEG–SPS–NBT additive system;
- (2)
- DFT calculations identify the nitro groups and the tetrazolium ring as the principal reactive sites: both accept back-donated electrons from Cu 3d orbitals, thereby reinforcing surface adsorption. The tetrazolium nitrogens coordinate strongly with Cu2+ ions, and the nitro oxygens act as hydrogen-bond acceptors for PEG hydroxyls, giving a robust NBT–PEG interaction with a binding energy of −30.98 kcal/mol;
- (3)
- MD simulations indicate that NBT alone adsorbs stably on Cu(111) through its nitro-substituted phenyl rings, with an adsorption energy of −128.27 kcal/mol. The presence of PEG increases this value to −191.51 kcal/mol and enables chemisorption via nitro, tetrazolium, and methoxy sites, an effect attributable to strong NBT–PEG intermolecular forces;
- (4)
- Electroplating experiments confirm that 30–40 ppm NBT yields void-free bottom-up filling in microvias. XPS verifies co-adsorption of PEG and NBT; the NBT anchors to the copper surface through its nitro, tetrazolium, methoxy, and aromatic sites. These mutually consistent results clarify the structure–function relationship that underlies the effectiveness of NBT as a leveler for microvia superfilling.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, K.; Wang, C.; Wang, J.; Hong, Y.; Chen, Y.; He, W.; Zhou, J.; Miao, H.; Chen, Q. Convection-Dependent Competitive Adsorption between SPS and EO/PO on Copper Surface for Accelerating Trench Filling. J. Electrochem. Soc. 2019, 166, D93–D98. [Google Scholar] [CrossRef]
- Lau, J.H. Recent Advances and New Trends in Flip Chip Technology. J. Electron. Packag. 2016, 138, 030802. [Google Scholar] [CrossRef]
- Kim, M.J.; Seo, Y.; Kim, H.C.; Lee, Y.; Choe, S.; Kim, Y.G.; Cho, S.K.; Kim, J.J. Galvanostatic Bottom-up Filling of TSV-like Trenches: Choline-Based Leveler Containing Two Quaternary Ammoniums. Electrochim. Acta 2015, 163, 174–181. [Google Scholar] [CrossRef]
- Tan, T.; Liu, R.; Guo, L.; He, Z.; Fan, X.; Ye, R.; Tao, C. Investigation of 2-Mercapto-1-Methylimidazole as a New Type of Leveler in Wafer Electroplating Copper. Materials 2025, 18, 1622. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Zhang, X.; Liu, C.Z.; Liu, H.Y. Effect of Reverse Pulse on Additives Adsorption and Copper Filling for Through Silicon Via. J. Electrochem. Soc. 2018, 166, D3006–D3012. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Li, R.; Peng, X.; Zhang, J.; Yang, P.; Wang, G.; Wang, B.; Broekmann, P.; An, M. Experimental and Theoretical Study of the New Leveler Basic Blue 1 during Copper Superconformal Growth. ACS Appl. Mater. Interfaces 2023, 15, 47628–47639. [Google Scholar] [CrossRef]
