Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis
Abstract
1. Introduction
2. Luminophores for Cellular Analysis
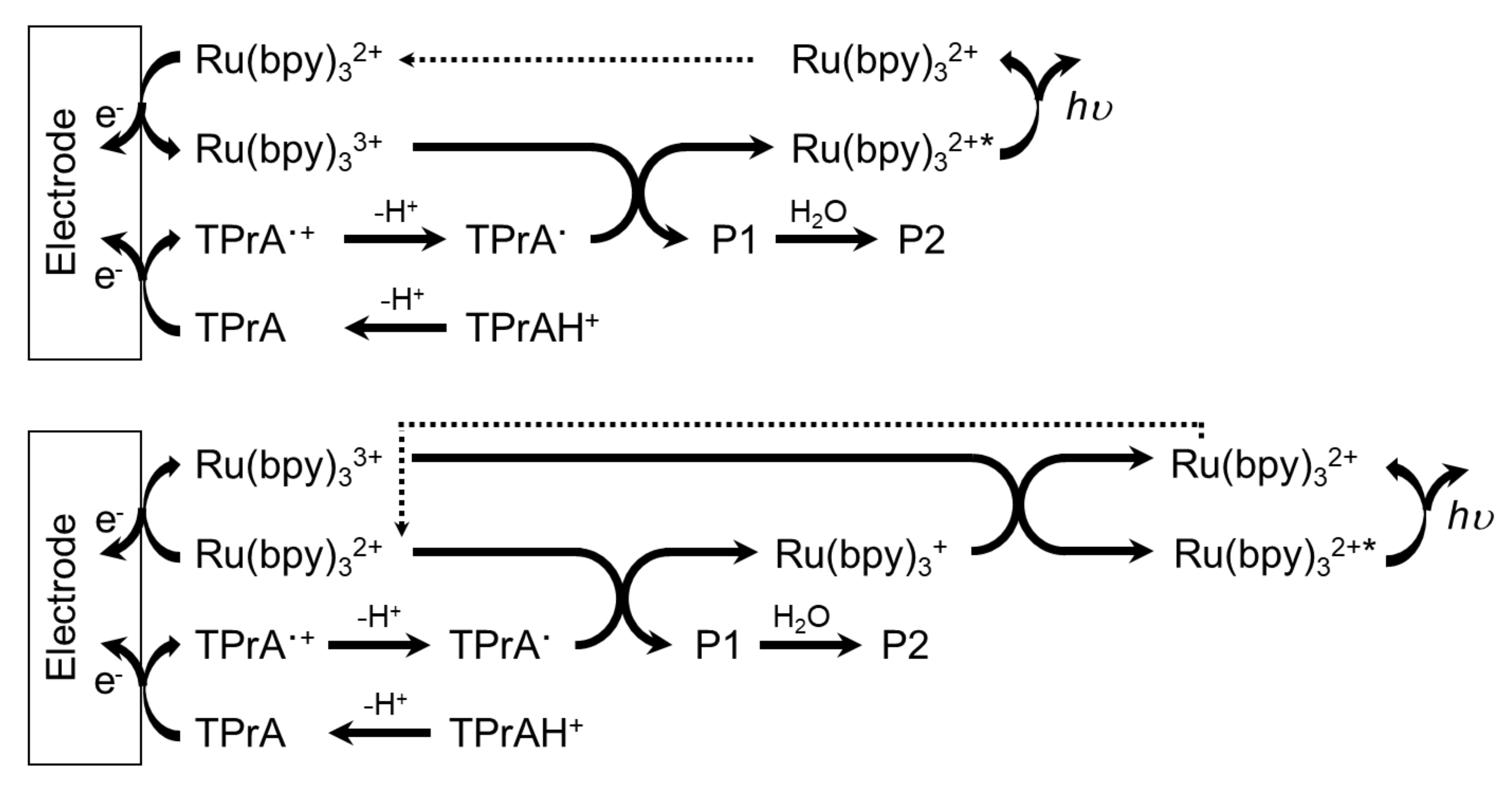
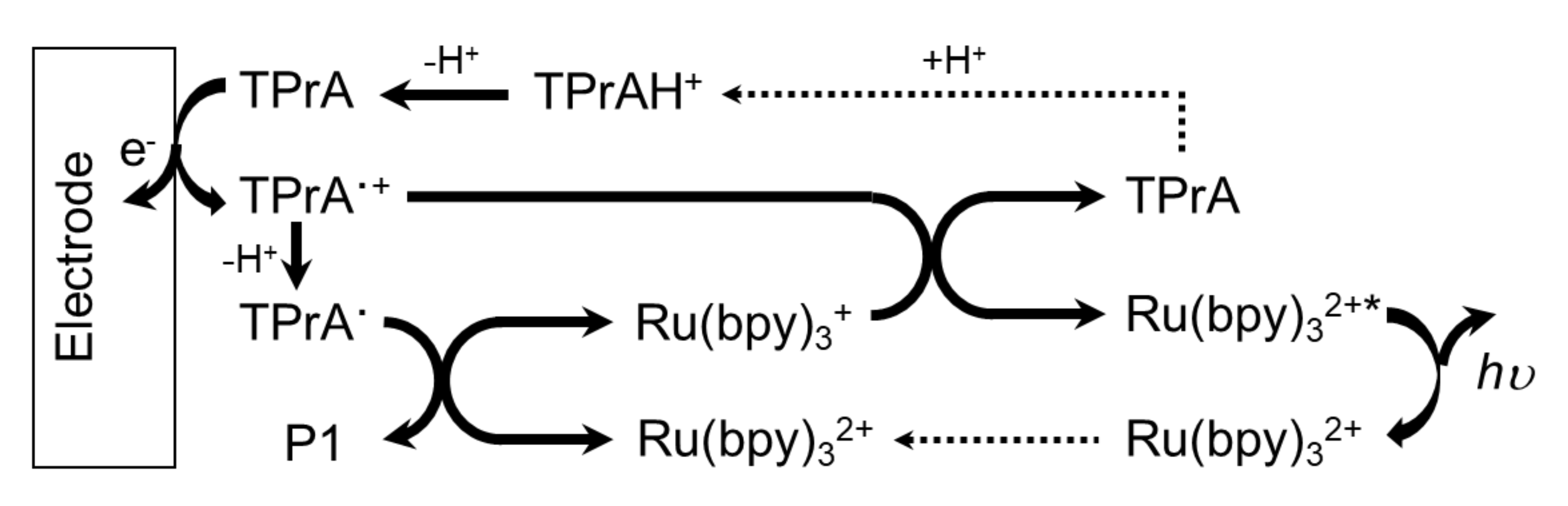
