Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polydimethylsiloxane (PDMS) Mold
2.2. Preparation of PCL and Base Polymer Solution
2.3. Fabrication of PCL-DMNs and SPCL-DMNs
2.4. Morphology and Mechanical Strength of PCL-DMNs and SPCL-DMNs
2.5. HPLC Analysis for Capsaicin
2.6. Skin Penetration of PCL-DMNs and SPCL-DMNs
2.7. Permeation of SPCL-DMNs
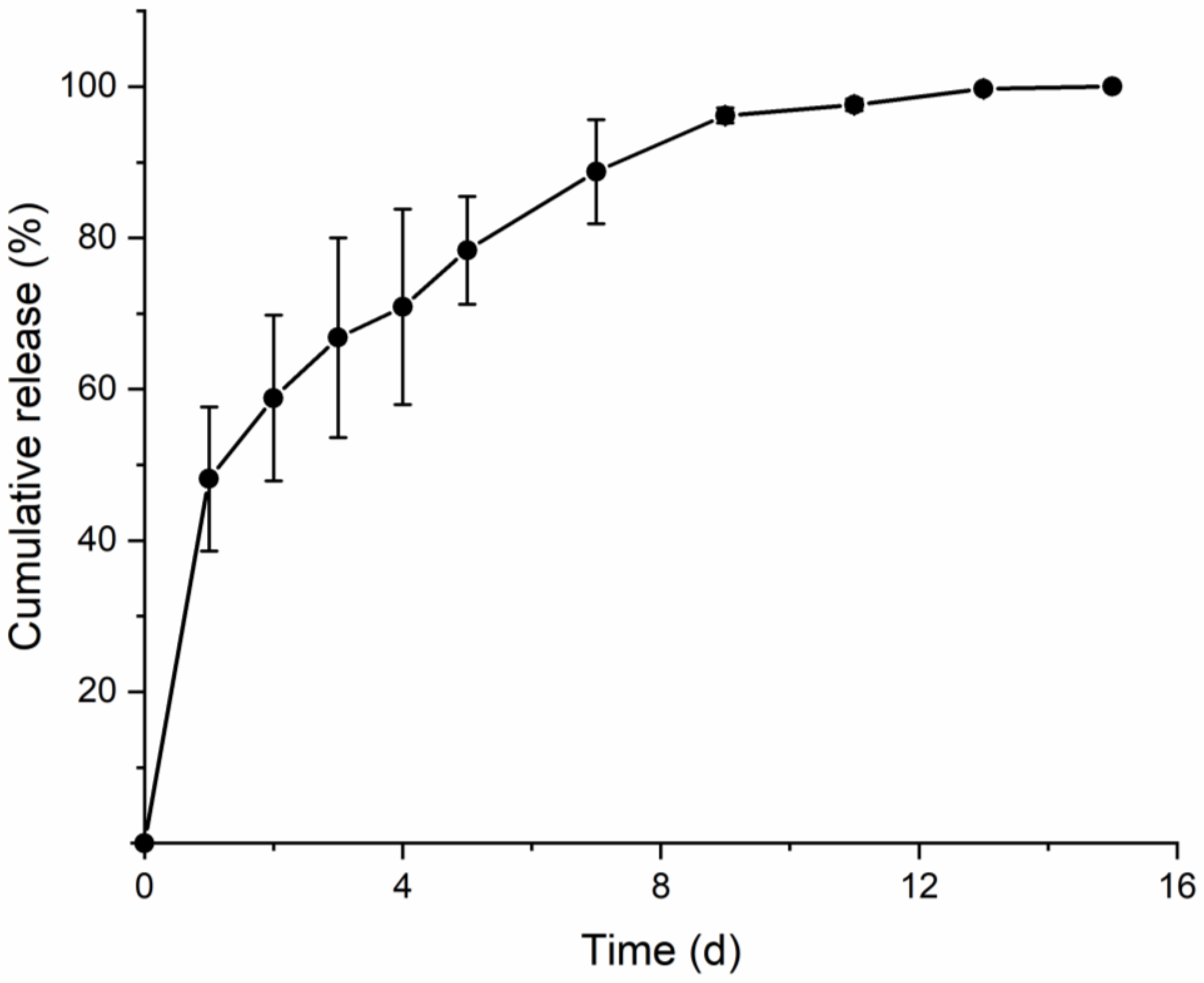
3. Results and Discussion
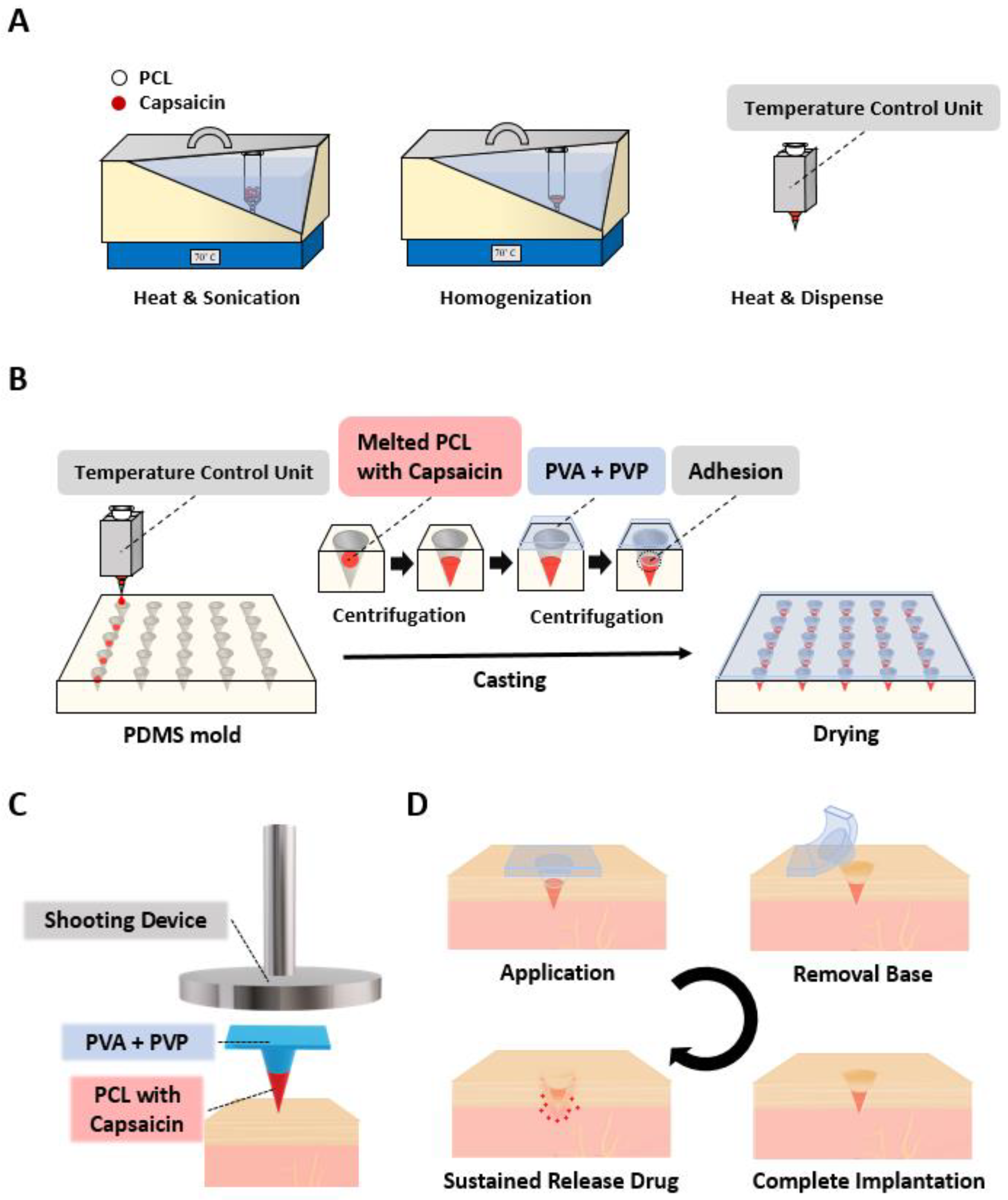
3.1. Fabrication and Application of SPCL-DMNs
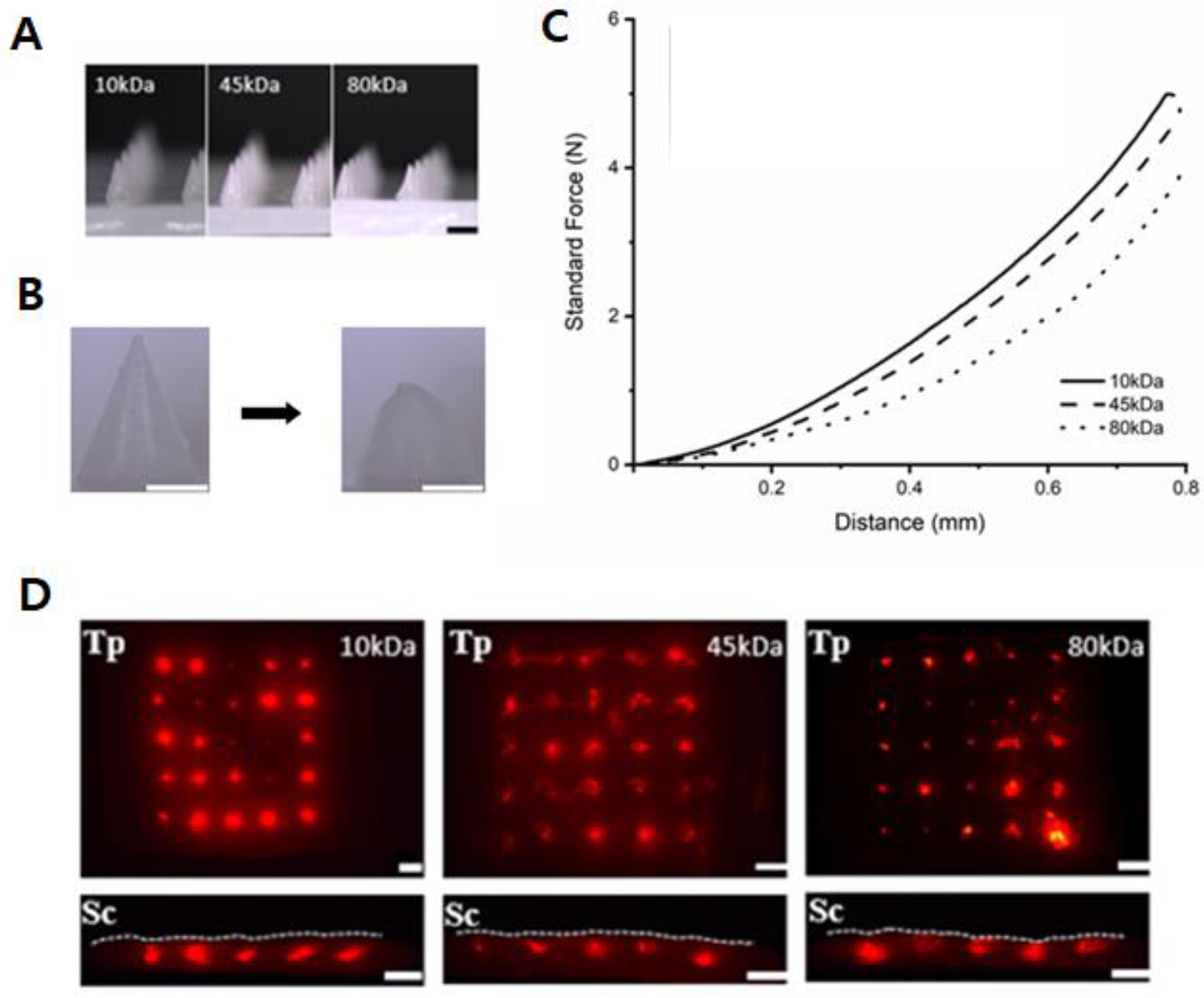
3.2. PCL-DMNs with Different Molecular Weights
3.3. Optimization of Base Polymer for SPCL-DMNs
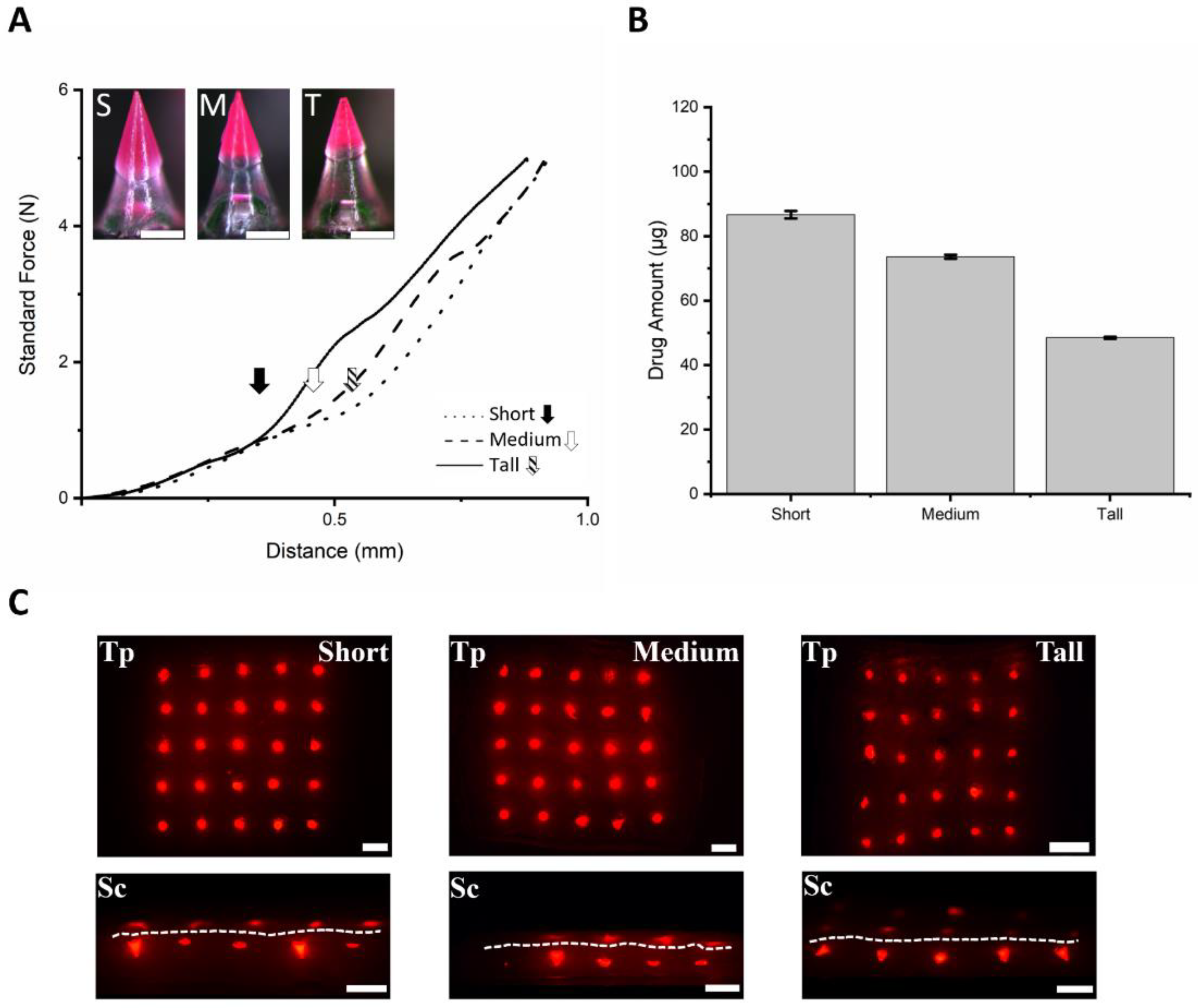
3.4. Fabrication of SPCL-DMNs with PVA and Sucrose Mixture
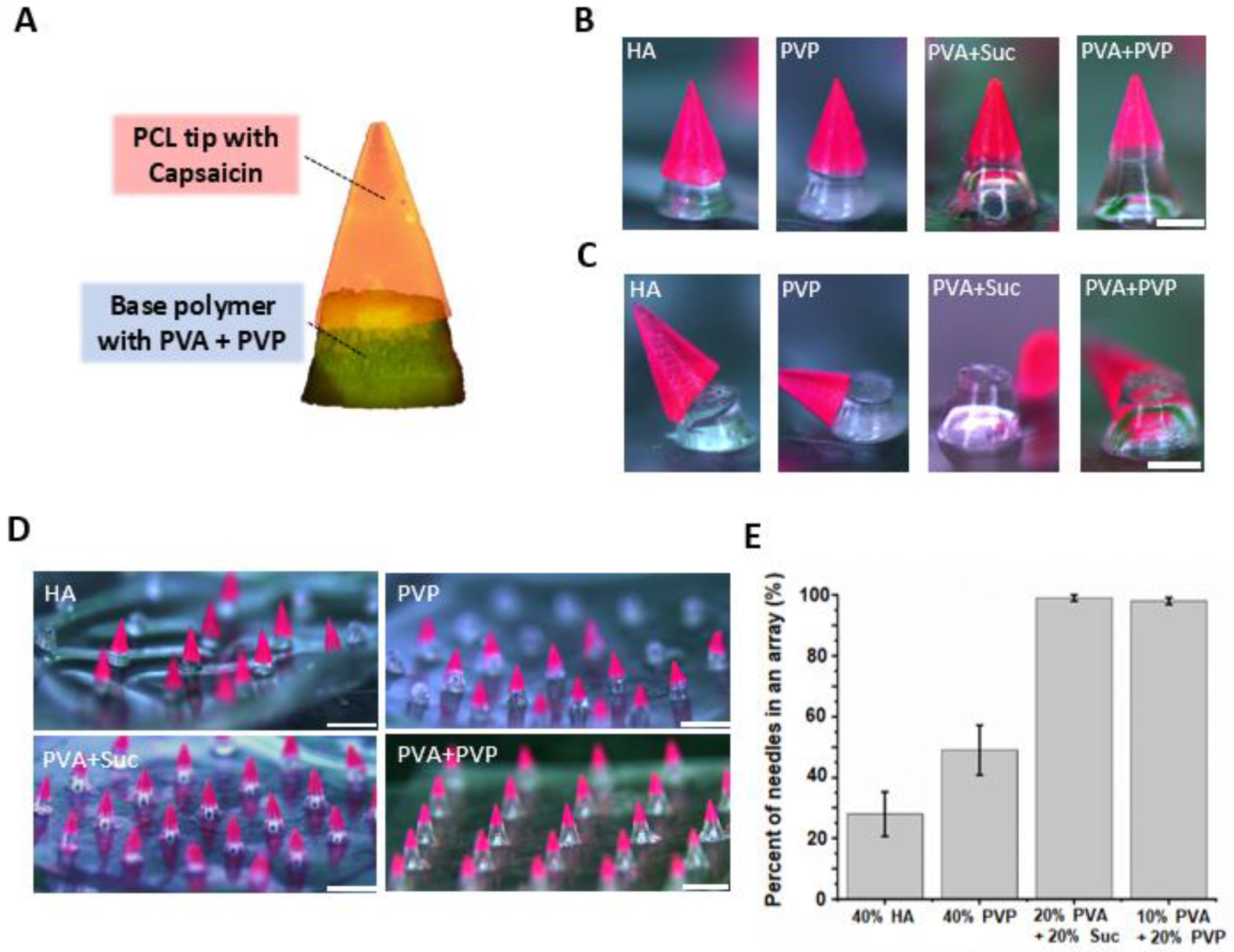
3.5. Fabrication of SPCL-DMNs with PVA and PVP Mixture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Fakhraei Lahiji, S.; Jang, J.; Jang, M.; Jung, H. Micro-Pillar Integrated Dissolving Microneedles for Enhanced Transdermal Drug Delivery. Pharmaceutics 2019, 11, E402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luo, H.; Lu, W.; Luan, H.; Wu, Y.; Luo, J.; Wang, Y.; Pi, J.; Lim, C.Y.; Wang, H. Rapidly dissolvable microneedle patches for transdermal delivery of exenatide. Pharm. Res. 2014, 31, 3348–3360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Shi, Y. Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020, 10, 14040–14049. [Google Scholar] [CrossRef]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 7, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of transdermal delivery of exendin-4 using novel tip-loaded microneedle arrays fabricated from hyaluronic acid. Mol. Pharm. 2016, 13, 272–279. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Devel. Ther. 2013, 7, 945–952. [Google Scholar]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef]
- Stefano, D.D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials Toxicity and Cell Death Modalities. J. Drug Deliv. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Cadete, A.; Olivera, A.; Besev, M.; Dhal, P.K.; Gonçalves, L.; Almeida, A.J.; Bastiat, G.; Benoit, J.P.; de la Fuente, M.; Garcia-Fuentes, M.; et al. Self-assembled hyaluronan nanocapsules for the intracellular delivery of anticancer drugs. Sci. Rep. 2019, 9, 11565. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Chen, B.Z.; Ashfaq, M.; Zhu, D.D.; Zhang, X.P.; Guo, X.D. Controlled delivery of insulin using rapidly separating microneedles fabricated from genipin-crosslinked gelatin. Macromol. Rapid Commun. 2018, 39, e1800075. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-pd1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Seo, I.H.; Lee, K.J.; Ryu, W. Rapid and repeatable fabrication of high A/R silk fibroin microneedles using thermally-drawn micromolds. Eur. J. Pharm. Biopharm. 2015, 94, 11–19. [Google Scholar] [CrossRef]