- Moffat, T.P.; Wheeler, D.; Huber, W.H.; Josell, D. Superconformal Electrodeposition of Copper. Electrochem. Solid-State Lett. 2001, 4, C26. [Google Scholar] [CrossRef]
- Moffat, T.P.; Bonevich, J.E.; Huber, W.H.; Stanishevsky, A.; Kelly, D.R.; Stafford, G.R.; Josell, D. Superconformal Electrodeposition of Copper in 500–590 Nm Features. J. Electrochem. Soc. 2000, 147, 4524. [Google Scholar] [CrossRef]
- Akolkar, R.; Landau, U. A Time-Dependent Transport-Kinetics Model for Additive Interactions in Copper Interconnect Metallization. J. Electrochem. Soc. 2004, 151, C702–C711. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, M.J.; Kim, J.J. Competitive Adsorption between Bromide Ions and Bis(3-Sulfopropyl)-Disulfide for Cu Microvia Filling. Electrochim. Acta 2021, 370, 137707. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, S.; Qiang, Y.; Chen, S.; Luo, L.; Gao, J.; Feng, L.; Qin, Z. 4,6-Dimethyl-2-Mercaptopyrimidine as a Potential Leveler for Microvia Filling with Electroplating Copper. RSC Adv. 2017, 7, 40342–40353. [Google Scholar] [CrossRef]
- Tao, Z.; Long, Z.; Tengxu, L.; Liu, G.; Tao, X. The Synergistic Effects of Additives on the Micro Vias Copper Filling. J. Electroanal. Chem. 2022, 918, 116456. [Google Scholar] [CrossRef]
- Dow, W.-P.; Yen, M.-Y.; Liao, S.-Z.; Chiu, Y.-D.; Huang, H.-C. Filling Mechanism in Microvia Metallization by Copper Electroplating. Electrochim. Acta 2008, 53, 8228–8237. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Jin, L.; Yang, J.-Q.; Li, W.-Q.; Wu, D.-Y.; Zhan, D.; Yang, F.-Z.; Sun, S.-G. A Transfer-Adsorption Model for Forward Understanding the Synergistic Effects of Additives in through-Hole Uniform Copper Thickening. J. Electroanal. Chem. 2023, 936, 117373. [Google Scholar] [CrossRef]
