Highly Efficient Inverted Organic Light-Emitting Devices with Li-Doped MgZnO Nanoparticle Electron Injection Layer
Abstract
1. Introduction
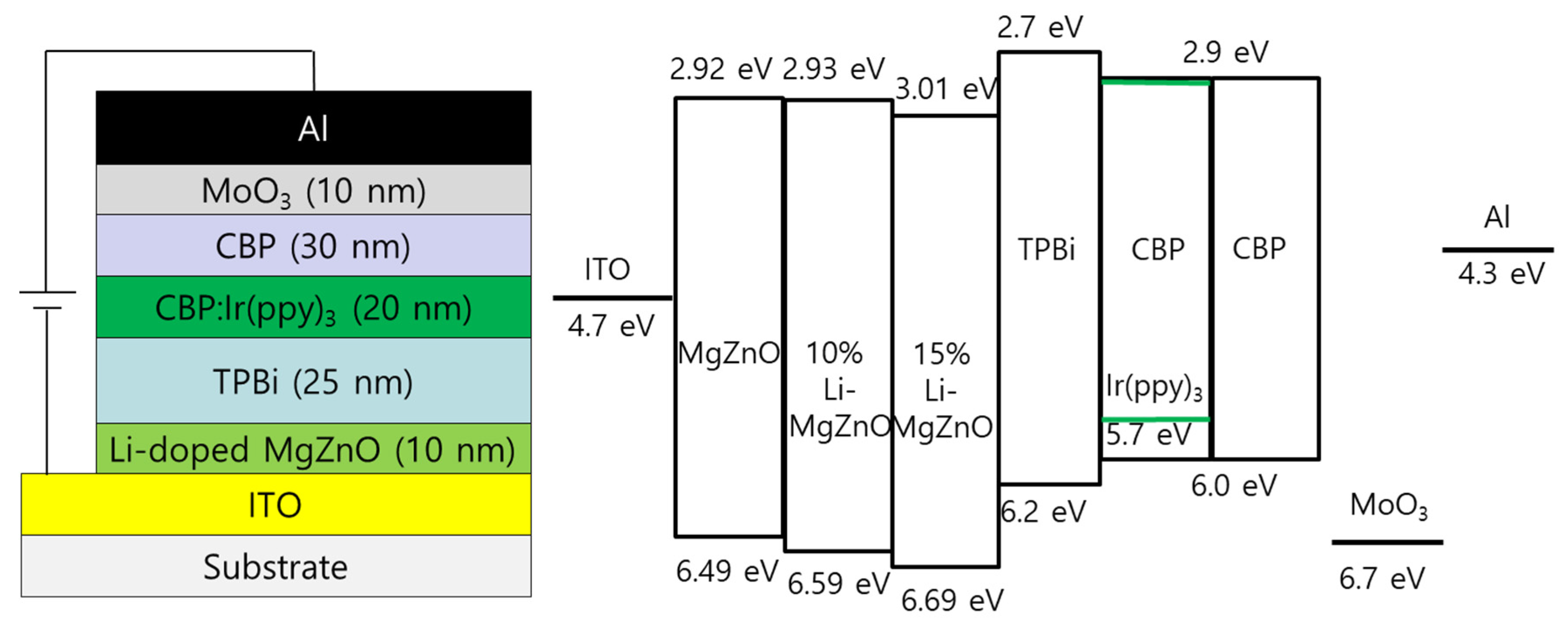
2. Materials and Methods
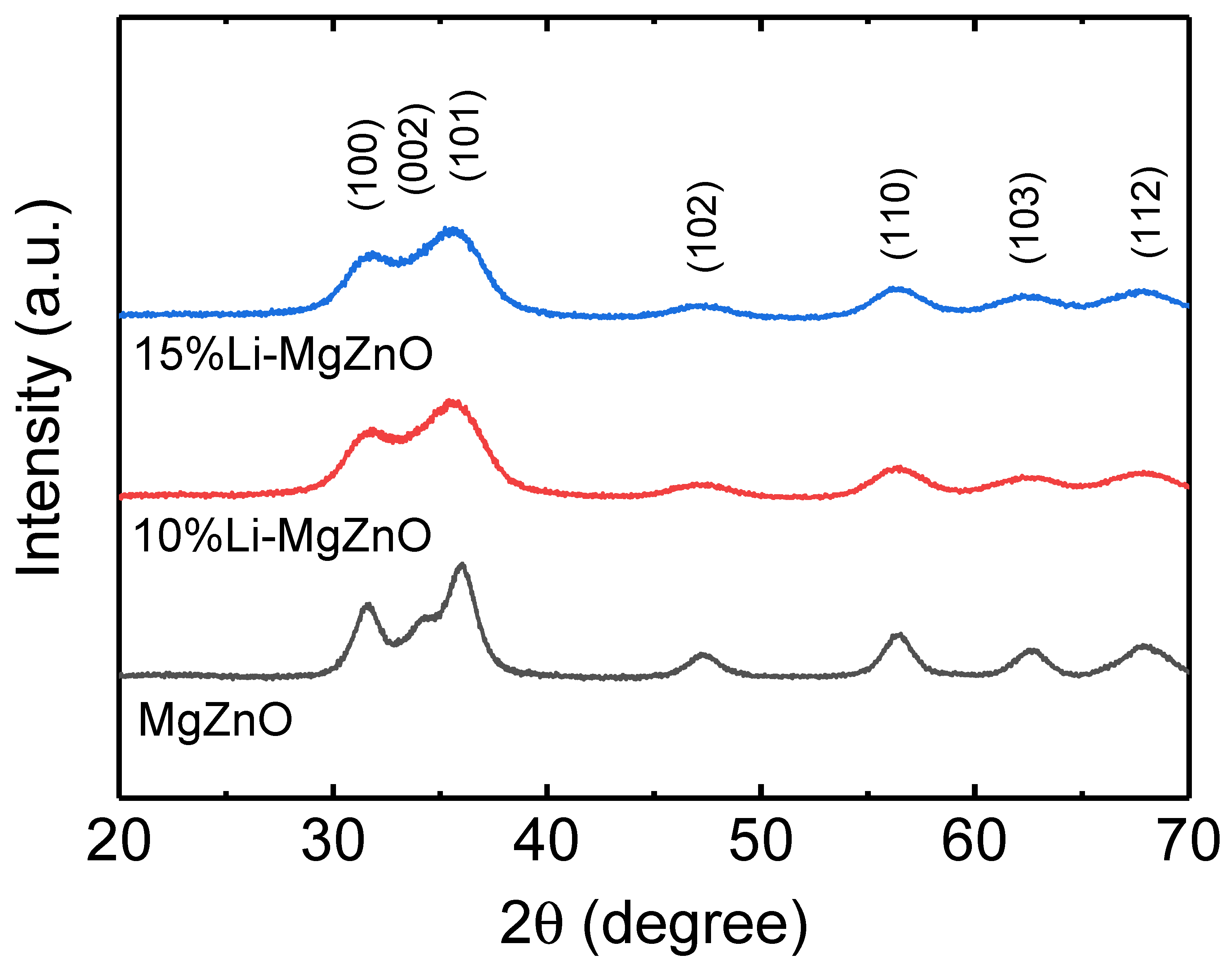
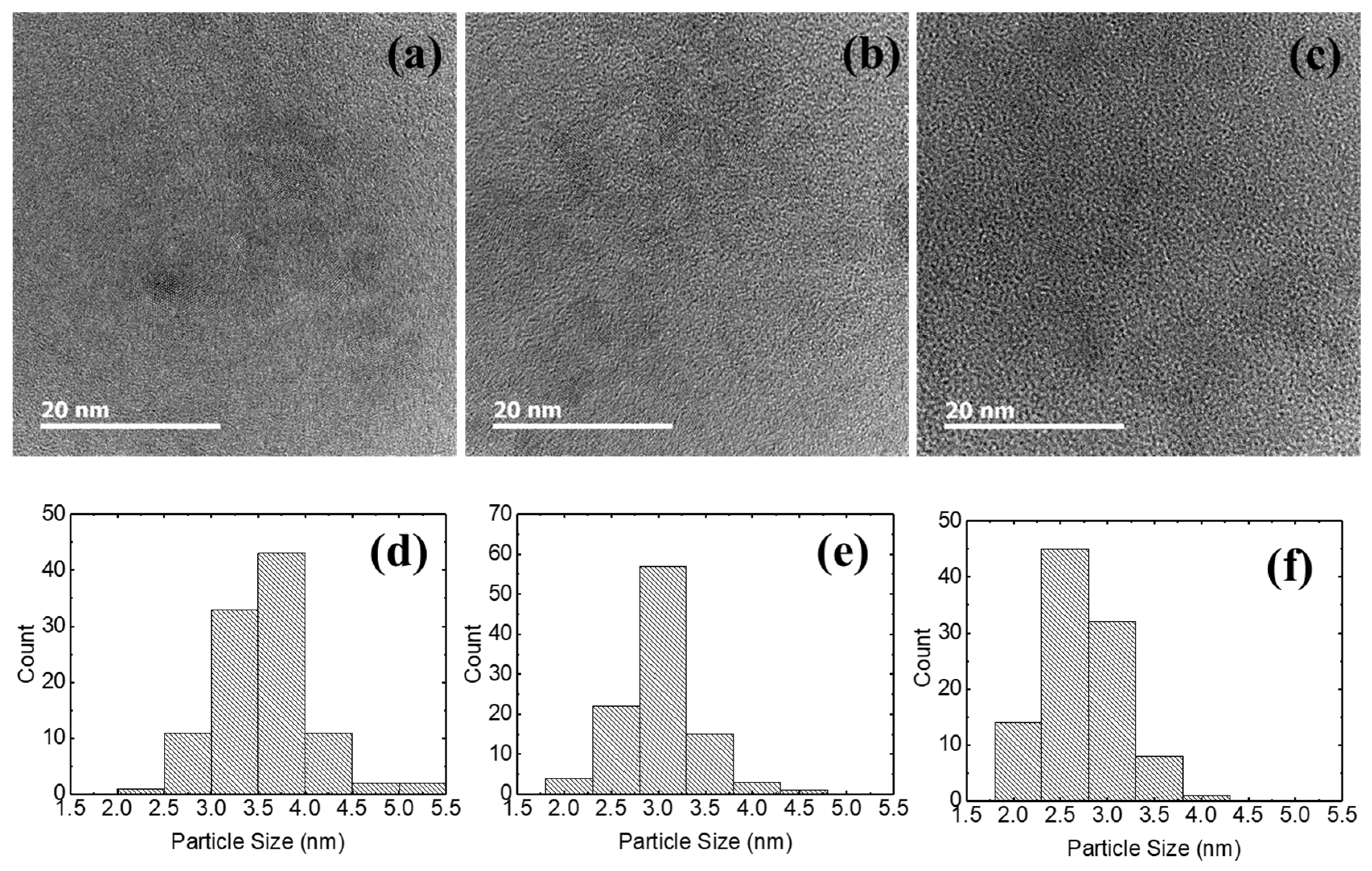
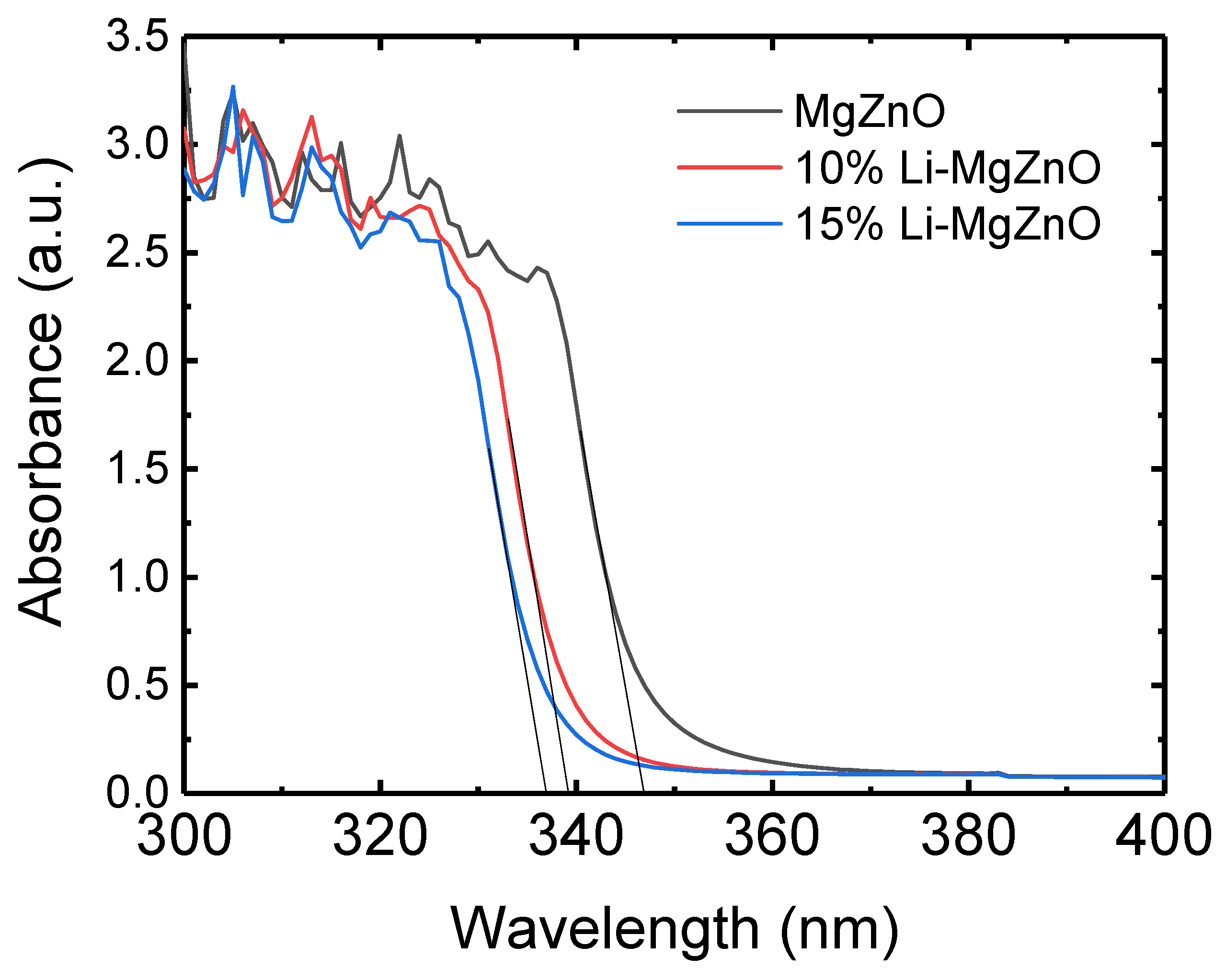
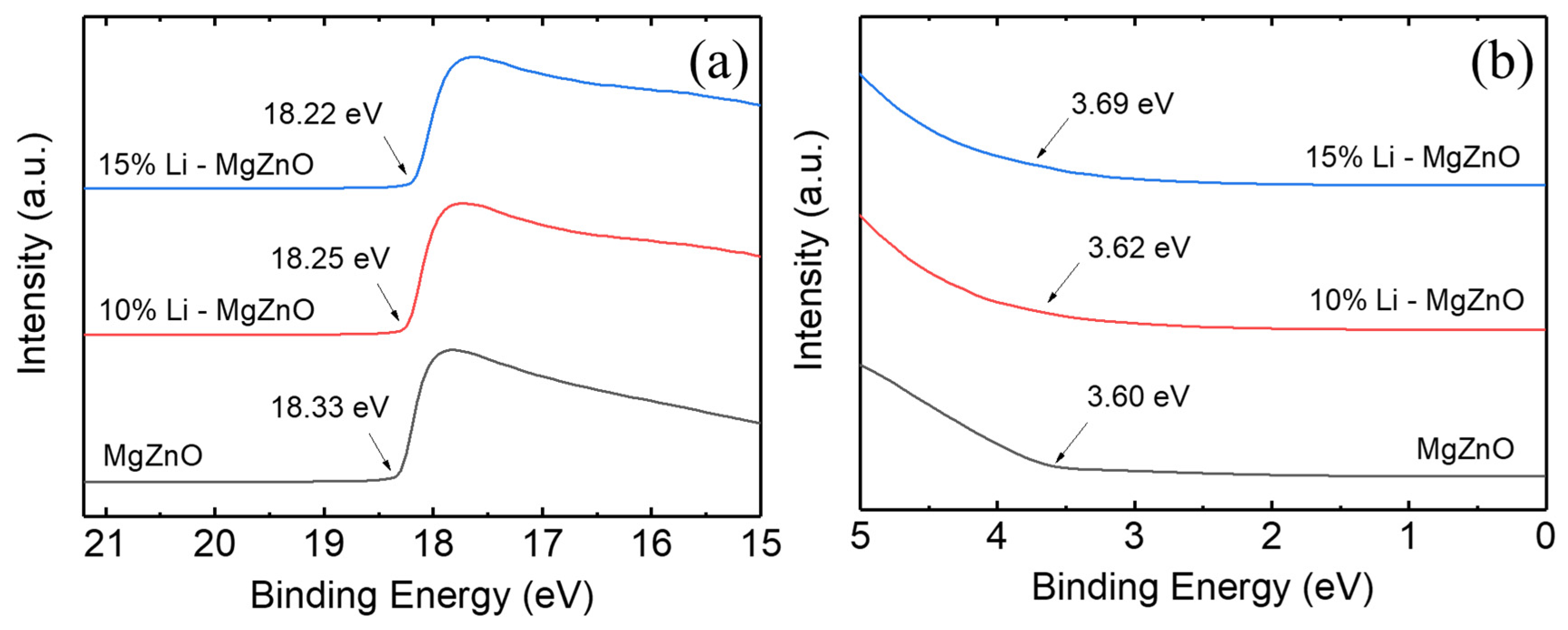
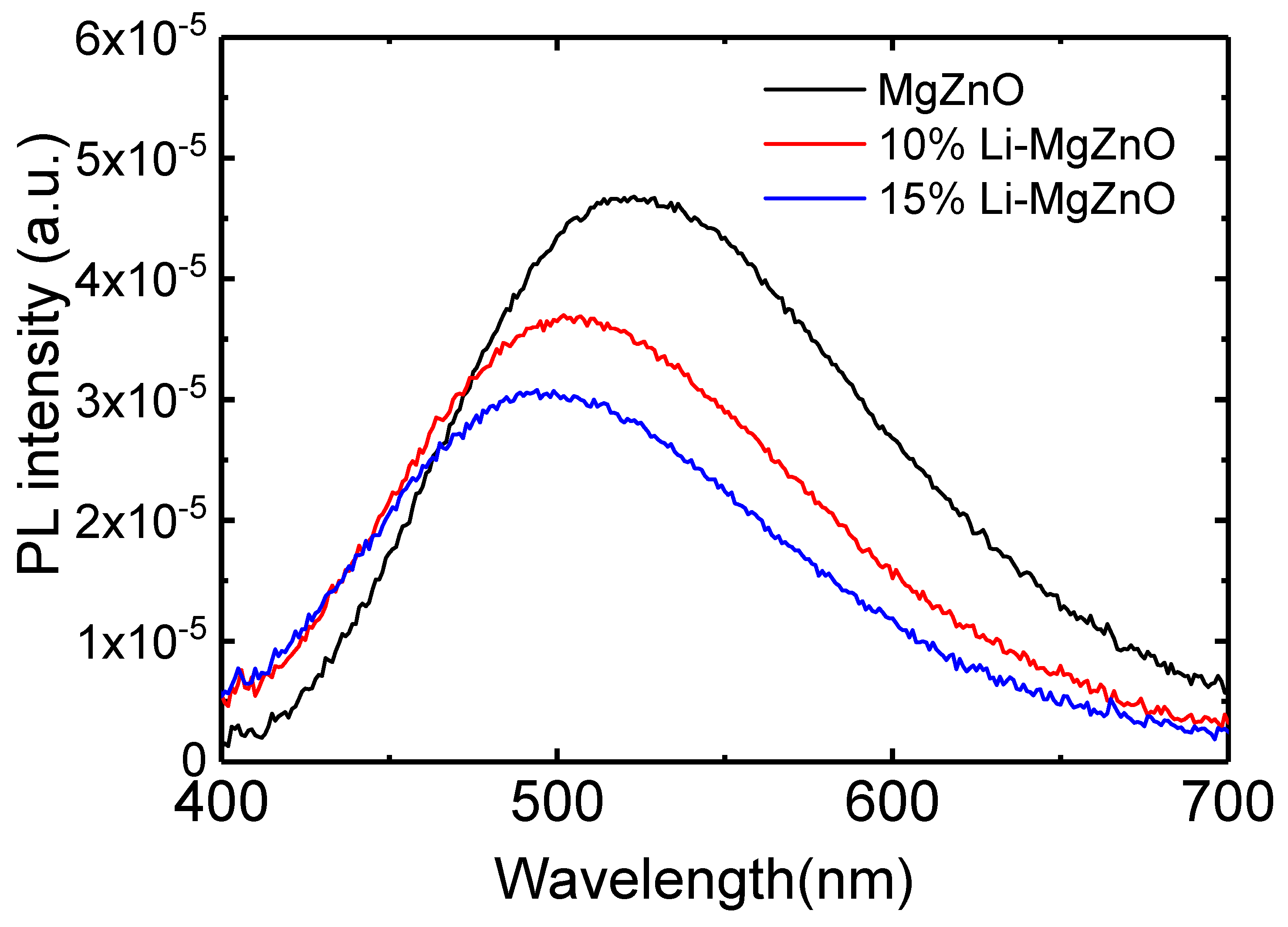
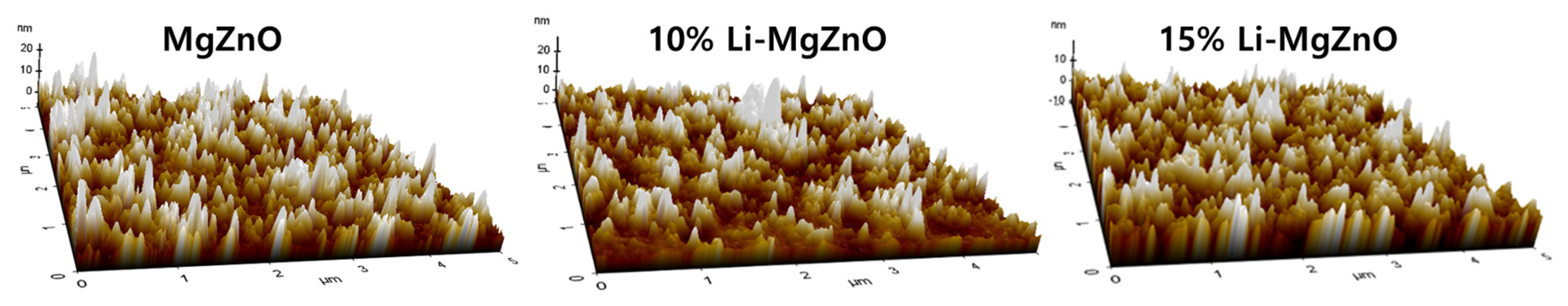
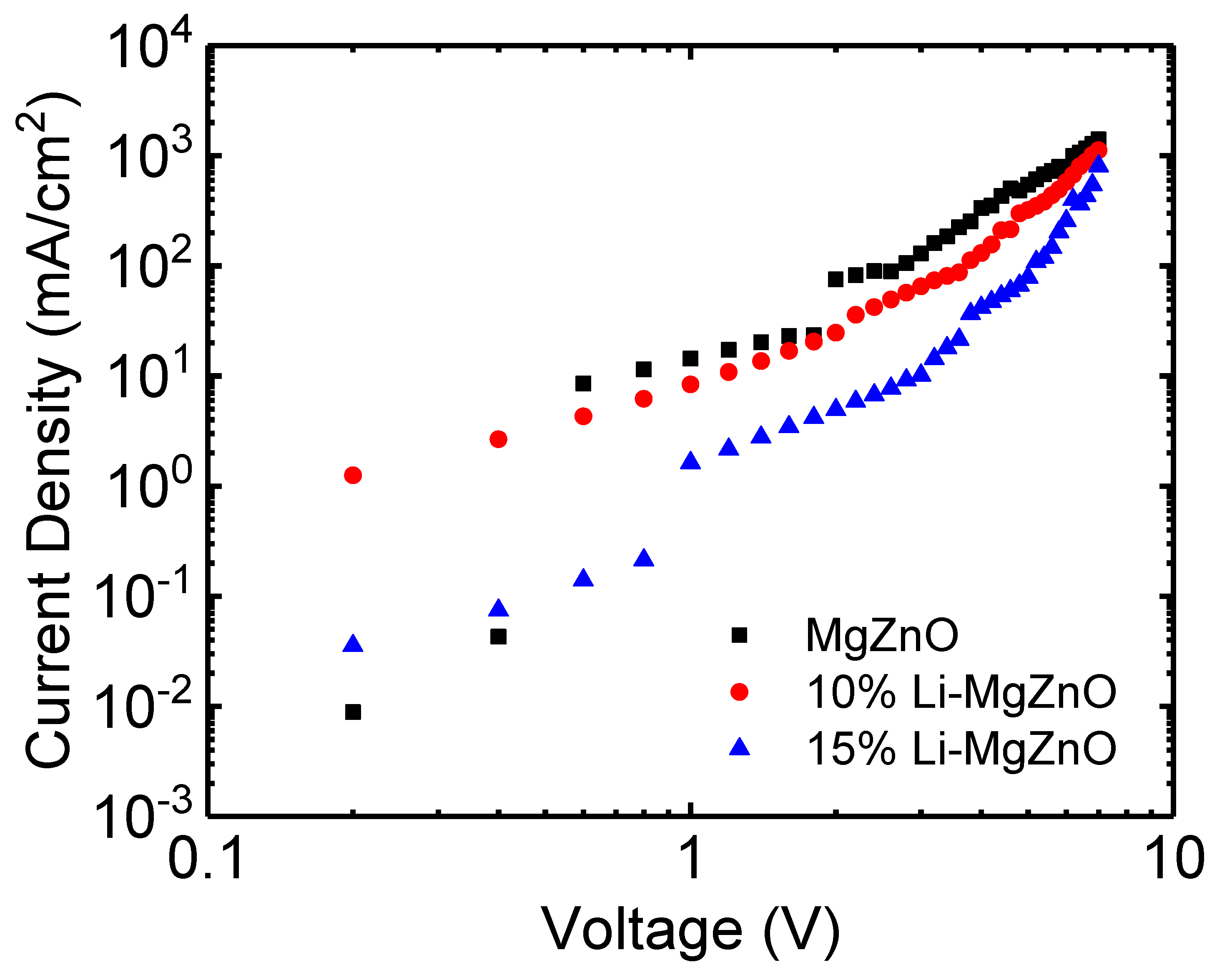
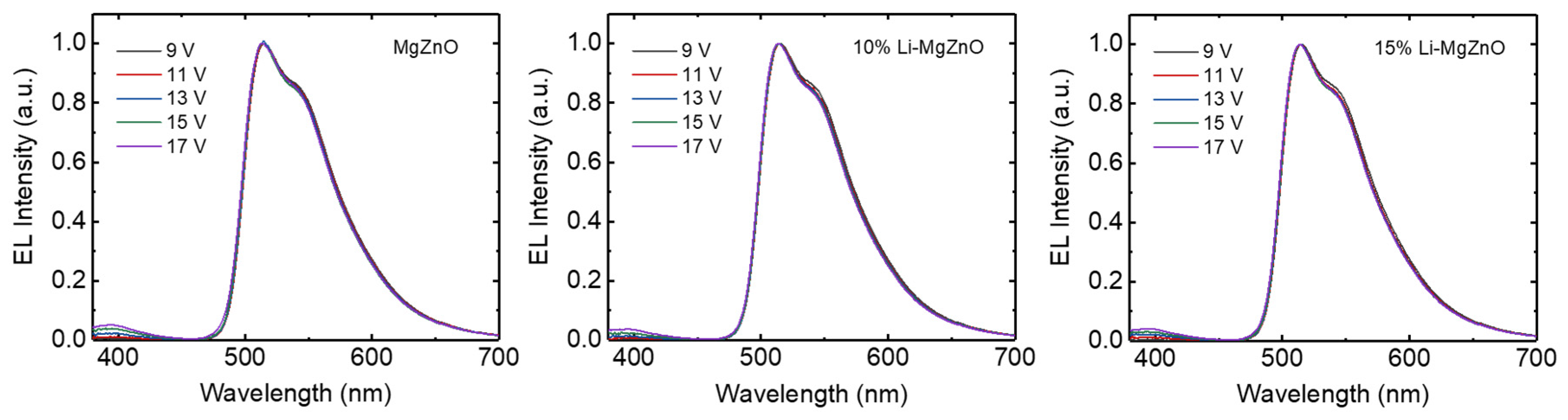
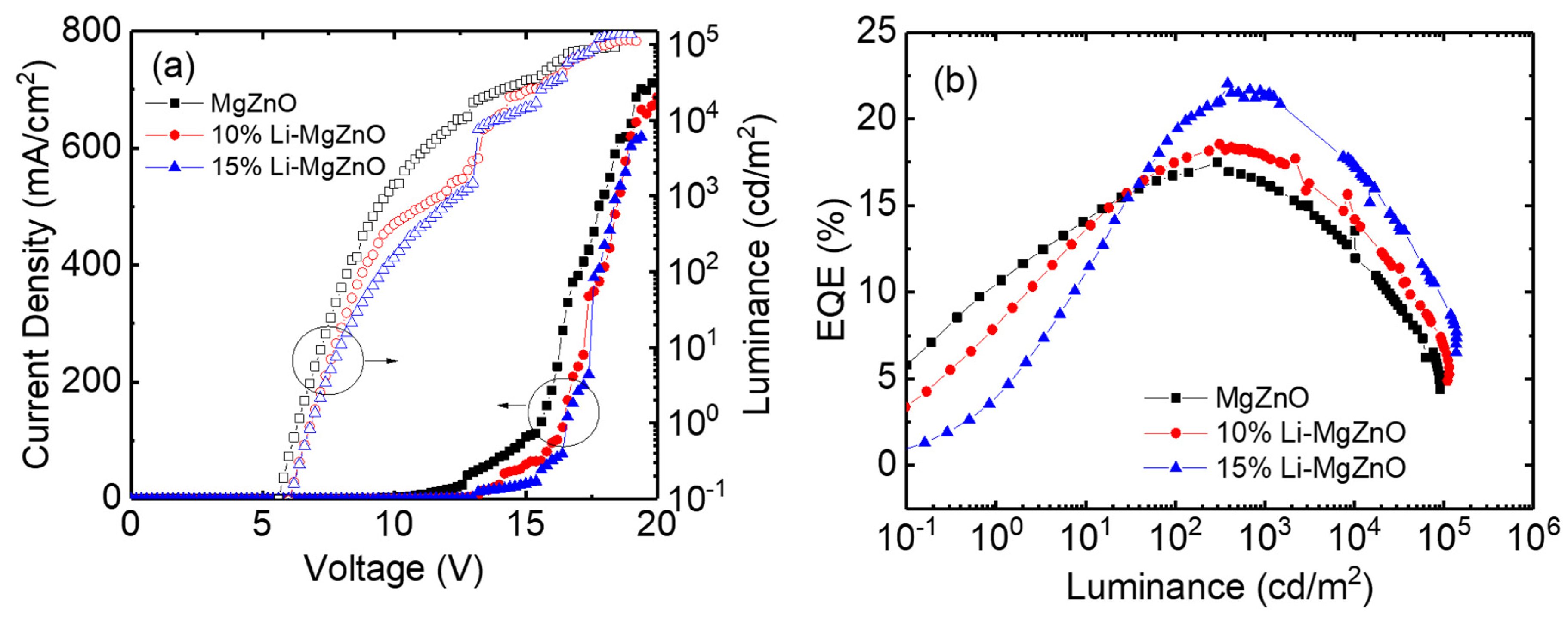
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A brief history of OLEDs-emitter development and industry milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef]
- Bauri, J.; Choudhary, R.B.; Mandal, G. Recent advances in efficient emissive materials-based OLED applications: A review. J. Mater. Sci. 2021, 56, 18837–18866. [Google Scholar] [CrossRef]
- Huseynova, G.; Lee, J.H.; Kim, Y.H.; Lee, J. Transparent organic light-emitting diodes: Advances, prospects, and challenges. Adv. Opt. Mater. 2021, 9, 2002040. [Google Scholar] [CrossRef]
- Chang, S.; Koo, J.H.; Yoo, J.; Kim, M.S.; Choi, M.K.; Kim, D.H. Flexible and stretchable light-emitting diodes and photodetectors for human-centric optoelectronics. Chem. Rev. 2024, 124, 768–859. [Google Scholar] [CrossRef]
- Ma, R.Q.; Hewitt, R.; Rajan, K.; Silvernail, J.; Urbanik, K.; Brown, J.J. Flexible active-matrix OLED displays: Challenges and progress. J. Soc. Inf. Disp. 2008, 16, 169–175. [Google Scholar] [CrossRef]
- Su, Y.; Geng, D.; Chen, Q.; Ji, H.; Li, M.; Shang, G.; Liu, L.; Chuai, X.; Huang, S.; Lu, N.; et al. Novel TFT-based emission driver in high performance AMOLED display applications. Org. Electron. 2021, 93, 106160. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.J.; Kim, H.S.; Park, J.S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Zhu, H.; Shin, E.S.; Liu, A.; Ji, D.; Xu, Y.; Noh, Y.Y. Printable semiconductors for backplane TFTs of flexible OLED displays. Adv. Func. Mater. 