- Pillay, V.; Seedat, A.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M. A review of polymeric refabrication techniques to modify polymer properties for biomedical and drug delivery applications. AAPS Pharm. Sci. Tech. 2013, 14, 692–711. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, M.J.; Baek, S.K.; Park, J.H.; Choi, S.O. Spray-formed layered polymer microneedles for controlled biphasic drug delivery. Polymers 2019, 11, E369. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Near-infrared light triggered and separable microneedles for transdermal delivery of metformin in diabetic rats. J. Mater. Chem. B 2017, 5, 9507–9513. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, D.; Gao, M.; Xu, B.; Jiang, G. Thermal ablation of separable microneedles for transdermal delivery of metformin on diabetic rats. Int. J. Polym. Mater. Polym. 2019, 68, 850–858. [Google Scholar] [CrossRef]
- Paul, D.; Dey, T.K.; Dhar, P. Nanoformulation and administration of PUFA-rich systems for applications in modern healthcare. In Nanostructures for Novel Therapy, 1st ed.; Ficai, D., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–200. [Google Scholar]
- Bae, W.G.; Ko, H.; So, J.Y.; Yi, H.; Lee, C.H.; Lee, D.H.; Ahn, Y.; Lee, S.H.; Lee, K.; Jun, J.; et al. Snake fang-inspired stamping patch for transdermal delivery of liquid formulations. Sci. Transl. Med. 2019, 11, eaaw3329. [Google Scholar] [CrossRef] [PubMed]