2.1. Ruthenium Based Systems
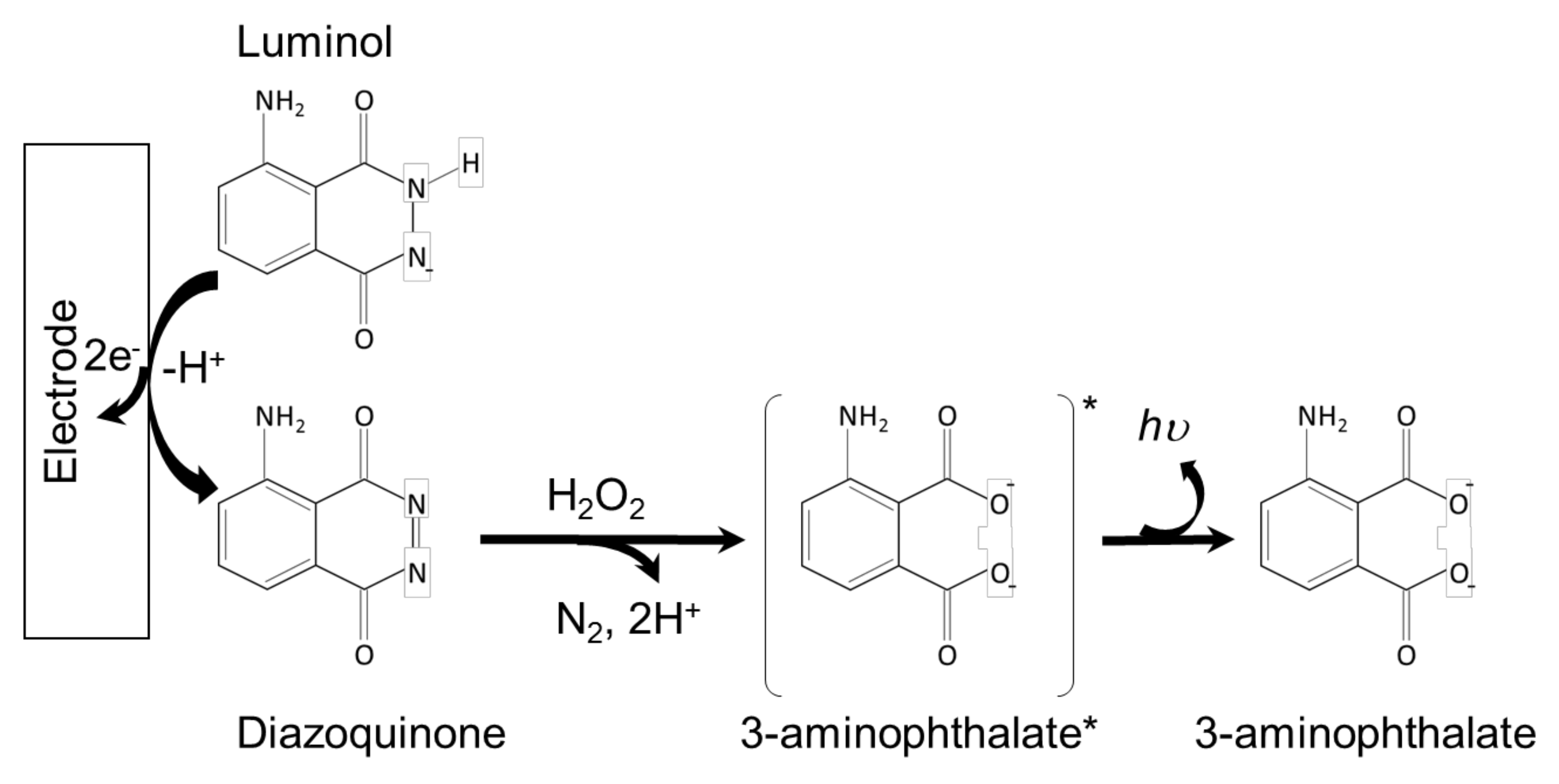
2.2. Luminol-Based Systems