- Li, Y.-B.; Wang, W.; Li, Y.-L. Adsorption Behavior and Related Mechanism of Janus Green B during Copper Via-Filling Process. J. Electrochem. Soc. 2009, 156, D119–D124. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Jin, L.; Li, G.; Yang, J.-Q.; Li, W.-Q.; Zhan, D.; Jiang, Y.-X.; Yang, F.-Z.; Sun, S.-G. Electrochemical and In-Situ FTIR Spectroscopic Studies of Gentian Violet as a Novel Leveler in through-Holes Metallization for Printed Circuit Board Applications. Electrochim. Acta 2022, 410, 140018. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Xu, J.; Li, J.; Lv, J.; Zhao, M.; Wang, L. Quinacridone Skeleton as a Promising Efficient Leveler for Smooth and Conformal Copper Electrodeposition. Dye. Pigment. 2020, 181, 108594. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, B.; Li, X.; Zou, P.; Du, K.; Zhou, N.; Wang, L. Investigation on Synthesis and Mechanism of a Sudan Ⅰ Copper Electrodeposition Leveler Based on Influencing Ion Diffusion Strategy. Dye. Pigment. 2024, 227, 112156. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, X.; Zhou, M.; Huang, W.; Xu, Q. The Effect of Quaternary Ammonium Salts with Different Chain Lengths on Copper Filling Behavior in Blind Holes of Printed Circuit Board. J. Micromech. Microeng. 2022, 32, 125004. [Google Scholar] [CrossRef]
- Zhu, H.P.; Zhu, Q.S.; Zhang, X.; Liu, C.Z.; Wang, J.J. Microvia Filling by Copper Electroplating Using a Modified Safranine T as a Leveler. J. Electrochem. Soc. 2017, 164, D645–D651. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Z.-Y.; Cai, Z.-Y.; Yang, J.-Q.; Zheng, A.-N.; Yang, F.-Z.; Wu, D.-Y.; Zhan, D. 1-(2-Pyridylazo)-2-Naphthol as a Synergistic Additive for Improving Throwing Power of through Hole Copper Electronic Electroplating. J. Ind. Eng. Chem. 2023, 125, 269–276. [Google Scholar] [CrossRef]
- Yang, J.-Q.; Qiu, J.-P.; Jin, L.; Wang, Z.-Y.; Song, T.; Zhao, Y.; Yang, X.-H.; Cheng, J.; Yang, F.-Z.; Zhan, D.-P. Molecular Structure Impacts of Tetrazole Derivatives on Their Diffusion and Adsorption Behaviors for Microvia Copper Void-Free Filling. Surf. Interfaces 2023, 44, 103679. [Google Scholar] [CrossRef]
- Wei, X.; Tang, S.; Ye, J.; Liu, X.; Dai, P.; He, W.; Huang, G.; Cai, Y.; Sun, C.; You, H. Experimental and Theoretical Study of Tetrazolium Derivatives as Levelers for Copper Superfilling in Microvias. Appl. Surf. Sci. 2025, 689, 162456. [Google Scholar] [CrossRef]
