Cell Culture on Low-Fluorescence and High-Resolution Photoresist
Abstract
1. Introduction
2. Material and Methods
2.1. SJI-001
2.2. Surface Modification by Plasma Treatment
2.3. Basic Surface Properties for Cell Culture and Analysis
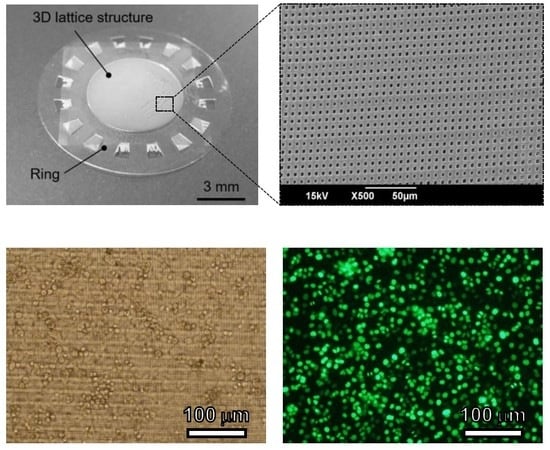
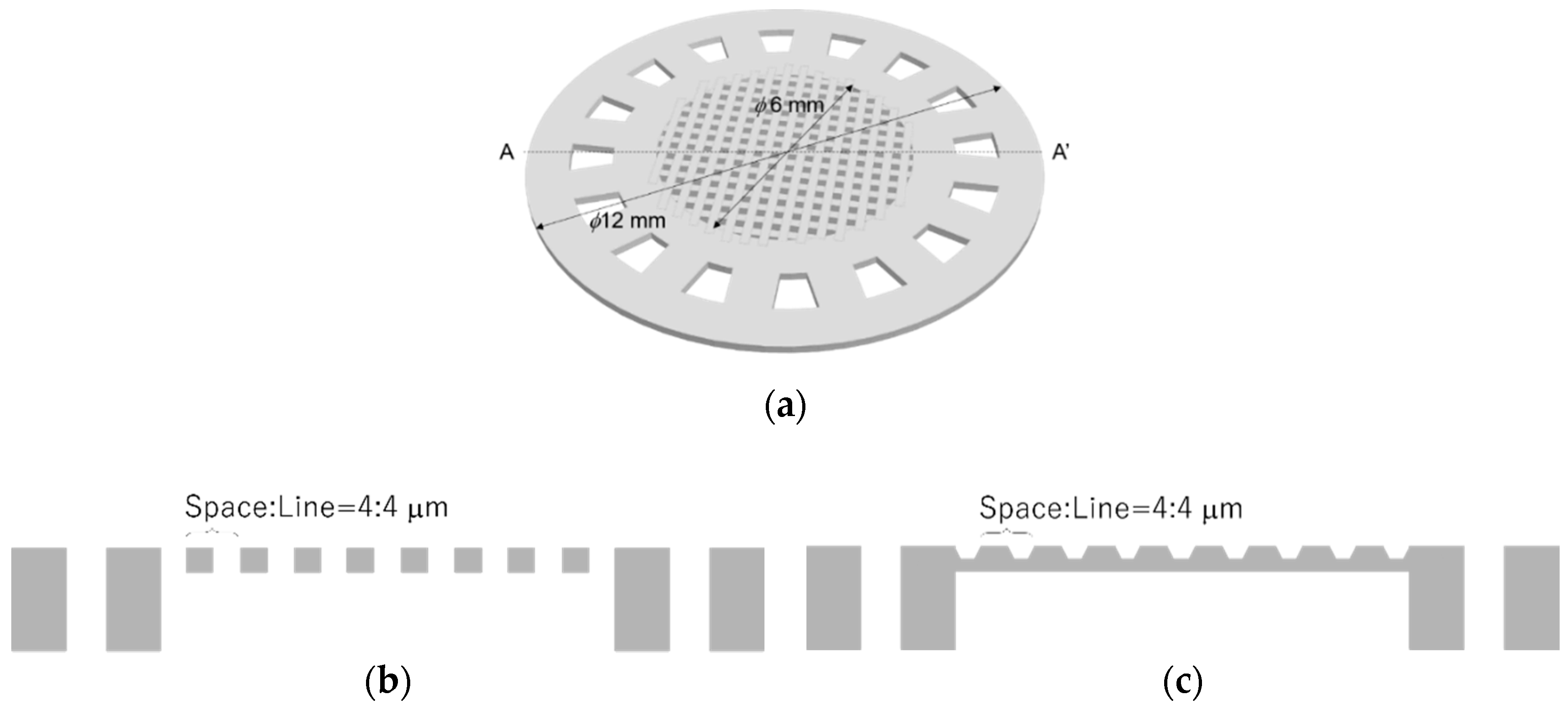
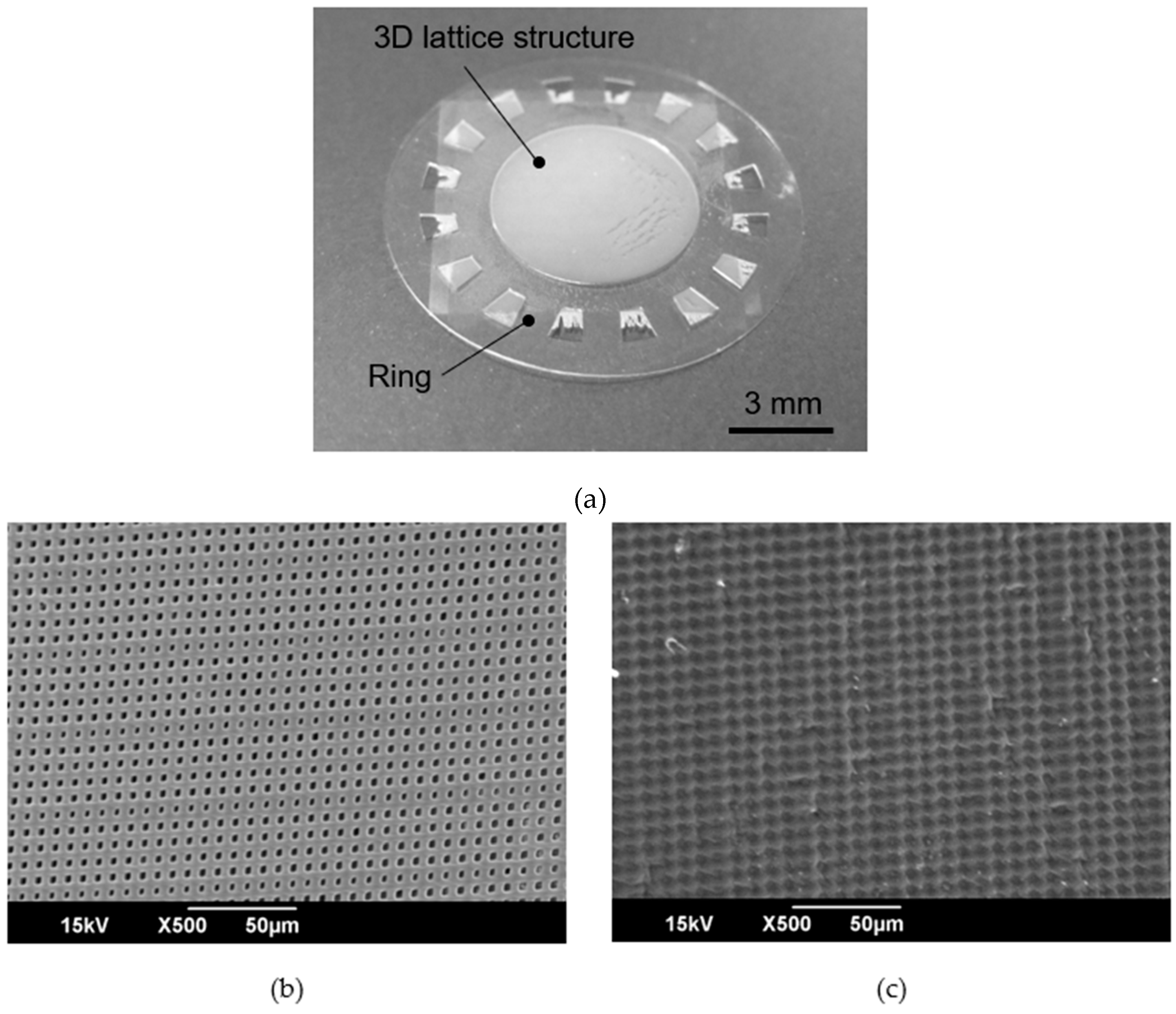
2.4. Cell Culture Plate
3. Results
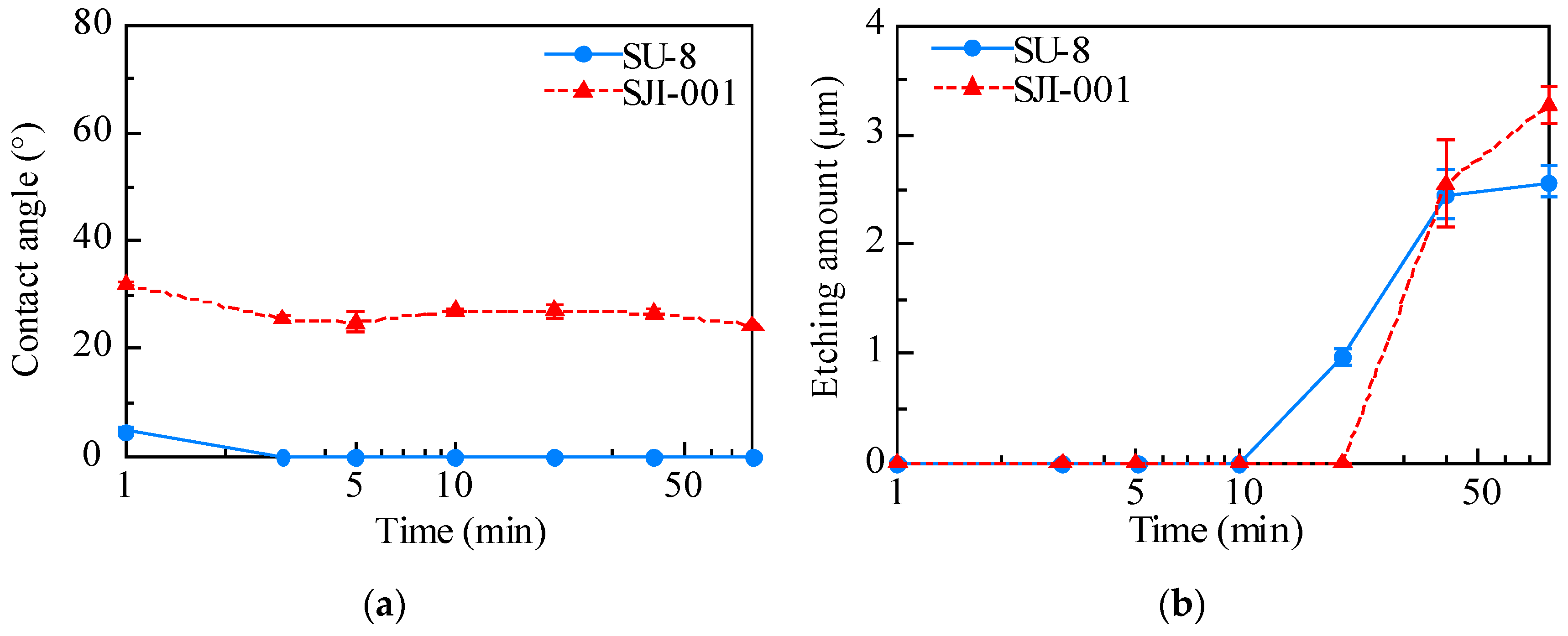
3.1. Surface Modification by Plasma Treatment
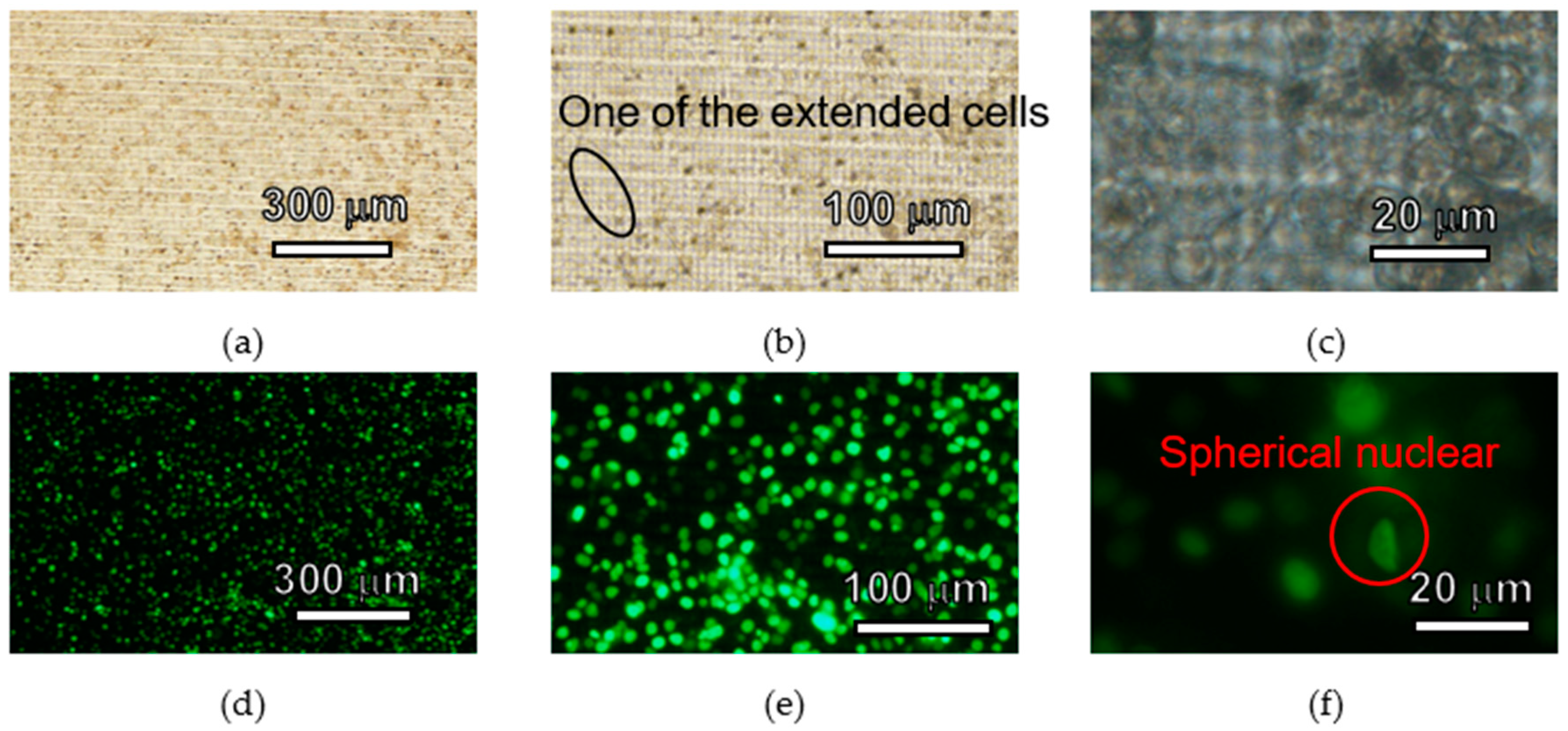
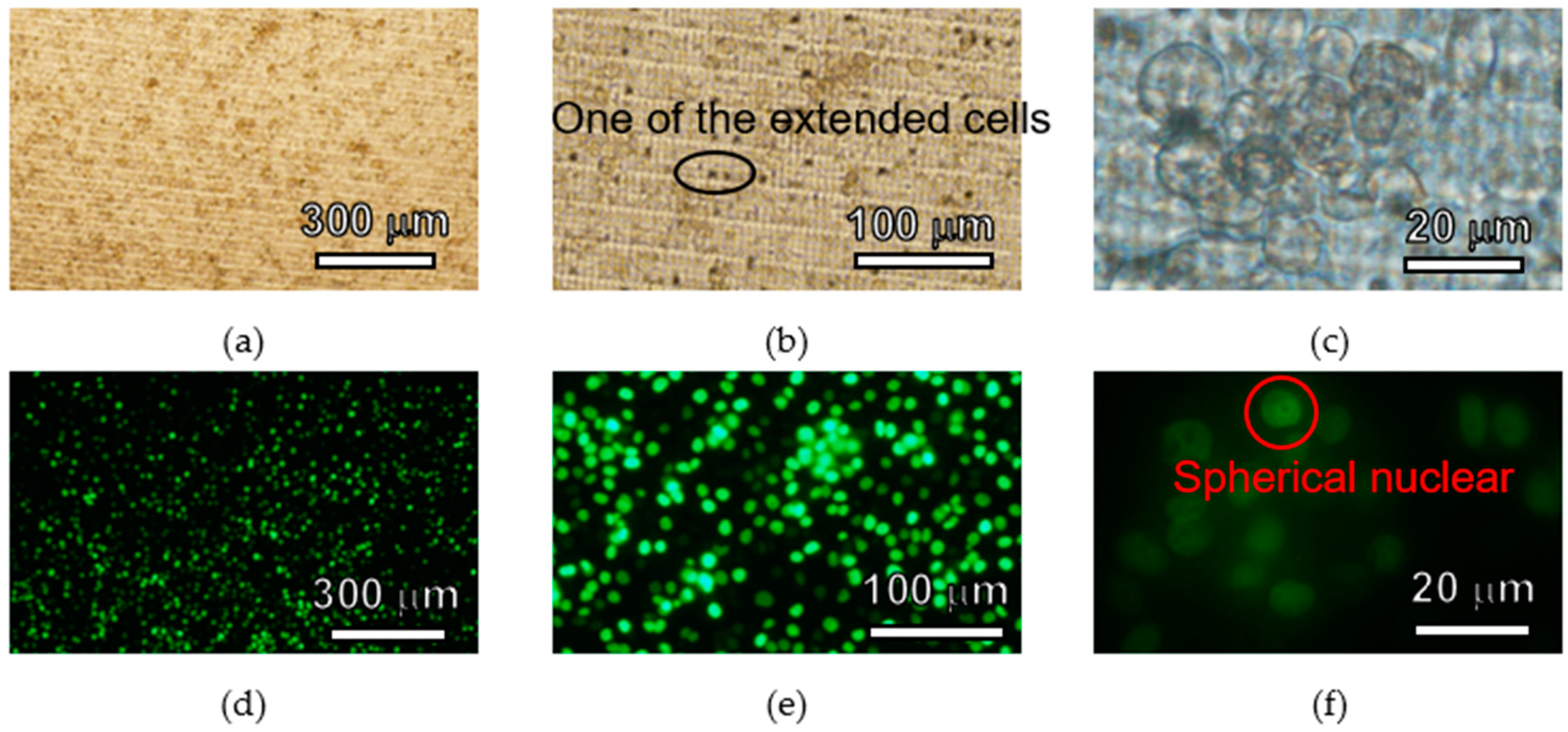
3.2. Basic Surface Properties for Cell Culture and Analysis
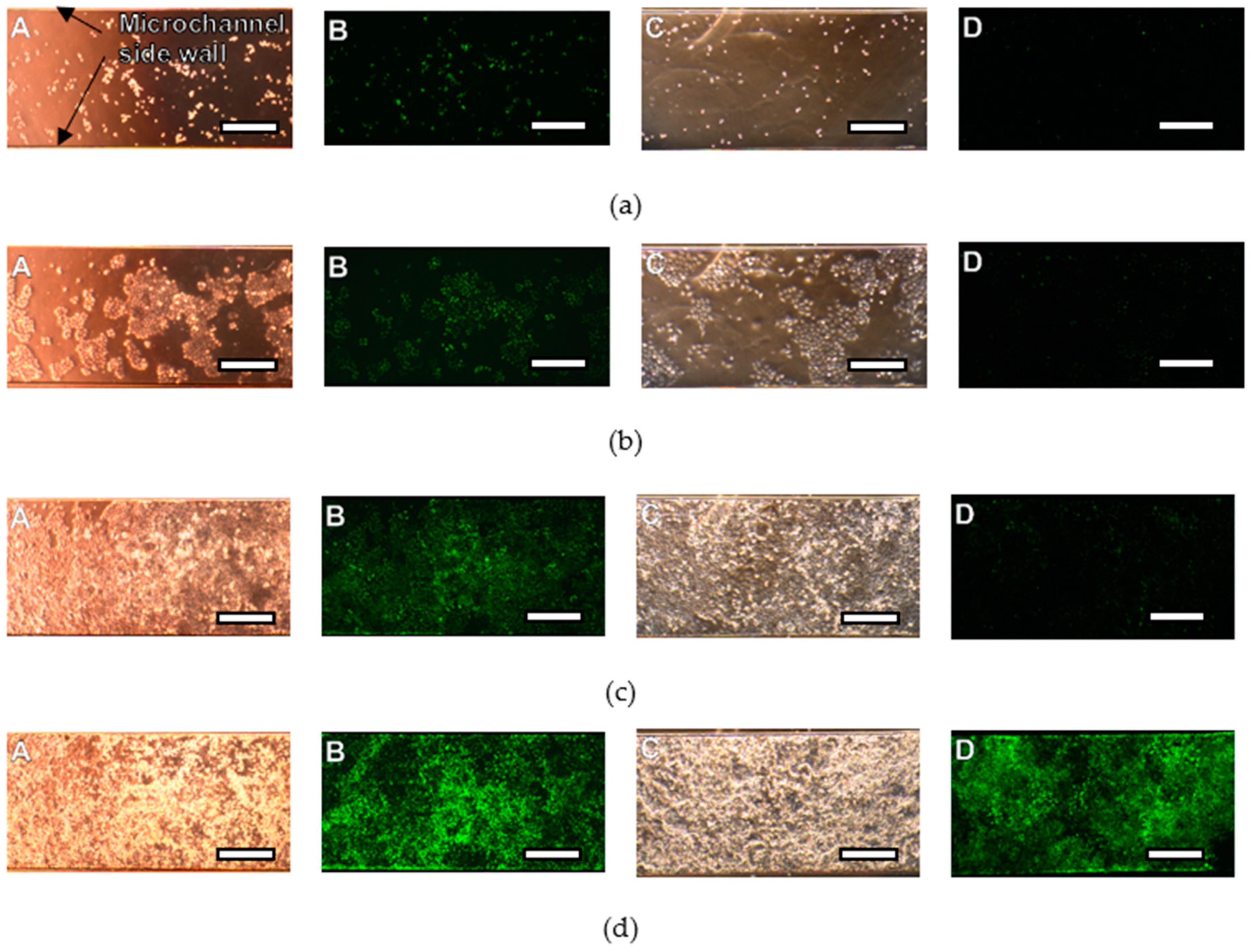
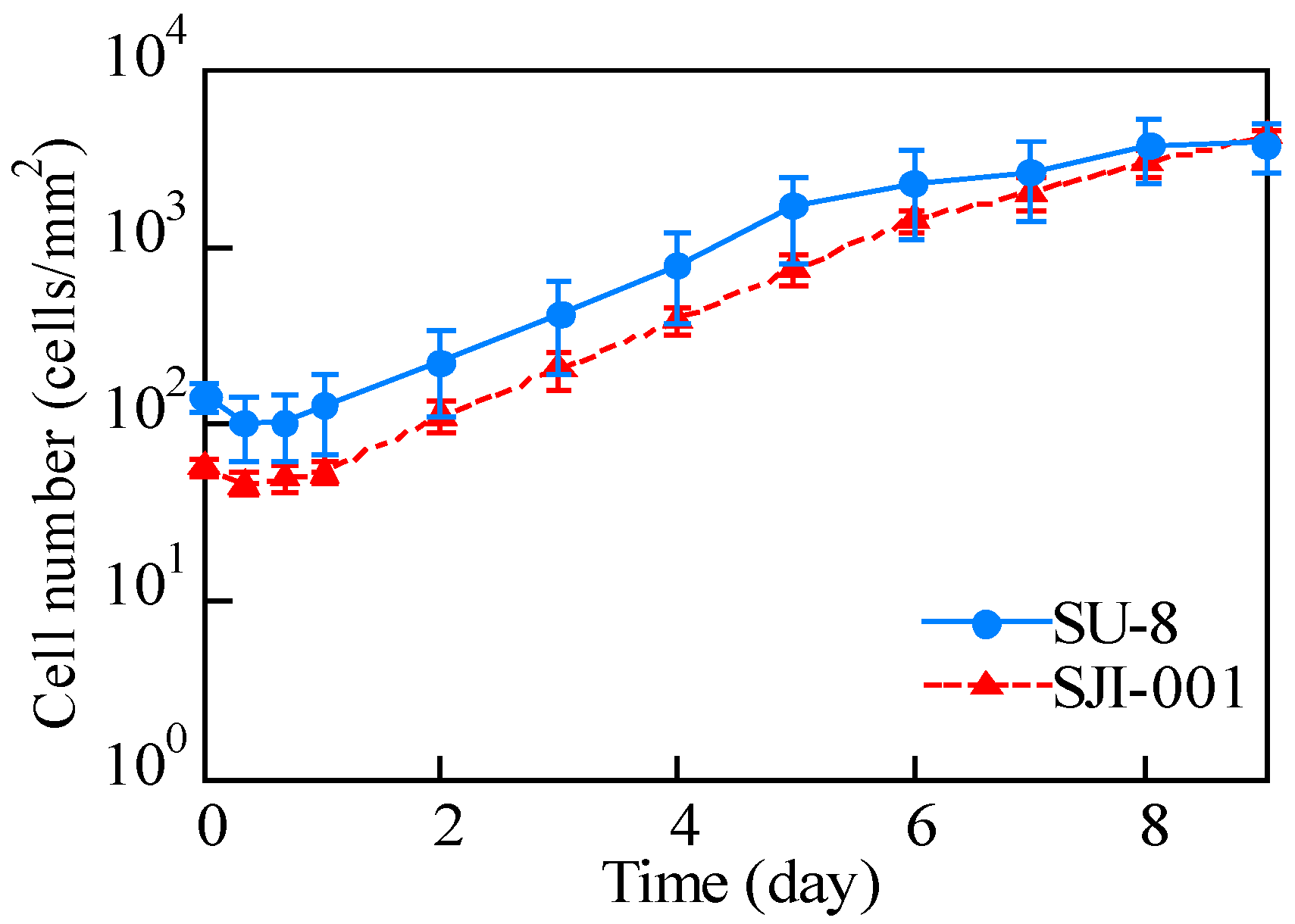
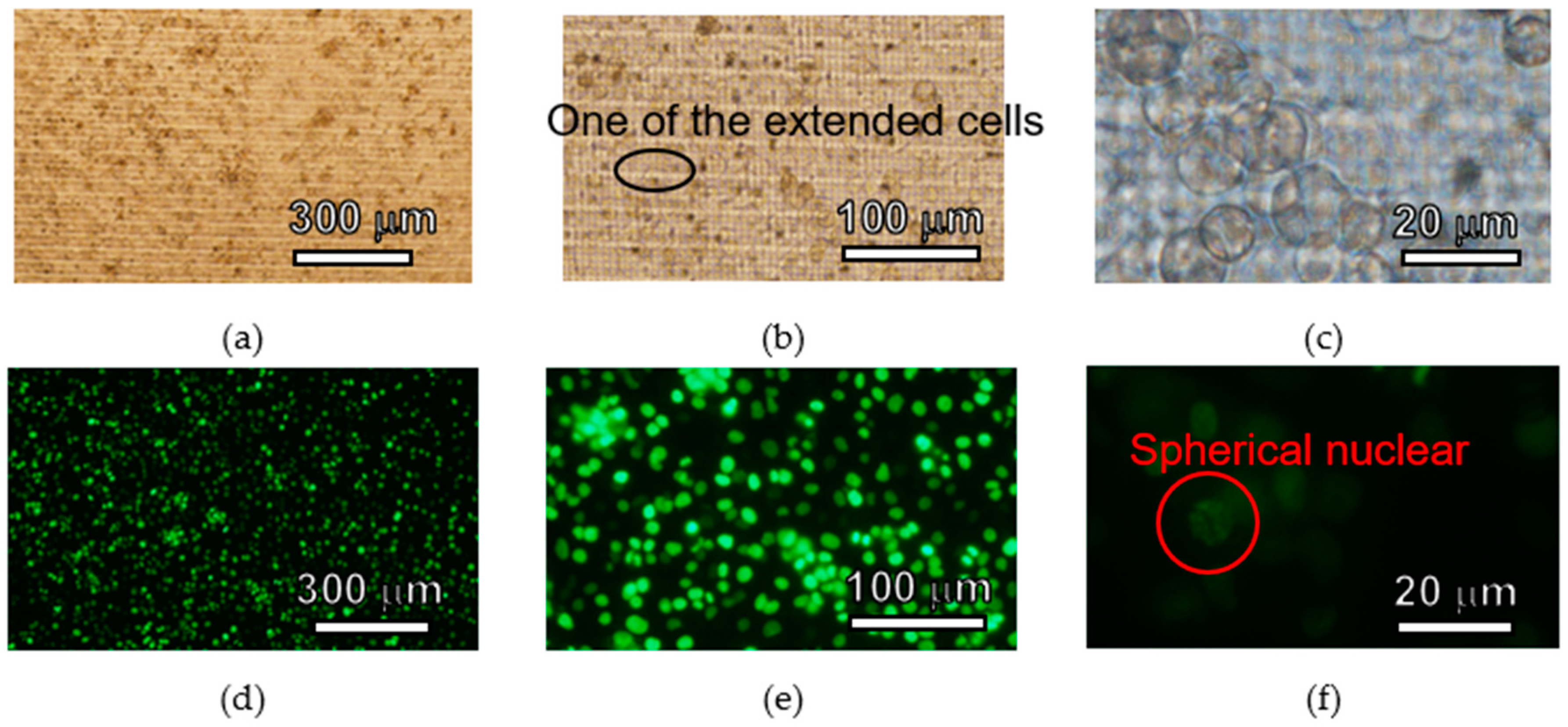
3.3. Cell Culture Plate
4. Discussion
4.1. Surface Modification by Plasma Treatment
4.2. Basic Surface Properties for Cell Culture and Analysis