2020, 30, 1904588. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, J.; Yang, L.; Qu, M.; Qi, D.C.; Zhang, K.H.L. Wide bandgap oxide semiconductors: From materials physics to optoelectronic devices. Adv. Mater. 2021, 33, 2006230. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.Y.; Chen, J.F.; Chen, S.Y.; Chen, C.J.; Chen, C.H. Highly efficient and stable inverted bottom-emission organic light emitting devices. Appl. Phys. Lett. 2006, 89, 053503. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Zhang, Y.; Fu, J.; Qin, Y.; Li, R.; Chen, Y.; Lai, W.; Zhang, X.; Huang, W. Efficient inverted organic light-emitting devices using a charge-generation unit as electron-injection layers. Org. Electron. 2021, 96, 106202. [Google Scholar] [CrossRef]
- Yang, H.I.; Jeong, S.H.; Cho, S.M.; Lampande, R.; Lee, K.M.; Hong, J.A.; Choi, J.W.; Kim, B.S.; Park, Y.; Pode, R.; et al. Efficient cathode contacts through Ag-doping in multifunctional strong nucleophilic electron transport layer for high performance inverted OLEDs. Org. Electron. 2021, 89, 106031. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, X.; Wan, M.; Wang, S.; Lian, H.; Xu, R.; Zhu, W. High-performance inverted organic light-emitting didoes with extremely low efficiency roll-off using solution-processed ZnS quantum dots as the electron injection layer. RSC Adv. 2019, 9, 6042–6047. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsu, M.K.; Wu, S.W.; Chen, M.H.; Lin, H.H.; Li, C.S.; Pi, T.W.; Chang, H.H.; Chen, N.P. Using lithium carbonate-based electron injection structures in high-performance inverted organic light-emitting diodes. Phys. Chem. Chem. Phys. 2015, 17, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, L.; Sun, Y.Q.; Xie, Y.M.; Deng, Y.L.; Wang, Z.K.; Lia, L.S. Inverted and large flexible organic light-emitting diodes with low operation voltage. J. Mater. Chem. C 2015, 3, 12399–12402. [Google Scholar] [CrossRef]