- Dow, W.-P.; Li, C.-C.; Su, Y.-C.; Shen, S.-P.; Huang, C.-C.; Lee, C.; Hsu, B.; Hsu, S. Microvia Filling by Copper Electroplating Using Diazine Black as a Leveler. Electrochim. Acta 2009, 54, 5894–5901. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yang, P.; An, M. Electrochemical Behaviors of Janus Green B in Through-Hole Copper Electroplating: An Insight by Experiment and Density Functional Theory Calculation Using Safranine T as a Comparison. Electrochim. Acta 2013, 92, 356–364. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, L.; Wang, W.; Wang, Z.; Zhao, C. Tetrazole Derived Levelers for Filling Electroplated Cu Microvias: Electrochemical Behaviors and Quantum Calculations. Electrochim. Acta 2015, 178, 546–554. [Google Scholar] [CrossRef]
- Teng, X.; Tao, Z.; Long, Z.; Liu, G.; Tao, X. 1-(4-Hydroxyphenyl)-2 H-Tetrazole-5-Thione as a Leveler for Acid Copper Electroplating of Microvia. RSC Adv. 2022, 12, 16153–16164. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Lei, Z.; Mou, Y.; Chen, M. Computational and Experiments Exploration of Convection on Cu Filling Characteristics of Multiple Aspect-Ratio Micro through-Holes. Electrochim. Acta 2022, 416, 140218. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, Y.; Mou, Y.; Chen, M. Promotion of High-Speed Copper-Filling Performance for Interconnections with Increasing Aspect-Ratio Using Compound Additives. Micromachines 2022, 13, 1539. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Z.; Peng, Y.; Su, P.; Luo, X.; Chen, M. Numerical and Experimental Investigation of 2-Thiazoline-2-Thiol and Nitrotetrazolium Blue Chloride Compound as Levelers for through-Hole Copper Electroplating. Mater. Today Commun. 2024, 40, 110003. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent Gradient Model Based on Hirshfeld Partition: A New Method for Visual Study of Interactions in Chemical Systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.R. Analysis of Current-Potential Hysteresis during Electrodeposition of Copper with Additives. J. Electrochem. Soc. 2001, 148, C726–C732. [Google Scholar] [CrossRef]
- Wei, X.-F.; Zhu, Q.-S.; Guo, J.-D.; Shang, J.-K. Obtaining Ultra-High Throwing Power in Cu Electroplating of Flexible Printed Circuit by Fast Consumption of a Suppressor. J. Solid State Electr. 2021, 26, 171–181. [Google Scholar] [CrossRef]
- Braun, T.M.; Josell, D.; Moffat, T.P. Microelectrode Studies of S-NDR Copper Electrodeposition: Potentiodynamic and Galvanodynamic Measurements and Simulations. J. Electrochem. Soc. 2020, 167, 082509. [Google Scholar] [CrossRef]