4.3. Cell Culture Plate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Qi-Chen, Z.; Rui-Zhi, N.; Yuan, M.; Jin-Ming, L. Recent developments in microfluidic chip for in vitro cell-based research. Anal. Chem. 2016, 44, 522–532. [Google Scholar]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Coord. Chem. 2014, 194, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Kishi, H.; Tokimitsu, Y.; Kondo, S.; Honda, R.; Rao, S.R.; Omori, M.; Tamiya, E.; Muraguchi, A. Single-cell microarray for analyzing cellular response. Anal. Chem. 2005, 77, 8050–8056. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Yatsushiro, S.; Yamaguchi, Y.; Abe, K.; Shinohara, Y.; Tamiya, E.; Baba, Y.; Kataoka, M. Accurate detection of carcinoma cells by use of a cell microarray chip. PLoS ONE 2012, 7, e32370. [Google Scholar] [CrossRef]
- Kaminaga, M.; Ishida, T.; Kadonosono, T.; Kizaka-Kondoh, S.; Omata, T. Microfluidic device for screening for target cell-specific binding molecules by using adherent cells. Micromachines 2019, 10, 41. [Google Scholar] [CrossRef]
- Ishida, T.; Shimamoto, T.; Kaminaga, M.; Kuchimaru, T.; Kizaka-Kondoh, S.; Omata, T. Microfluidic high-migratory cell collector suppressing artifacts caused by microstructures. Micromachines 2019, 10, 116. [Google Scholar] [CrossRef]
- Escobedo, C. On-chip nanohole array based sensing: A review. Lab Chip 2013, 13, 2445–2463. [Google Scholar] [CrossRef]
- Miyajima, H.; Mehregany, M. High-aspect-ratio photolithography for MEMS applications. J. MEMS 1995, 4, 220–229. [Google Scholar] [CrossRef]
- He, W.; Halberstadtb, C.R.; Gonsalves, K.E. Lithography application of a novel photoresist for patterning of cells. Biomaterials 2004, 25, 2055–2063. [Google Scholar] [CrossRef]