- Chen, J.; Shi, C.; Fu, Q.; Zhao, F.; Hu, Y.; Feng, Y.; Ma, D. Solution-processable small molecules as efficient universal bipolar host for blue, green and red phosphorescent inverted OLEDs. J. Mater. Chem. 2012, 22, 5164–5170. [Google Scholar] [CrossRef]
- Lee, H.; Kang, C.M.; Park, M.; Kwak, J.; Lee, C. Improved efficiency of inverted organic light-emitting diodes using tin dioxide nanoparticles as an electron injection layer. ACS Appl. Mater. Interfaces 2013, 5, 1977–1981. [Google Scholar] [CrossRef]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size tunable ZnO nanoparticles to enhance electron injection in solution processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Kirkwood, N.; Singh, B.; Mulvaney, P. Enhancing quantum dot LED efficiency by tuning mobility in the ZnO electron transport layer. Adv. Mater. Interfaces 2016, 3, 1600868. [Google Scholar] [CrossRef]
- Sessolo, M.; Bolink, H.J. Hybrid organic-inorganic light-emitting diodes. Adv. Mater. 2011, 23, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Yang, M. Solution processed polymer light-emitting diodes utilizing a ZnO/organic ionic interlayer with Al cathode. Appl. Phys. Lett. 2010, 97, 243302. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Choudhury, K.R.; Bera, D.; So, F.; Xue, J.; Holloway, P.H. Electroluminescence from light-emitting polymer/ZnO nanoparticle heterojunction at sub-bandgap voltages. Nano Today 2010, 5, 384–389. [Google Scholar] [CrossRef]
- Chiba, T.; Pu, Y.J.; Hirasawa, M.; Masuhara, A.; Sasabe, H.; Kido, J. Solution-processed inorganic-organic hybrid electron injection layer for polymer light-emitting devices. ACS Appl. Mater. Interfaces 2012, 4, 6104–6108. [Google Scholar] [CrossRef]