- Piyasin, P.; Yensano, R.; Pinitsoontorn, S. Size-controllable melt-electrospun polycaprolactone (PCL) fibers with a sodium chloride additive. Polymers 2019, 11, E1768. [Google Scholar] [CrossRef]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Heimowska, A.; Morawska, M.; Janiszewska, A.B. Biodegradation of poly(ε-caprolactone) in natural water environments. Pol. J. Chem. Technol. 2017, 19, 120–126. [Google Scholar] [CrossRef]
- Kurniawan, D.; Nor, F.M.; Lee, H.Y.; Lim, J.Y. Elastic properties of polycaprolactone at small strains are significantly affected by strain rate and temperature. Proc. Inst. Mech. Eng. H 2011, 225, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- Chen, Z.H.; Ren, X.L.; Zhou, H.H.; Li, X.D. The role of hyaluronic acid in biomineralization. Front. Mater. Sci. 2012, 6, 283–296. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- Djonlagic, J.; Nikolic, M.S. Biodegradable polyesters: Synthesis and physical properties. In A Handbook of Applied Biopolymer Technology Synthesis, Degradation & Application, 1st ed.; Sharma, S.K., Mudhoo, A., Eds.; RSC Publishing: London, UK, 2011; Volume 12, pp. 164–166. [Google Scholar]