- Ornoff, D.M.; Wang, Y.; Proctor, A.; Shah, A.S.; Allbritton, N.L. Co-fabrication of chitosan and epoxy photoresist to form microwell arrays with permeable hydrogel bottoms. Biomaterials 2016, 74, 77–88. [Google Scholar] [CrossRef]
- Lorenz, H.; Despont, M.; Fahrni, N.; LaBianca, N.; Renaud, P.; Vettiger, P. SU-8: A low-cost negative resist for MEMS. J. Micromech. Microeng. 1997, 7, 121–124. [Google Scholar] [CrossRef]
- Lee, J.B.; Choi, K.; Yoo, K. Innovative SU-8 lithography techniques and their applications. Micromachines 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Probst, C.; Grünberger, A.; Wiechert, W.; Kohlheyer, D. Polydimethylsiloxane (PDMS) sub-micron traps for single-cell analysis of bacteria. Micromachines 2013, 4, 357–369. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Wang, S.; Maryu, G.; Yang, O. Building dynamic cellular machineries in droplet-based artificial cells with single-droplet tracking and analysis. Anal. Chem. 2019, 91, 9813–9818. [Google Scholar] [CrossRef]
- Escobedo, C.; Vincent, S.; Choudhury, A.I.K.; Campbell, J.; Brolo, A.G.; Sinton, D.; Gordon, R. Integrated nanohole array surface plasmon resonance sensing device using a dual-wavelength source. J. Micromech. Microeng. 2011, 21, 115001. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, P.; Liu, K.; Escobedo, C. A versatile bonding method for PDMS and SU-8 and its application towards a multifunctional microfluidic device. Micromachines 2016, 7, 230. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, K.; Suzuki, T. Integration method of microchannel and vertical micromesh structure for three-dimensional cell culture by using inclined exposure and inclined oxygen ashing. Micromachines 2018, 9, 681. [Google Scholar] [CrossRef]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater. Sci. Eng. C 2013, 33, 4453–4459. [Google Scholar] [CrossRef]