- Lee, B.R.; Jung, E.D.; Park, J.S.; Nam, Y.S.; Min, S.H.; Kim, B.S.; Lee, K.M.; Jeong, J.R.; Friend, R.H.; Kim, J.S.; et al. Highly efficient inverted polymer light-emitting diodes using surface modifications of ZnO layer. Nat. Commun. 2014, 5, 4840. [Google Scholar] [CrossRef]
- Pu, Y.J.; Morishita, N.; Chiba, T.; Ohisa, S.; Igarashi, M.; Masuhara, A.; Kido, J. Efficient electron injection by size- and shape-controlled zinc oxide nanoparticles in organic light-emitting devices. ACS Appl. Mater. Interfaces 2015, 7, 25373–25377. [Google Scholar] [CrossRef]
- Murat, Y.; Langer, E.; Barnes, J.P.; Laurent, J.Y.; Wantz, G.; Hirsch, L.; Maindron, T. Bright and efficient inverted organic light-emitting diodes with improved solution-processed electron-transport interlayers. Org. Electron. 2017, 48, 377–381. [Google Scholar] [CrossRef]
- Kaçar, R.; Mucur, S.P.; Yildiz, F.; Dabak, S.; Tekin, E. Solution processed ternary blend nano-composite charge regulation layer to enhance inverted OLED performances. Appl. Phys. Lett. 2018, 112, 163302. [Google Scholar] [CrossRef]
- Khairnar, N.; Kwon, H.; Park, S.; Lee, H.; Park, J. Tailoring the size and shape of ZnO nanoparticles for enhanced performance of OLED device. Nanomaterials 2023, 13, 2816. [Google Scholar] [CrossRef]
- Lu, L.P.; Kabra, D.; Friend, R.H. Barium hydroxide as an interlayer between zinc oxide and a luminescent conjugated polymer for light-emitting didoes. Adv. Funct. Mater. 2012, 22, 4165–4171. [Google Scholar] [CrossRef]
- Hőfle, S.; Schienle, A.; Bernhard, C.; Bruns, M.; Lemmer, U.; Colsmann, A. Solution processed, white emitting tandem organic light-emitting diodes with inverted device architecture. Adv. Mater. 2014, 26, 5155–5159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Feng, H.; Yu, Z.; Zhang, J.; Liu, S.; Zhang, L.; Xie, W. An efficient and stable hybrid organic light-emitting device based on an inorganic metal oxide hole transport layer and an electron transport layer. J. Mater. Chem. C 2019, 7, 1991–1998. [Google Scholar] [CrossRef]
