A Fluidics-Based Biosensor to Detect and Characterize Inhibition Patterns of Organophosphate to Acetylcholinesterase in Food Materials
Abstract
1. Introduction
| Acetylthiocholine + AChE + H2O → Thiocholine + Acetic acid | (i) |
| Thiocholine + Electrolysis → Dithiobischoline + 2H+ + 2e− | (ii) |
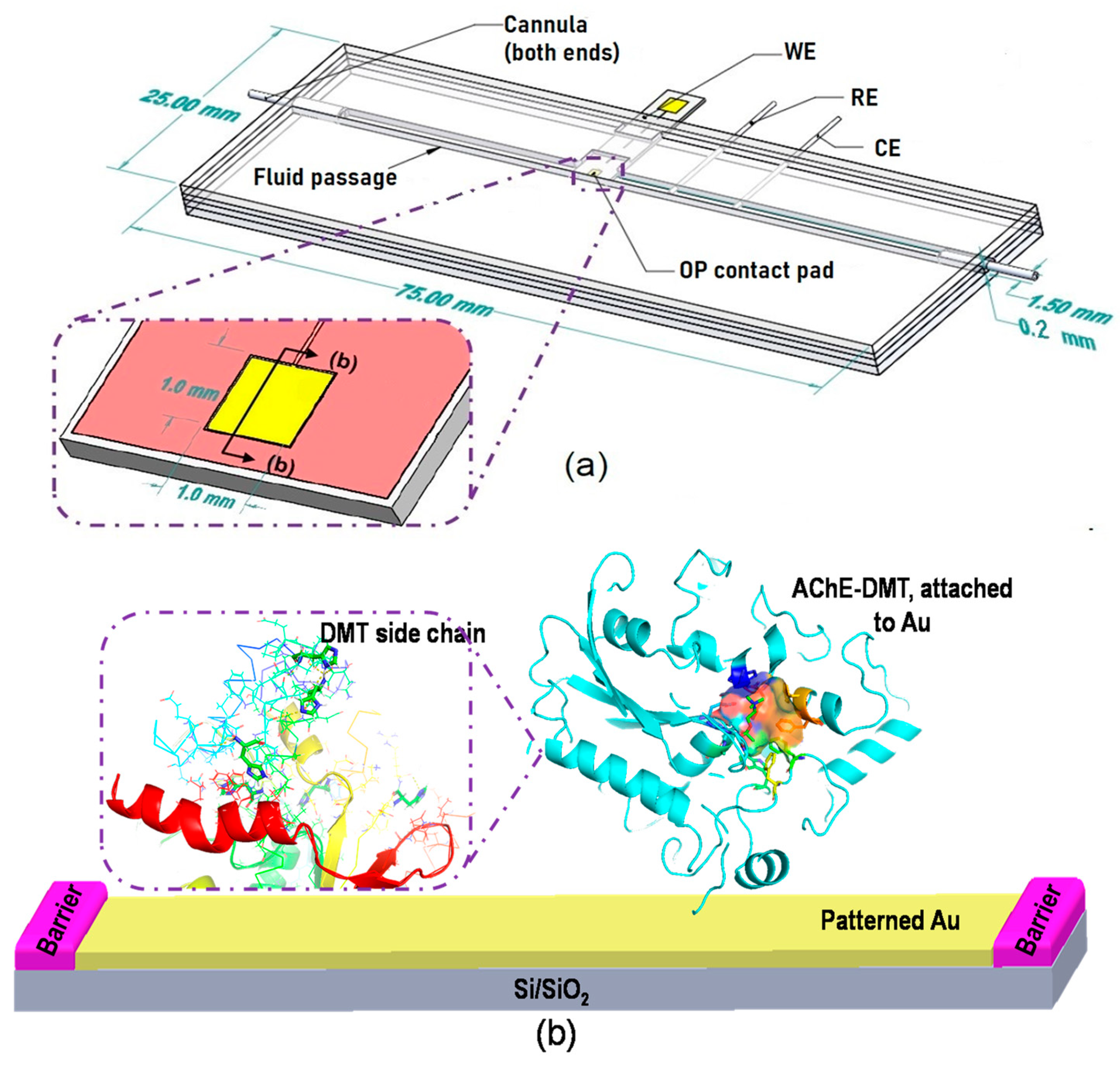
2. Materials and methods
2.1. Materials
2.2. Concentration-Response and Time-Response Study for AChE Inhibition
2.3. Immobilization of AChE onto Composited Electrodes
2.4. Characterization of Modified Electrodes
2.5. Fabrication of Chip-Based Biosensor
2.6. Electrochemical Detection of Dimethoate
2.7. Preparation of Food Samples
2.8. Analysis of Inhibition Patterns of Dimethoate against AChE
3. Results and Discussion
3.1. Characterizations of AChE-Modified Electrode
3.2. Measuring AChE Activity on Electrodes
3.3. Detecting Dimethoate and Determining Inhibition Patterns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melgar, M.J.; Santaeufemia, M.; Garcia, M.A. Organophosphorus pesticide residues in raw milk and infant formulas from Spanish northwest. J. Environ. Sci. Health B 2010, 45, 595–600. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, H.; Kannan, K. Organophosphorus Flame Retardants and Plasticizers in Breast Milk from the United States. Environ. Sci. Technol. Lett. 2019, 6, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Kandimalla, R.J.L.; Sharma, D.R.; Kaushal, A.; Ruban, A.; Sunkaria, A.; Vallamkondu, J.; Chiarugi, A.; Reddy, P.H.; Gill, K.D. Cell cycle activation in p21 dependent pathway: An alternative mechanism of organophosphate induced dopaminergic neurodegeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Santed, F.; Colomina, M.T.; Herrero Hernández, E. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016, 74, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, J.R.; Remy, M.T.; Erickson, C.M.; Dutca, L.M.; Brat, D.J.; Pieper, A.A. Occupational-like organophosphate exposure disrupts microglia and accelerates deficits in a rat model of Alzheimer’s disease. NPJ Aging Mech. Dis. 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Cockburn, M.; Ly, T.T.; Bronstein, J.M.; Ritz, B. The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occup. Environ. Med. 2014, 71, 275. [Google Scholar] [CrossRef]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Cholinergic Crisis Caused by Cholinesterase Inhibitors: A Retrospective Nationwide Database Study. J. Med. Toxicol. 2018, 14, 237–241. [Google Scholar] [CrossRef]
- Naughton, S.X.; Terry, A.V., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef]
- Aguilar-Garduño, C.; Lacasaña, M.; Blanco-Muñoz, J.; Rodríguez-Barranco, M.; Hernández, A.F.; Bassol, S.; González-Alzaga, B.; Cebrián, M.E. Changes in male hormone profile after occupational organophosphate exposure. A longitudinal study. Toxicology 2013, 307, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Seidler, F.J. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull. 2007, 72, 232–274. [Google Scholar] [CrossRef]