- Walcazc, R.; Sniadek, P.; Dziuban, J.A. SU-8 photoresist as material of optical passive components integrated with analytical microsystems for real-time polymerase chain reaction. Opt. Appl. 2011, 41, 873–884. [Google Scholar]
- Tamai, H.; Maruo, K.; Ueno, H.; Terao, K.; Kotera, H.; Suzuki, T. Development of low-fluorescence thick photoresist for high-aspect-ratio microstructure in bio-application. Biomaicrofluidics 2015, 9, 022405. [Google Scholar] [CrossRef]
- Cooke, M.J.; Phillips, S.R.; Shah, D.S.H.; Athey, D.; Lakey, J.H.; Przyborski, S.A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology 2008, 56, 71–79. [Google Scholar] [CrossRef]
- Hennemeyer, M.; Walther, F.; Kerstan, S.; Schürzinger, K.; Gigler, A.M.; Stark, R.W. Cell proliferation assays on plasma activated SU-8. Microelectron. Eng. 2008, 85, 1298–1301. [Google Scholar] [CrossRef]
- Giljean, S.; Bigerelle, M.; Anselme, K.; Haidara, H. New insights on contact angle/roughness dependence on high surface energy materials. Appl. Surf. Sci. 2011, 257, 9631–9638. [Google Scholar] [CrossRef]
- Inoue, M.; Okonogi, A.; Terao, K.; Takao, H.; Shimokawa, F.; Oohira, F.; Kotera, H.; Suzuki, T. Cell Culture on MEMS Materials in Micro-Environment Limited by a Physical Condition. Micro Nano Lett. 2012, 7, 725–728. [Google Scholar] [CrossRef]
- Ueno, H.; Inoue, M.; Okonogic, A.; Koterad, H.; Suzuki, T. Correlation between Cells-on-Chips materials and cell adhesion/proliferation focused on material’s surface free energy. Colloids Surf. A 2019, 565, 188–194. [Google Scholar] [CrossRef]
- Histone H2B-GFP Expressing HeLa Cell Line. Available online: https://www.merckmillipore.com/JP/en/product/Histone-H2B-GFP-expressing-HeLa-Cell-Line,MM_NF-SCC117 (accessed on 27 May 2020).