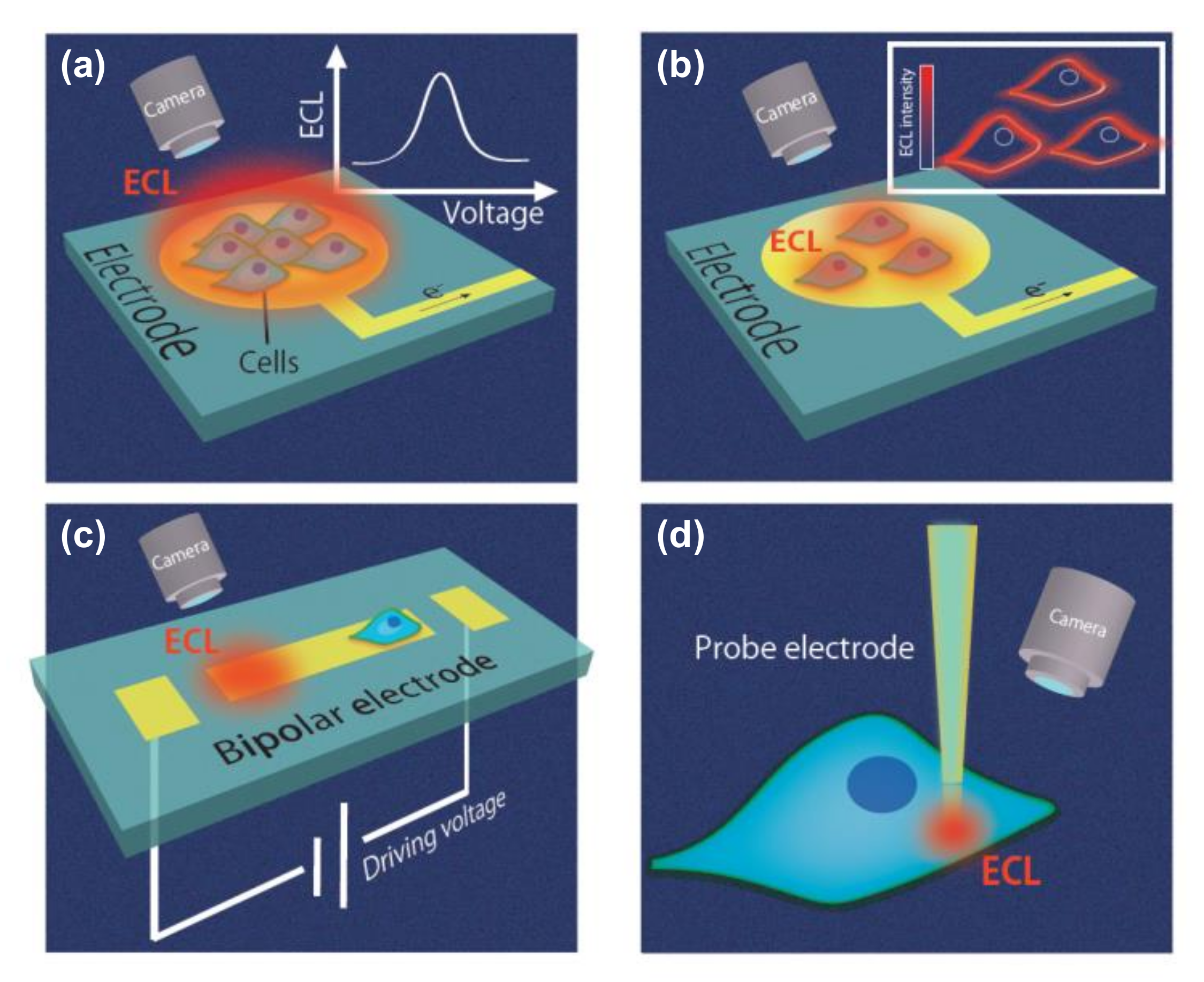
3. Electrochemiluminescence (ECL) Microscopy for Cell Imaging