- Dorri, S.A.; Hosseinzadeh, H.; Abnous, K.; Hasani, F.V.; Robati, R.Y.; Razavi, B.M. Involvement of brain-derived neurotrophic factor (BDNF) on malathion induced depressive-like behavior in subacute exposure and protective effects of crocin. Iran. J. Basic Med. Sci. 2015, 18, 958–966. [Google Scholar] [PubMed]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 24799–24814. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.-Z.; Yang, H.-J.; Li, Y.-F.; Lin, C.-L.; Chang, S.-Y.; Sung, F.-C.; Tai, S.C.W. The Long-Term Effects of Organophosphates Poisoning as a Risk Factor of CVDs: A Nationwide Population-Based Cohort Study. PLoS ONE 2015, 10, e0137632. [Google Scholar] [CrossRef]
- Lin, J.N.; Lin, C.L.; Lin, M.C.; Lai, C.H.; Lin, H.H.; Yang, C.H.; Kao, C.H. Increased Risk of Dementia in Patients With Acute Organophosphate and Carbamate Poisoning: A Nationwide Population-Based Cohort Study. Medicine 2015, 94, e1187. [Google Scholar] [CrossRef]
- Wiesner, J.; Kříž, Z.; Kuča, K.; Jun, D.; Koča, J. Acetylcholinesterases—the structural similarities and differences. J. Enzym. Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Tota, S.; Kamat, P.K.; Shukla, R.; Nath, C. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav. Brain Res. 2011, 221, 207–215. [Google Scholar] [CrossRef]
- Scremin, O.U.; Jenden, D.J. Chapter 22: Acetylcholine turnover and release: The influence of energy metabolism and systemic choline availability. In Progress in Brain Research; Cuello, A.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 98, pp. 191–195. [Google Scholar]
- Bordone, M.P.; Salman, M.M.; Titus, H.E.; Amini, E.; Andersen, J.V.; Chakraborti, B.; Diuba, A.V.; Dubouskaya, T.G.; Ehrke, E.; Espindola de Freitas, A.; et al. The energetic brain—A review from students to students. J. Neurochem. 2019, 151, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Savy, C.Y.; Campbell, M.; Dodds, R.; Gomes, L.K.; Laws, G.; Watson, A.; Blain, P.G.; Morris, C.M.; Gartside, S.E. Mechanism for the acute effects of organophosphate pesticides on the adult 5-HT system. Chem. Biol. Interact. 2016, 245, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Satake, T.; Suzuki, H. Microfluidic Device for Coulometric Detection of Organophosphate Pesticides. Anal. Sci. 2015, 31, 591–595. [Google Scholar] [CrossRef]
- Pavlov, V.; Xiao, Y.; Willner, I. Inhibition of the Acetycholine Esterase-Stimulated Growth of Au Nanoparticles: Nanotechnology-Based Sensing of Nerve Gases. Nano Lett. 2005, 5, 649–653. [Google Scholar] [CrossRef]
- Harshit, D.; Charmy, K.; Nrupesh, P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017, 230, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Prins, J.M.; George, K.M. Mass spectrometric analyses of organophosphate insecticide oxon protein adducts. Environ. Health Perspect. 2010, 118, 11–19. [Google Scholar] [CrossRef]