- Zhou, L.; Xiang, H.Y.; Zhu, Y.F.; Ou, Q.D.; Wang, Q.K.; Hu, R.; Huang, X.B.; Tang, J.X. Multifunctional silver nanoparticle interlayer-modified ZnO as the electron-injection layer for efficient inverted organic light-emitting diodes. ACS Appl. Mater. Interfaces 2019, 11, 9251–9258. [Google Scholar] [CrossRef]
- Chiba, T.; Pu, Y.J.; Kido, J. Solution-processable electron injection materials for organic light-emitting devices. J. Mater. Chem. C 2015, 3, 11567–11576. [Google Scholar] [CrossRef]
- Eun, Y.B.; Jang, G.P.; Yang, J.H.; Kim, S.Y.; Chae, Y.B.; Ha, M.Y.; Moon, D.G.; Kim, C.K. Performance improvement of quantum dot light-emitting diodes using a ZnMgO electron transport layer with a core/shell structure. Materials 2023, 16, 600. [Google Scholar] [CrossRef]
- Alexandrov, A.; Zvaigzne, M.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Al-, Ga-, Mg-, or Li-doped zinc oxide nanoparticles as electron transport layers for quantum dot light-emitting diodes. Sci. Rep. 2020, 10, 7496. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Cho, S.; Kim, J.; Shin, H.; Jang, J. Li and Mg co-doped zinc oxide electron transporting layer for highly efficient quantum dot light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 24028–24036. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Chen, D.; Ye, Y.; Gao, Y.; Peng, X.; Jin, Y. Inverted quantum dot light-emitting diodes with conductive interlayers of zirconium acetylacetonate. J. Mater. Chem. C 2019, 7, 3154–3159. [Google Scholar] [CrossRef]
- Manzhi, P.; Alam, M.B.; Kumari, R.; Krishna, R.; Singh, R.K.; Srivastava, R.; Sinha, O.P. Li-doped ZnO nanostructures for the organic light emitting diode application. Vacuum 2017, 146, 462–467. [Google Scholar] [CrossRef]