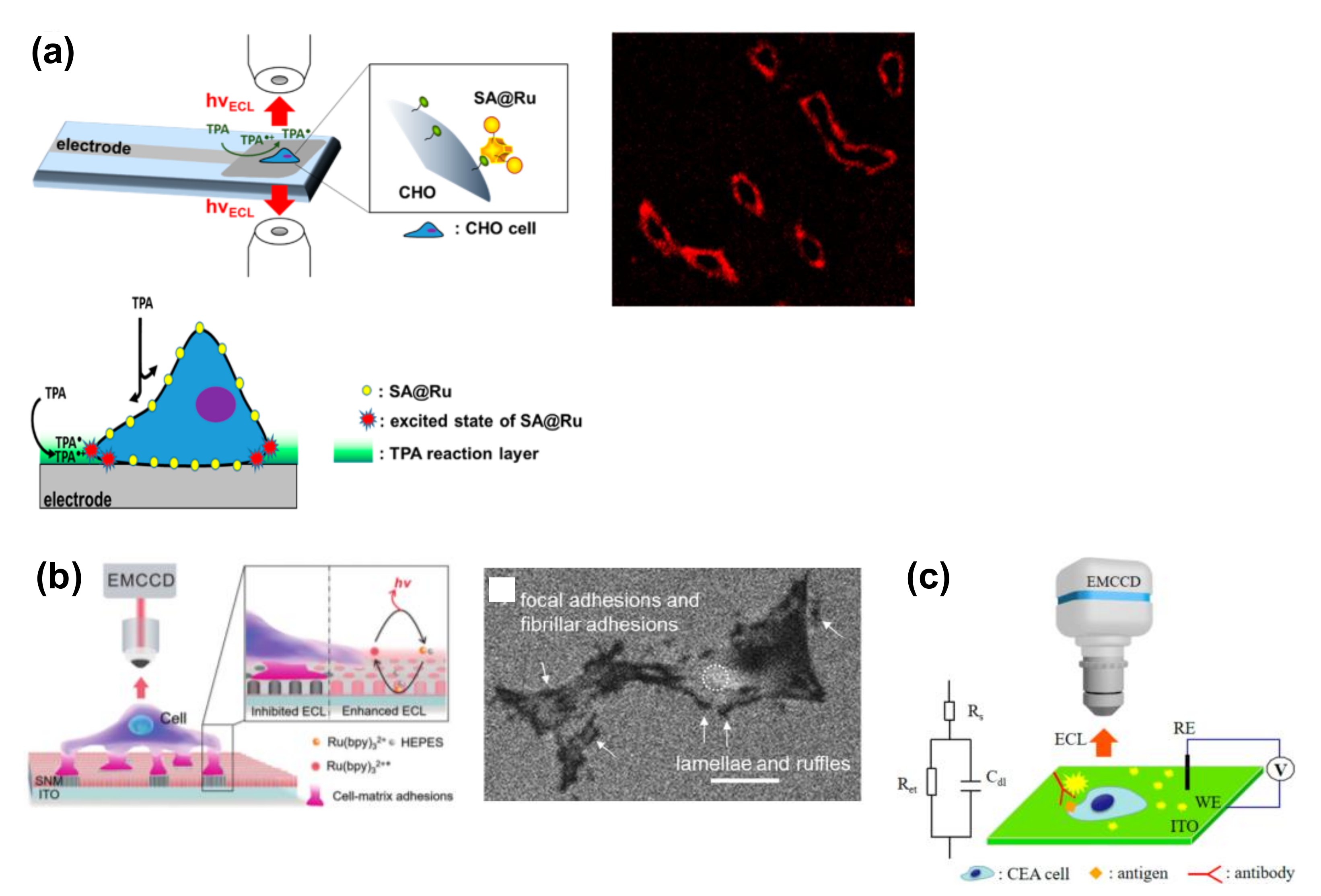
3.1. Imaging of Cell Membranes and Cell Adhesion
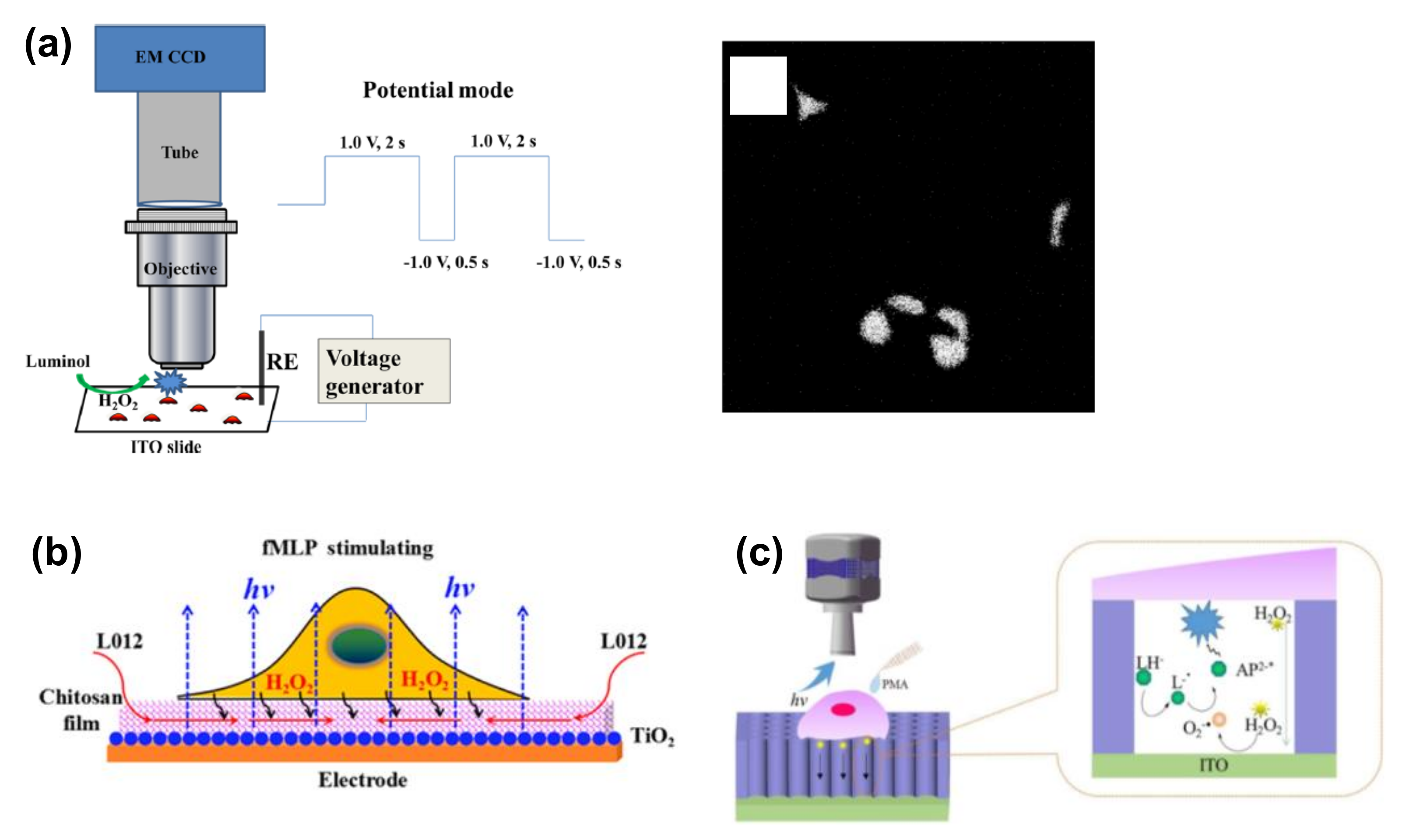