- Su, H.; Yeh, I.-J.; Wu, Y.-H.; Jiang, Z.-H.; Shiea, J.; Lee, C.-W. Rapid identification of organophosphorus pesticides on contaminated skin and confirmation of adequate decontamination by ambient mass spectrometry in emergency settings. Rapid Commun. Mass Spectrom. 2020, 34, e8562. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Yang, J.; Wang, L.; Fang, Q.; Zhang, G.; Liu, F. Development of an Enzyme Linked Immunosorbent Assay and an Immunochromatographic Assay for Detection of Organophosphorus Pesticides in Different Agricultural Products. PLoS ONE 2013, 7, e53099. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.K.; Seravalli, J.; Arbuckle, T.; Quinn, D.M. Molecular recognition in acetylcholinesterase catalysis: Free-energy correlations for substrate turnover and inhibition by trifluoro ketone transition-state analogs. Biochemistry 1994, 33, 8566–8576. [Google Scholar] [CrossRef]
- Aldewachi, H.; Al-Zidan, R.N.; Conner, M.T.; Salman, M.M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.; Araújo, M.E. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Nordberg, A.; Darreh-Shori, T.; Peskind, E.; Soininen, H.; Mousavi, M.; Eagle, G.; Lane, R. Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients. Curr. Alzheimer Res. 2009, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Musilek, K.; Kuca, K. Progress of biosensors based on cholinesterase inhibition. Curr. Med. Chem. 2009, 16, 1790–1798. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Hu, H.; Shu, W.; Yang, L.; Zhang, J. Acetylcholinesterase electrochemical biosensors with graphene-transition metal carbides nanocomposites modified for detection of organophosphate pesticides. PLoS ONE 2020, 15, e0231981. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef]
- Wooten, M.; Karra, S.; Zhang, M.; Gorski, W. On the Direct Electron Transfer, Sensing, and Enzyme Activity in the Glucose Oxidase/Carbon Nanotubes System. Anal. Chem. 2014, 86, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, J.; Chidambaram, R. Acetylcholinesterase with mesoporous silica: Covalent immobilization, physiochemical characterization, and its application in food for pesticide detection. J. Cell. Biochem. 2019, 120, 10777–10786. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Manjakkal, L.; Yang, X.; Huerta, M.; Le, T.; Thiel, L.; Chiao, J.C.; Cao, H.; Dahiya, R. Flexible Iridium Oxide Based pH Sensor Integrated With Inductively Coupled Wireless Transmission System for Wearable Applications. Ieee Sens. J. 2020, 20, 5130–5138. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-Oxidized Nanocellulose Participating as Crosslinking Aid for Alginate-Based Sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Ma, X.; Sim, S.J. Gold nanostar based biosensor detects epigenetic alterations on promoter of real cells. Biosens. Bioelectron. 2015, 66, 497–503. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Z.; Yu, L.; Li, C.M. Fast prototyping of a customized microfluidic device in a non-clean-room setting by cutting and laminating Parafilm®. Rsc. Adv. 2016, 6, 85468–85472. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, Y.; Henry, C.S. Laminated and infused Parafilm®—Paper for paper-based analytical devices. Sens. Actuators B Chem. 2018, 255, 3654–3661. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Taylor, P.; Camp, S.; Radić, Z. Acetylcholinesterase. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 5–7. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Setua, P.; Sarkar, N. Photoinduced Electron Transfer in a Protein−Surfactant Complex: Probing the Interaction of SDS with BSA. J. Phys. Chem. B 2006, 110, 16607–16617. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Kriebel, J.K.; Love, J.C. Molecular engineering of surfaces using self-assembled monolayers. Sci. Prog. 2005, 88, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.J.; Yuk, J.S.; Jung, S.H.; Zhavnerko, G.K.; Kim, Y.M.; Ha, K.S. Investigation of selective protein immobilization on charged protein array by wavelength interrogation-based SPR sensor. Mol. Cells 2003, 15, 333–340. [Google Scholar] [PubMed]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Hanoian, P.; Liu, C.T.; Hammes-Schiffer, S.; Benkovic, S. Perspectives on electrostatics and conformational motions in enzyme catalysis. Acc. Chem. Res. 2015, 48, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Gerhardt, G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Schartner, J.; Güldenhaupt, J.; Katharina Gaßmeyer, S.; Rosga, K.; Kourist, R.; Gerwert, K.; Kötting, C. Highly stable protein immobilization via maleimido-thiol chemistry to monitor enzymatic activity. Analyst 2018, 143, 2276–2284. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Faizullin, D.A.; Zuev, Y.F.; Weisel, J.W. The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef]
- Javid, A.; Kumar, M.; Yoon, S.; Lee, J.H.; Han, J.G. Size-controlled growth and antibacterial mechanism for Cu:C nanocomposite thin films. Phys. Chem. Chem. Phys. 2017, 19, 237–244. [Google Scholar] [CrossRef]
- Mazzotta, E.; Rella, S.; Turco, A.; Malitesta, C. XPS in development of chemical sensors. Rsc Adv. 2015, 5, 83164–83186. [Google Scholar] [CrossRef]
- Shimada, H.; Kiyozumi, Y.; Koga, Y.; Ogata, Y.; Katsuda, Y.; Kitamura, Y.; Iwatsuki, M.; Nishiyama, K.; Baba, H.; Ihara, T. A novel cholinesterase assay for the evaluation of neurotoxin poisoning based on the electron-transfer promotion effect of thiocholine on an Au electrode. Sens. Actuators B Chem. 2019, 298, 126893. [Google Scholar] [CrossRef]
- Jha, N.; Ramaprabhu, S. Development of Au nanoparticles dispersed carbon nanotube-based biosensor for the detection of paraoxon. Nanoscale 2010, 2, 806–810. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Valerio, L.G. CHAPTER 11—Onchidal and Fasciculins. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 143–152. [Google Scholar] [CrossRef]
- Giese, B.; Graber, M.; Cordes, M. Electron transfer in peptides and proteins. Curr. Opin. Chem. Biol. 2008, 12, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, F.; Dong, T.; Du, L.; Zhang, D.; Gao, J. Charge-Transfer Knowledge Graph among Amino Acids Derived from High-Throughput Electronic Structure Calculations for Protein Database. ACS Omega 2018, 3, 4094–4104. [Google Scholar] [CrossRef]
- Pal, S.; Sahoo, M.; Veettil, V.T.; Tadi, K.K.; Ghosh, A.; Satyam, P.; Biroju, R.K.; Ajayan, P.M.; Nayak, S.K.; Narayanan, T.N. Covalently Connected Carbon Nanotubes as Electrocatalysts for Hydrogen Evolution Reaction through Band Engineering. ACS Catal. 2017, 7, 2676–2684. [Google Scholar] [CrossRef]