- Manzhi, P.; Kumari, R.; Alam, M.B.; Umapathy, G.R.; Krishna, R.; Ojha, S.; Srivastava, R.; Sinha, O.P. Mg-doped ZnO nanostructures for efficient organic light emitting diode. Vacuum 2019, 166, 370–376. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, C.Y.; Lee, K.H.; An, K.S.; Song, W.; Kim, J.; Oh, M.S.; Do, Y.R.; Yang, H. Performance improvement of quantum dot-light-emitting diodes enabled by an alloyed ZnMgO nanoparticle electron transport layer. Chem. Mater. 2015, 27, 197–204. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Feng, D.; Chen, L.; Fang, Y.; Shen, H.; Du, Z. Bandgap tunable Zn1-xMgxO thin films as electron transport layers for high performance quantum dot light-emitting diodes. J. Mater. Chem. C 2017, 5, 4724–4730. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Kun, Y.; Tuo, K.; Shang, J.; Du, W.; Qi, H.; Liu, S. The Zn1−xMgxO electron transport layer for charge balance in high-brightness inverted quantum-dot light-emitting diodes. J. Mater. Sci. Mater. Electron. 2024, 35, 754. [Google Scholar] [CrossRef]
- Wang, L.; Lin, J.; Liu, X.; Cao, S.; Wang, Y.; Zhao, J.; Zou, B. Mg-doped ZnO nanoparticle films as the interlayer between the ZnO electron transport layer and InP quantum dot layer for light-emitting diodes. J. Phys. Chem. C 2020, 124, 8758–8765. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Moyen, E.; Kim, J.H.; Kim, J.; Jang, J. ZnO nanoparticles for quantum-dot-based light-emitting diodes. ACS Appl. Nano Mater. 2020, 3, 5203–5211. [Google Scholar] [CrossRef]
- Ahmoum, H.; Boughrara, M.; Su’ait, M.S.; Kerouad, M. Effect of position and concentration of Li on ZnO physical properties: Density functional investigation. Chem. Phys. Lett. 2019, 719, 45–53. [Google Scholar] [CrossRef]
- Vidya, R.; Ravindran, P.; Fjellvåg, H. Ab-initio studies on Li-doping, Li-pairs, and complexes between Li and intrinsic defects in ZnO. J. Appl. Phys. 2012, 111, 123713. [Google Scholar] [CrossRef]