3.2. Imaging of Released Cellular Molecules
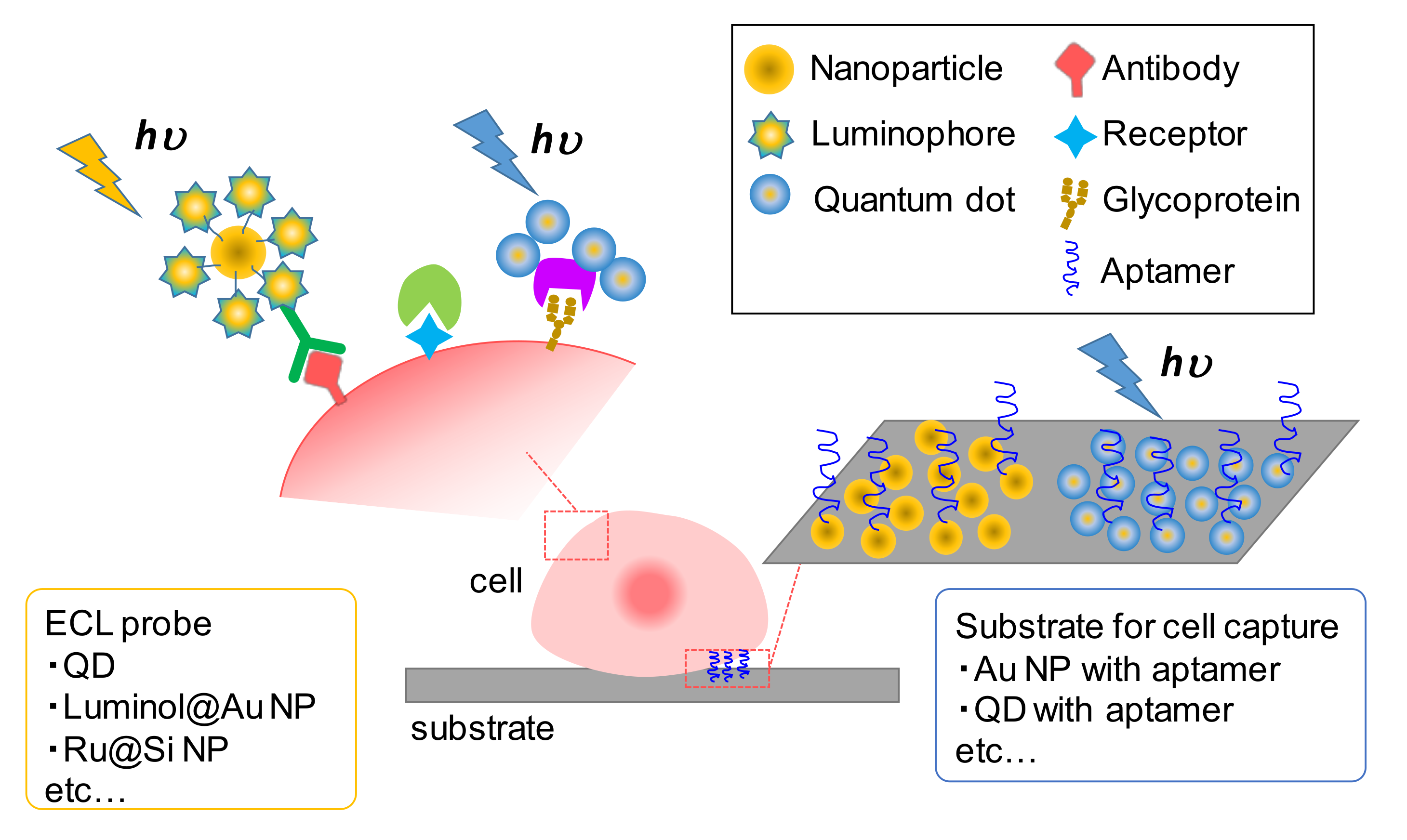