- Lu, X.; Yim, W.-L.; Suryanto, B.H.R.; Zhao, C. Electrocatalytic Oxygen Evolution at Surface-Oxidized Multiwall Carbon Nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef]
- Yang, C.; Jacobs, C.B.; Nguyen, M.D.; Ganesana, M.; Zestos, A.G.; Ivanov, I.N.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Venton, B.J. Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine. Anal. Chem. 2016, 88, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Du, D.; Lin, Y. Highly Sensitive and Selective Immuno-Capture/Electrochemical Assay of Acetylcholinesterase Activity in Red Blood Cells: A Biomarker of Exposure to Organophosphorus Pesticides and Nerve Agents. Environ. Sci. Technol. 2012, 46, 1828–1833. [Google Scholar] [CrossRef]
- Bucur, M.-P.; Bucur, B.; Radu, G.-L. Critical evaluation of acetylthiocholine iodide and acetylthiocholine chloride as substrates for amperometric biosensors based on acetylcholinesterase. Sensors 2013, 13, 1603–1613. [Google Scholar] [CrossRef]
- Hart, J.P.; Hartley, I.C. Voltammetric and amperometric studies of thiocholine at a screen-printed carbon electrode chemically modified with cobalt phthalocyanine: Studies towards a pesticide sensor. Analyst 1994, 119, 259–263. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Gomez, M.E.; Kaifer, A.E. Voltammetric behavior of a ferrocene derivative: A comparison using surface-confined and diffusion-controlled species. J. Chem. Educ. 1992, 69, 502. [Google Scholar] [CrossRef]
- Hou, S.; Ou, Z.; Chen, Q.; Wu, B. Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol-gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens. Bioelectron. 2012, 33, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Ly, K.H.; Robinson, W.E.; Heidary, N.; Zhang, J.Z.; Reisner, E. Advancing Techniques for Investigating the Enzyme–Electrode Interface. Acc. Chem. Res. 2019, 52, 1439–1448. [Google Scholar] [CrossRef]
- Lenina, O.A.; Zueva, I.V.; Zobov, V.V.; Semenov, V.E.; Masson, P.; Petrov, K.A. Slow-binding reversible inhibitor of acetylcholinesterase with long-lasting action for prophylaxis of organophosphate poisoning. Sci. Rep. 2020, 10, 16611. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, S.V.; Masson, P. Slow-binding inhibitors of acetylcholinesterase of medical interest. Neuropharmacology 2020, 177, 108236. [Google Scholar] [CrossRef]
- Leatherbarrow, R.J. Use of nonlinear regression to analyze enzyme kinetic data: Application to situations of substrate contamination and background subtraction. Anal. Biochem. 1990, 184, 274–278. [Google Scholar] [CrossRef]