- Hirai, T.; Harada, Y.; Hashimoto, S.; Itoh, T.; Ohno, N. Luminescence of excitons in mesoscopic ZnO particles. J. Lumin. 2005, 112, 196–199. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experimental and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Viswanatha, R.; Sapra, S.; Satpati, B.; Satyam, P.V.; Dev, B.N.; Sarma, D.D. Understanding the quantum size effects in ZnO nanocrystals. J. Mater. Chem. 2004, 14, 661–668. [Google Scholar] [CrossRef]
- Gil, B.; Kavokin, A.V. Giant exciton-light coupling in ZnO quantum dots. Appl. Phys. Lett. 2002, 81, 748–750. [Google Scholar] [CrossRef]
- Igityan, A.; Aghamalyan, N.; Petrosyan, S.; Badalyan, G.; Kafadaryan, Y. Resistive switching in Li-doped ZnO films. Phys. Status Solidi A 2018, 215, 1700353. [Google Scholar] [CrossRef]
- Aida, M.S.; Hjiri, M. Temperature-dependent photoluminescence of Li-doped ZnO. J. Mater. Sci. Mater. Electron. 2020, 31, 10521–10530. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, J.; Zhao, J.; Yang, Z.; Li, C.; Guan, X.; Yang, W.; Shang, M.; Wu, T. Enhancing the performance of quantum dot light-emitting diodes using room-temperature-processed Ga-doped ZnO nanoparticles as the electron transport layer. ACS Appl. Mater. Interfaces 2017, 9, 15605–15614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y.; Xiang, D. Origin of visible photoluminescence of ZnO quantum dots: Defect-dependent and size-dependent. J. Phys. Chem. C 2010, 114, 9651–9658. [Google Scholar] [CrossRef]
- Li, N.; Li, T.; Li, L.; Li, J. Efficient and stable OLEDs with inverted device structure utilizing solution-processed ZnO-based electron injection layer. Adv. Opt. Mater. 2023, 11, 2300467. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, H.; Hwang, H.A.; Park, H.J.; Moon, D.G. Effect of oxidizing agent on the synthesis of ZnO nanoparticles for inverted phosphorescent organic light-emitting devices without multiple interlayers. Nanomaterials 2024, 14, 622. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, L.; Li, F.; Chen, Y. Nanostructured hybrid ZnO@CdS nanowalls grown in situ for inverted polymer solar cells. J. Mater. Chem. C 2014, 2, 1018–1027. [Google Scholar] [CrossRef]