- Ko, P.-T.; Lee, I.-C.; Chen, M.-C.; Tsai, S.W. Polymer microneedles fabricated from PCL and PCL/PEG blends for transdermal delivery of hydrophilic compounds. J. Taiwan Inst. Chem. Eng. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yeh, I.-T.; Niyama, E.; Huang, W.-R.; Ebara, M.; Wu, C.-S. Electrospun poly(ε-caprolactone) nanofibrous mesh for imiquimod delivery in melanoma therapy. Polymers 2018, 10, 231. [Google Scholar] [CrossRef]
- Romero, V.; Lara, J.R.; Otero-Espinar, F.; Salgado, M.H.; Modolo, N.S.P.; Barros, G.A.M. Capsaicin topical cream (8%) for the treatment of myofascial pain syndrome. Rev. Bras. Anestesiol. 2019, 69, 432–438. [Google Scholar] [CrossRef]
- Illigens, B.M.; Gibbons, C.H. A human model of small fiber neuropathy to study wound healing. PLoS ONE 2013, 8, e54760. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.L.; Schnitzer, T.J.; Lipstein, E.; Seibold, J.R.; Stevens, R.M.; Levy, M.D.; Albert, D.; Renold, F. Treatment of arthritis with topical capsaicin: A double-blind trial. Clin. Ther. 1991, 13, 383–395. [Google Scholar]
- Tokumura, F.; Umekage, K.; Sado, M.; Otsuka, S.; Suda, S.; Taniguchi, M.; Yamori, A.; Nakamura, A.; Kawai, J.; Oka, K. Skin irritation due to repetitive application of adhesive tape: The influence of adhesive strength and seasonal variability. Skin Res. Technol. 2005, 11, 102–106. [Google Scholar] [CrossRef]