- Feng, Z.V.; Li, X.; Gewirth, A.A. Inhibition Due to the Interaction of Polyethylene Glycol, Chloride, and Copper in Plating Baths: A Surface-Enhanced Raman Study. J. Phys. Chem. B 2003, 107, 9415–9423. [Google Scholar] [CrossRef]
- Josell, D.; Wheeler, D.; Moffat, T.P. Modeling Extreme Bottom-Up Filling of Through Silicon Vias. J. Electrochem. Soc. 2012, 159, D570–D576. [Google Scholar] [CrossRef]
- Yan, J.-J.; Chang, L.-C.; Lu, C.-W.; Dow, W.-P. Effects of Organic Acids on Through-Hole Filling by Copper Electroplating. Electrochim. Acta 2013, 109, 1–12. [Google Scholar] [CrossRef]
- Kondo, K.; Matsumoto, T.; Watanabe, K. Role of Additives for Copper Damascene Electrodeposition. J. Electrochem. Soc. 2004, 151, C250–C255. [Google Scholar] [CrossRef]
- Takeuchi, K.; Suda, A.; Ushioda, S. Local Variation of the Work Function of Cu(111) Surface Deduced from the Low Energy Photoemission Spectra. Surf. Sci. 2001, 489, 100–106. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, S.; Wang, C.; Hong, Y.; Chen, Y.; Zhang, H.; Zhou, G.; He, W.; Ai, K.; Peng, Y. Computational Analysis and Experimental Evidence of Two Typical Levelers for Acid Copper Electroplating. Electrochim. Acta 2018, 273, 318–326. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, S.; Wang, C.; Hong, Y.; Zhou, G.; Chen, Y.; He, W.; Peng, Y.; Xiao, D. A Comparison of Typical Additives for Copper Electroplating Based on Theoretical Computation. Comp. Mater. Sci. 2018, 147, 95–102. [Google Scholar] [CrossRef]
- Li, X.; Zou, P.; Chen, X.; Wang, L. Quantum Chemical Calculations and Molecular Dynamics Simulations to Investigate the Mechanism of Interaction of Six Dye Levelers with Copper Surface. J. Electroanal. Chem. 2024, 961, 118230. [Google Scholar] [CrossRef]
- Fu, R.; Lu, T.; Chen, F.W. Comparing Methods for Predicting the Reactive Site of Electrophilic Substitution. Acta Phys.-Chim. Sin. 2014, 30, 628–639. [Google Scholar] [CrossRef]
- Brinck, T.; Carlqvist, P.; Stenlid, J.H. Local Electron Attachment Energy and Its Use for Predicting Nucleophilic Reactions and Halogen Bonding. J. Phys. Chem. A 2016, 120, 10023–10032. [Google Scholar] [CrossRef]
- Peng, X.; Li, L.; Jiang, J.; Li, Y.; Li, X.; Wang, G.; Liu, A.; Zhang, M.; Li, R.; An, M. Mechanistic Insight into the Janus Green B on Electrochemical Roughening Layer of Copper Foil. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134523. [Google Scholar] [CrossRef]