- Hofbeck, T.; Yersin, H. The triplet state of fac-Ir(ppy)3. Inorg. Chem. 2010, 49, 9290–9299. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.H.; Sun, M.C.; Chou, H.H.; Li, C.H. Efficient pure-white organic light-emitting diodes with a solution-processed, binary-host employing single emission layer. Appl. Phys. Lett. 2006, 88, 141101. [Google Scholar] [CrossRef]
- Hwang, H.A.; Park, H.J.; Moon, D.G. Highly efficient inverted phosphorescent organic light-emitting devices with ZnO nanoparticles electron injection layer. Synth. Met. 2022, 287, 117078. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Jiang, C.; Fang, Y.; Bai, P.; Wang, Y. Study on electron transport characterization in TPBi thin films and OLED application. J. Phys. Chem. C 2021, 125, 16753–16758. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.-J.; Kim, G.-E.; Park, C.-J.; Lee, S.-B.; Kim, S.-Y.; Moon, D.-G. Highly Efficient Inverted Organic Light-Emitting Devices with Li-Doped MgZnO Nanoparticle Electron Injection Layer. Micromachines 2025, 16, 617. https://doi.org/10.3390/mi16060617
Yoo H-J, Kim G-E, Park C-J, Lee S-B, Kim S-Y, Moon D-G. Highly Efficient Inverted Organic Light-Emitting Devices with Li-Doped MgZnO Nanoparticle Electron Injection Layer. Micromachines. 2025; 16(6):617. https://doi.org/10.3390/mi16060617
Chicago/Turabian StyleYoo, Hwan-Jin, Go-Eun Kim, Chan-Jun Park, Su-Been Lee, Seo-Young Kim, and Dae-Gyu Moon. 2025. "Highly Efficient Inverted Organic Light-Emitting Devices with Li-Doped MgZnO Nanoparticle Electron Injection Layer" Micromachines 16, no. 6: 617. https://doi.org/10.3390/mi16060617
APA StyleYoo, H.-J., Kim, G.-E., Park, C.-J., Lee, S.-B., Kim, S.-Y., & Moon, D.-G. (2025). Highly Efficient Inverted Organic Light-Emitting Devices with Li-Doped MgZnO Nanoparticle Electron Injection Layer. Micromachines, 16(6), 617. https://doi.org/10.3390/mi16060617