- Rasband, W.S.; Image, J. U.S. National Institutes of Health; Bethesda: Rockville, MD, USA, 2018. [Google Scholar]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1969, 2, 66–81. [Google Scholar] [CrossRef]
- Kaelble, D.H.; Uy, K.C. A reinterpretation of organic liquid- polytetrafluoroethylene surface interactions. J. Adhes. 1969, 1, 50–60. [Google Scholar] [CrossRef]
- Chou, S.; Lai, J.; Cho, C.; Lee, C. Relationships between surface roughness/stiffness of chitosan coatings and fabrication of corneal keratocyte spheroids: Effect of degree of deacetylation. Colloids Surf. B 2016, 142, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Mastumoto, S.; Yamamura, S. Control of cell adhesion and detachment on a nanostructured scaffold composed of light-responsive gas-generating film. Sens. Mater. 2019, 31, 89–98. [Google Scholar]
- Lai, J.; Tu, I. Adhesion, phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights. Acta Biomater. 2012, 8, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamiltona, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sasaki, N.; Svahn, H.A.; Sato, K. Microfluidics for nano-pathophysiology. Adv. Drug Deliv. Rev. 2014, 74, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Sato, K.; Yoshimura, E. Micro total bioassay system for ingested substances: Assessment of intestinal absorption, hepatic metabolism, and bioactivity. Anal. Chem. 2010, 82, 9983–9988. [Google Scholar] [CrossRef]
- Esch, M.B.; Sung, J.H.; Yang, J.; Yu, C.; Yu, J.; March, J.C.; Shuler, M.L. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 2012, 14, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; Chen, P.; Li, Y.; Yan, S.; Wang, J.; Du, W.; Liu, B. Wound-on-a-chip: High-throughput 3D wound healing assay with a novel SU-8 mesh chip. Sens. Actuators B 2019, 280, 86–93. [Google Scholar] [CrossRef]
- Kennedy, O.O.; Kurosawa, O.; Yamazaki, S.; Oana, H.; Kotera, H.; Nakauchi, H.; Washizu, M. Cell adhesion minimization by a novel mesh culture method mechanically directs trophoblast differentiation and self-assembly organization of human pluripotent stem cells. Tissue Eng. Part C Methods 2015, 21, 1105–1115. [Google Scholar]
- Villaluenga, J.P.G.; Tabe-Mohammadi, A. A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. J. Membr. Sci. 2000, 169, 159–174. [Google Scholar] [CrossRef]
- Williams, J.D.; Wang, W. Study on the postbaking process and the effects on UV lithography of high aspect ratio SU-8 microstructures. J. Micro/Nanolithography MEMS MOEMS 2004, 3, 563–568. [Google Scholar] [CrossRef]
- Curtis, A.S.G.; Forrester, J.V.; Mcinnes, C.; Lawrie, F. Adhesion of Cells to Polystyrene Surfaces. J. Cell Biol. 1983, 97, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]

| SU-8 | SJI-001 | |
|---|---|---|
| Adhesion rate (%) | 68 | 74 |
| Doubling time (h) | 27.9 | 25.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.; Maruo, K.; Inoue, M.; Kotera, H.; Suzuki, T. Cell Culture on Low-Fluorescence and High-Resolution Photoresist. Micromachines 2020, 11, 571. https://doi.org/10.3390/mi11060571
Ueno H, Maruo K, Inoue M, Kotera H, Suzuki T. Cell Culture on Low-Fluorescence and High-Resolution Photoresist. Micromachines. 2020; 11(6):571. https://doi.org/10.3390/mi11060571
Chicago/Turabian StyleUeno, Hidetaka, Katsuya Maruo, Masatoshi Inoue, Hidetoshi Kotera, and Takaaki Suzuki. 2020. "Cell Culture on Low-Fluorescence and High-Resolution Photoresist" Micromachines 11, no. 6: 571. https://doi.org/10.3390/mi11060571
APA StyleUeno, H., Maruo, K., Inoue, M., Kotera, H., & Suzuki, T. (2020). Cell Culture on Low-Fluorescence and High-Resolution Photoresist. Micromachines, 11(6), 571. https://doi.org/10.3390/mi11060571