- Lipkowski, P.; Grabowski, S.J.; Robinson, T.L.; Leszczynski, J. Properties of the C−H···H Dihydrogen Bond: An Ab Initio and Topological Analysis. J. Phys. Chem. A 2004, 108, 10865–10872. [Google Scholar] [CrossRef]
- Jenkins, S.; Morrison, I. The Chemical Character of the Intermolecular Bonds of Seven Phases of Ice as Revealed by Ab Initio Calculation of Electron Densities. Chem. Phys. Lett. 2000, 108, 10865–10872. [Google Scholar] [CrossRef]
- Iravani, D.; Esmaeili, N.; Berisha, A.; Akbarinezhad, E.; Aliabadi, M.H. The Quaternary Ammonium Salts as Corrosion Inhibitors for X65 Carbon Steel under Sour Environment in NACE 1D182 Solution: Experimental and Computational Studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 656, 130544. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Abbout, S.; Dagdag, O.; Benkhaya, S.; Berisha, A.; Erramli, H.; Elharfi, A. Trifunctional Epoxy Polymer as Corrosion Inhibition Material for Carbon Steel in 1.0 M HCl: MD Simulations, DFT and Complexation Computations. Inorg. Chem. Commun. 2020, 115, 107858. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yang, P.; Zhang, B.; An, M. Through-Hole Copper Electroplating Using Nitrotetrazolium Blue Chloride as a Leveler. J. Electrochem. Soc. 2013, 160, D85–D88. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Wang, X.; Wei, X.; Lv, J.; Wang, L. Novel 2,5-Bis(6-(Trimethylamonium)Hexyl)-3,6-Diaryl-1,4-Diketopyrrolo[3,4-c]Pyrrole Pigments as Levelers for Efficient Electroplating Applications. Dye. Pigment. 2021, 186, 109064. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Yun, H.S.; Lee, C.-G. Temperature-Dependent Green Biosynthesis and Characterization of Silver Nanoparticles Using Balloon Flower Plants and Their Antibacterial Potential. J. Mol. Struct. 2019, 1177, 302–309. [Google Scholar] [CrossRef]
- Ahmed, M.; Byrne, J.A.; McLaughlin, J.A.D. Glycine Adsorption onto DLC and N-DLC Thin Films Studied by XPS and AFM. e-J. Surf. Sci. Nanotechnol. 2009, 7, 217–224. [Google Scholar] [CrossRef]
- Seo, J.; Chang, W.S.; Kim, T.-S. Adhesion Improvement of Graphene/Copper Interface Using UV/Ozone Treatments. Thin Solid Film. 2015, 584, 170–175. [Google Scholar] [CrossRef]