- Lachapelle, J.-M.; Ring, J.; Darsow, U.; Maibach, H.; Rustemeyer, T. Patch testing methodology. In Patch Testing and Pricking Testing: A Practical Guide Official Publication of the ICDRG, 2nd ed.; Lachapelle, J.-M., Maibach, H.I., Eds.; Springer: Berlin, Germany, 2009; pp. 33–70. [Google Scholar]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 2019, 3, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller, W.H. Structure and function of the skin. In Equine Dermatology, 1st ed.; Scott, D.W., Miller, W.H., Eds.; Elsevier: Amsterdam, Nederland, 2003; pp. 1–58. [Google Scholar]
- Demuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef]
- Xi, H.; Chen, D.; Lv, L.; Zhong, P.; Lin, Z.; Chang, J.; Wang, H.; Wang, B.; Ma, X.; Zhang, C. High performance transient organic solar cells on biodegradable polyvinyl alcohol composite substrate. RSC Adv. 2017, 7, 52930–52937. [Google Scholar] [CrossRef]
- Bernal, A.; Kuritka, I.; Saha, P. Poly(vinyl alcohol)-poly(vinyl pyrrolidone) blends: Preparation and characterization for a prospective medical application. J. Appl. Polym. Sci. 2013, 127, 3560–3568. [Google Scholar] [CrossRef]
- Vinayakumar, K.B.; Nadiger, G.; R Shetty, V.; Dinesh, N.S.; Nayak, M.M.; Rajanna, K. Packaged Peristaltic Micropump for Controlled Drug Delivery Application. Rev. Sci. Instrum. 2017, 88, 015102. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pissinato Pere, C.P.; Okereke, M.; Douroumis, D. Optimisation of Design and Manufacturing Parameters of 3D Printed Solid Microneedles for Improved Strength, Sharpness, and Drug Delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef]