- Yu, R.; Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun, Northeast China. Food Control. 2016, 60, 353–360. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, P.; Hou, S.; Wu, P.; Xue, J. Fluorescence sensor for facile and visual detection of organophosphorus pesticides using AIE fluorogens-SiO2-MnO2 sandwich nanocomposites. Talanta 2019, 198, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mane, P.C.; Shinde, M.D.; Varma, S.; Chaudhari, B.P.; Fatehmulla, A.; Shahabuddin, M.; Amalnerkar, D.P.; Aldhafiri, A.M.; Chaudhari, R.D. Highly sensitive label-free bio-interfacial colorimetric sensor based on silk fibroin-gold nanocomposite for facile detection of chlorpyrifos pesticide. Sci. Rep. 2020, 10, 4198. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Barry, R.; Petersen, C.; Timchalk, C.; Gassman, P.L.; Lin, Y. Nanoparticle-based electrochemical immunosensor for the detection of phosphorylated acetylcholinesterase: An exposure biomarker of organophosphate pesticides and nerve agents. Chem. (Weinh. Bergstr. Ger.) 2008, 14, 9951–9959. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, H.; Qiao, F.; Liu, P.; Wang, X.; Ai, S. Quantum dot immobilized acetylcholinesterase for the determination of organophosphate pesticides using graphene-chitosan nanocomposite modified electrode. Anal. Methods 2013, 5, 2866–2872. [Google Scholar] [CrossRef]
- Selvolini, G.; Băjan, I.; Hosu, O.; Cristea, C.; Săndulescu, R.; Marrazza, G. DNA-Based Sensor for the Detection of an Organophosphorus Pesticide: Profenofos. Sensors 2018, 18, 2035. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Shukla, S.; Bajpai, V.K.; Han, Y.-K.; Huh, Y.S.; Ghosh, A.; Gandhi, S. Microfluidic-based graphene field effect transistor for femtomolar detection of chlorpyrifos. Sci. Rep. 2019, 9, 276. [Google Scholar] [CrossRef]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]






| Sensing Receptors | Sensing Methods | Detection Limit |
|---|---|---|
| Aptamers, enzymes | Localized surface plasmon resonance (LSPR) | 58–1835 ppm [74] |
| Fluorescence | 1 mg mL−1 [75] | |
| Nanoplasmonic | 10 ppb [76] | |
| Cyclic voltammograms | 0.2 nM [52] 8.0 pM [77] | |
| Amperometry | 0.27 μM [78,79] 9 nM [56] | |
| Field-effect transistor | 1.8 fM [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.S.; Nguyen, X.A.; Marsh, P.; Chu, S.S.; Lau, M.P.H.; Nguyen, A.H.; Cao, H. A Fluidics-Based Biosensor to Detect and Characterize Inhibition Patterns of Organophosphate to Acetylcholinesterase in Food Materials. Micromachines 2021, 12, 397. https://doi.org/10.3390/mi12040397
Pham DS, Nguyen XA, Marsh P, Chu SS, Lau MPH, Nguyen AH, Cao H. A Fluidics-Based Biosensor to Detect and Characterize Inhibition Patterns of Organophosphate to Acetylcholinesterase in Food Materials. Micromachines. 2021; 12(4):397. https://doi.org/10.3390/mi12040397
Chicago/Turabian StylePham, Dang Song, Xuan Anh Nguyen, Paul Marsh, Sung Sik Chu, Michael P. H. Lau, Anh H. Nguyen, and Hung Cao. 2021. "A Fluidics-Based Biosensor to Detect and Characterize Inhibition Patterns of Organophosphate to Acetylcholinesterase in Food Materials" Micromachines 12, no. 4: 397. https://doi.org/10.3390/mi12040397
APA StylePham, D. S., Nguyen, X. A., Marsh, P., Chu, S. S., Lau, M. P. H., Nguyen, A. H., & Cao, H. (2021). A Fluidics-Based Biosensor to Detect and Characterize Inhibition Patterns of Organophosphate to Acetylcholinesterase in Food Materials. Micromachines, 12(4), 397. https://doi.org/10.3390/mi12040397