- Vimal Kumar, K.; Appa Rao, B.V.; Hebalkar, N.Y. Phosphorylated Chitin as a Chemically Modified Polymer for Ecofriendly Corrosion Inhibition of Copper in Aqueous Chloride Environment. Res. Chem. Intermed. 2017, 43, 5811–5828. [Google Scholar] [CrossRef]
- Batich, C.D.; Donald, D.S. X-Ray Photoelectron Spectroscopy of Nitroso Compounds: Relative Ionicity of the Closed and Open Forms. J. Am. Chem. Soc. 1984, 106, 2758–2761. [Google Scholar] [CrossRef]
- Nohira, H.; Tsai, W.; Besling, W.; Young, E.; Petry, J.; Conard, T.; Vandervorst, W.; De Gendt, S.; Heyns, M.; Maes, J.; et al. Characterization of ALCVD-Al2O3 and ZrO2 Layer Using X-Ray Photoelectron Spectroscopy. J. Non-Cryst. Solids 2002, 303, 83–87. [Google Scholar] [CrossRef]
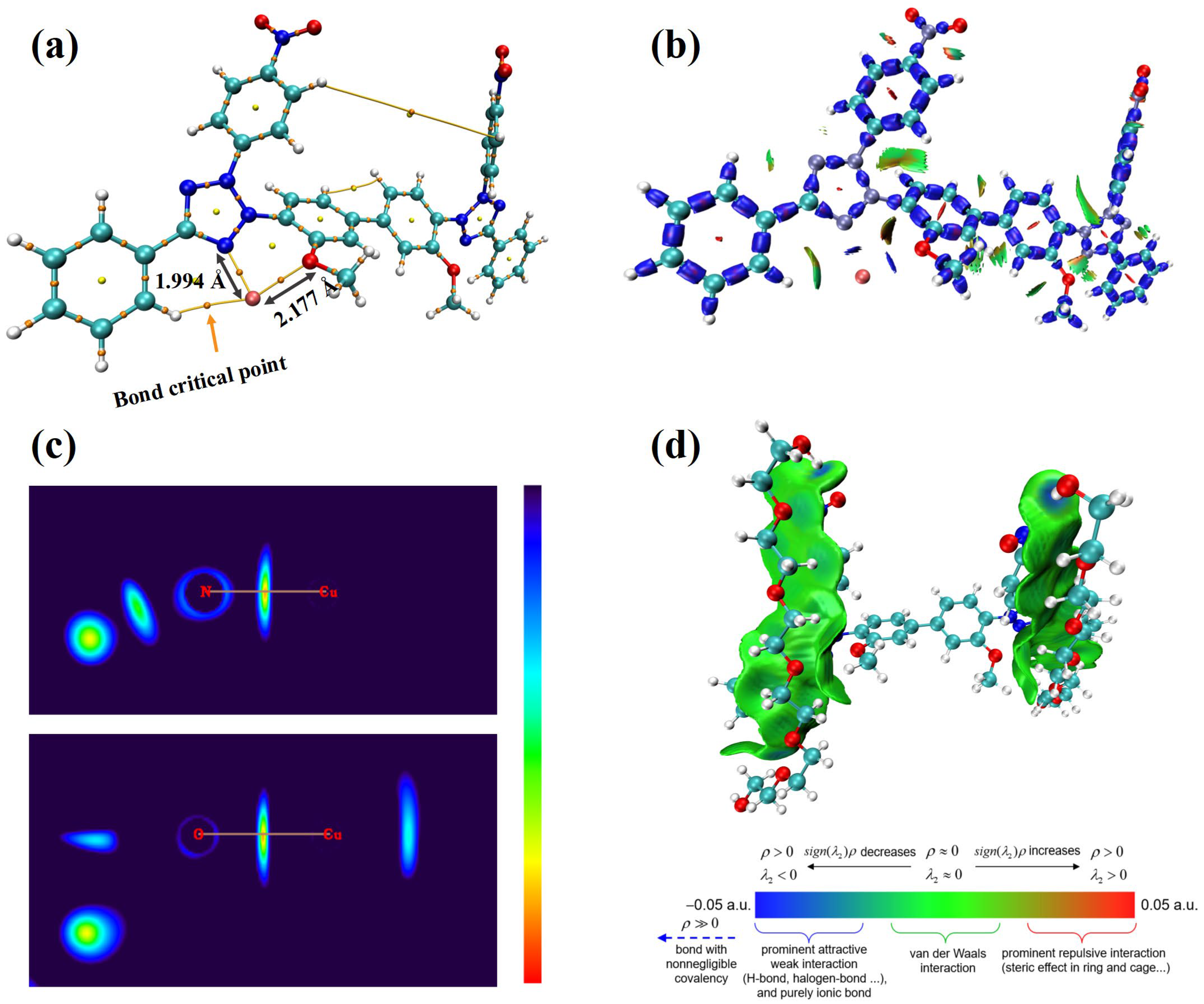
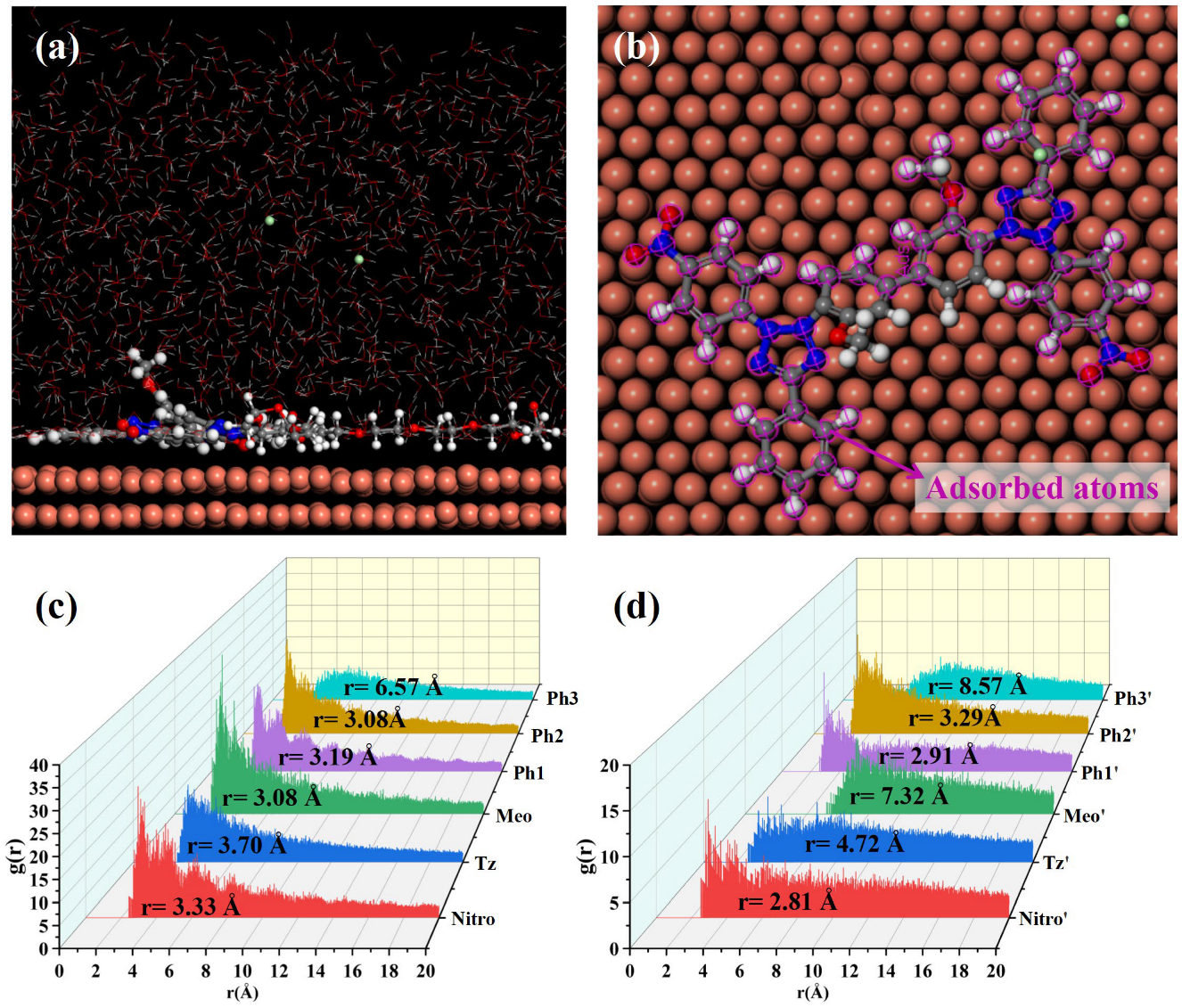
| Bond-Atoms | ρBCP × 102 (a.u.) | ∇2ρBCP × 102 (a.u.) | HBCP × 103 (a.u.) | VrBCP × 102 (a.u.) |
|---|---|---|---|---|
| Cu2+–N | 8.07 | 50.64 | −1.37 | −12.93 |
| Cu2+–O | 4.77 | 26.66 | 2.91 | −6.08 |
| NBT | 0 ppm | 10 ppm | 20 ppm | 30 ppm | 40 ppm |
|---|---|---|---|---|---|
| Rq (nm) | 113.0 | 87.7 | 53.8 | 56.8 | 26.7 |
| Ra (nm) | 131.9 | 105.1 | 71.0 | 74.5 | 35.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, D.; Wei, X.; Ye, J.; Lin, M.; Tang, S.; You, H. Integrated Electrochemical and Computational Elucidation of Nitro Blue Tetrazolium Chloride as an Efficient Leveler for Copper Microvia Superfilling. Micromachines 2025, 16, 721. https://doi.org/10.3390/mi16060721
Xing D, Wei X, Ye J, Lin M, Tang S, You H. Integrated Electrochemical and Computational Elucidation of Nitro Blue Tetrazolium Chloride as an Efficient Leveler for Copper Microvia Superfilling. Micromachines. 2025; 16(6):721. https://doi.org/10.3390/mi16060721
Chicago/Turabian StyleXing, Dong, Xiangfu Wei, Jinge Ye, Mingsong Lin, Shengchang Tang, and Hui You. 2025. "Integrated Electrochemical and Computational Elucidation of Nitro Blue Tetrazolium Chloride as an Efficient Leveler for Copper Microvia Superfilling" Micromachines 16, no. 6: 721. https://doi.org/10.3390/mi16060721
APA StyleXing, D., Wei, X., Ye, J., Lin, M., Tang, S., & You, H. (2025). Integrated Electrochemical and Computational Elucidation of Nitro Blue Tetrazolium Chloride as an Efficient Leveler for Copper Microvia Superfilling. Micromachines, 16(6), 721. https://doi.org/10.3390/mi16060721