- McCormick, C. Polymer-free drug-eluting stents. In Functionalised Cardiovascular Stents, 1st ed.; Wall, J.G., Podbielska, H., Wawrzyńska, M., Eds.; Elsevier: Amsterdam, The Netherland, 2018; pp. 57–74. [Google Scholar]
- Szurkowska, K.; Laskus, A.; Kolmas, J. Hydroxyapatite-based materials for potential use in bone tissue infections. In Hydroxyapatite-Advances in Composite Nanomaterials, Biomedical Applications and its Technological Facets, 1st ed.; Thirumalai, J., Ed.; Intech: London, UK, 2017; pp. 109–135. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eum, J.; Kim, Y.; Um, D.J.; Shin, J.; Yang, H.; Jung, H. Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin. Micromachines 2021, 12, 167. https://doi.org/10.3390/mi12020167
Eum J, Kim Y, Um DJ, Shin J, Yang H, Jung H. Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin. Micromachines. 2021; 12(2):167. https://doi.org/10.3390/mi12020167
Chicago/Turabian StyleEum, Jaehong, Youseong Kim, Daniel Junmin Um, Jiwoo Shin, Huisuk Yang, and Hyungil Jung. 2021. "Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin" Micromachines 12, no. 2: 167. https://doi.org/10.3390/mi12020167
APA StyleEum, J., Kim, Y., Um, D. J., Shin, J., Yang, H., & Jung, H. (2021). Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin. Micromachines, 12(2), 167. https://doi.org/10.3390/mi12020167