4. ECL Sensors for Cell Analysis
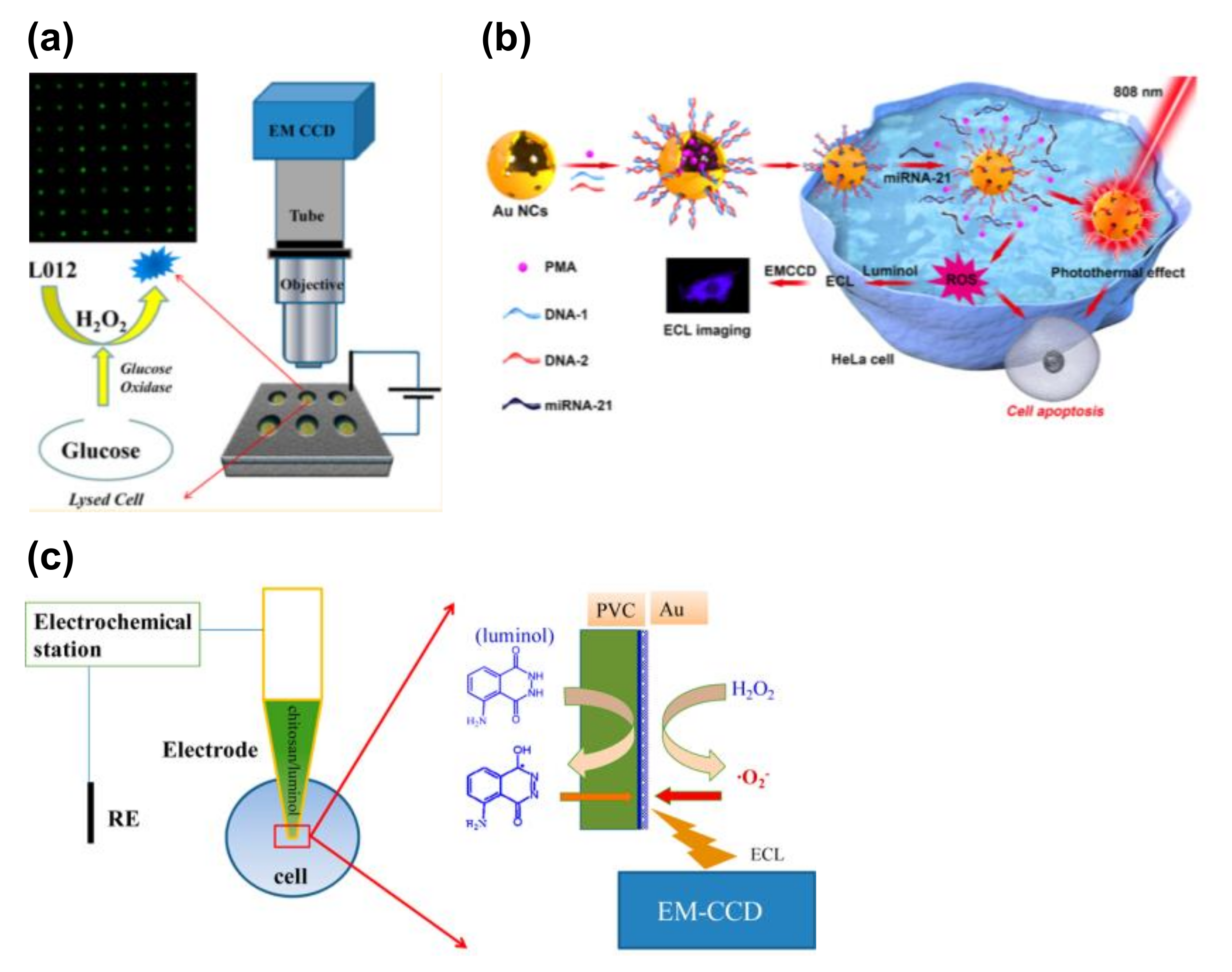
4.1. ECL Detection of Intracellular Molecules
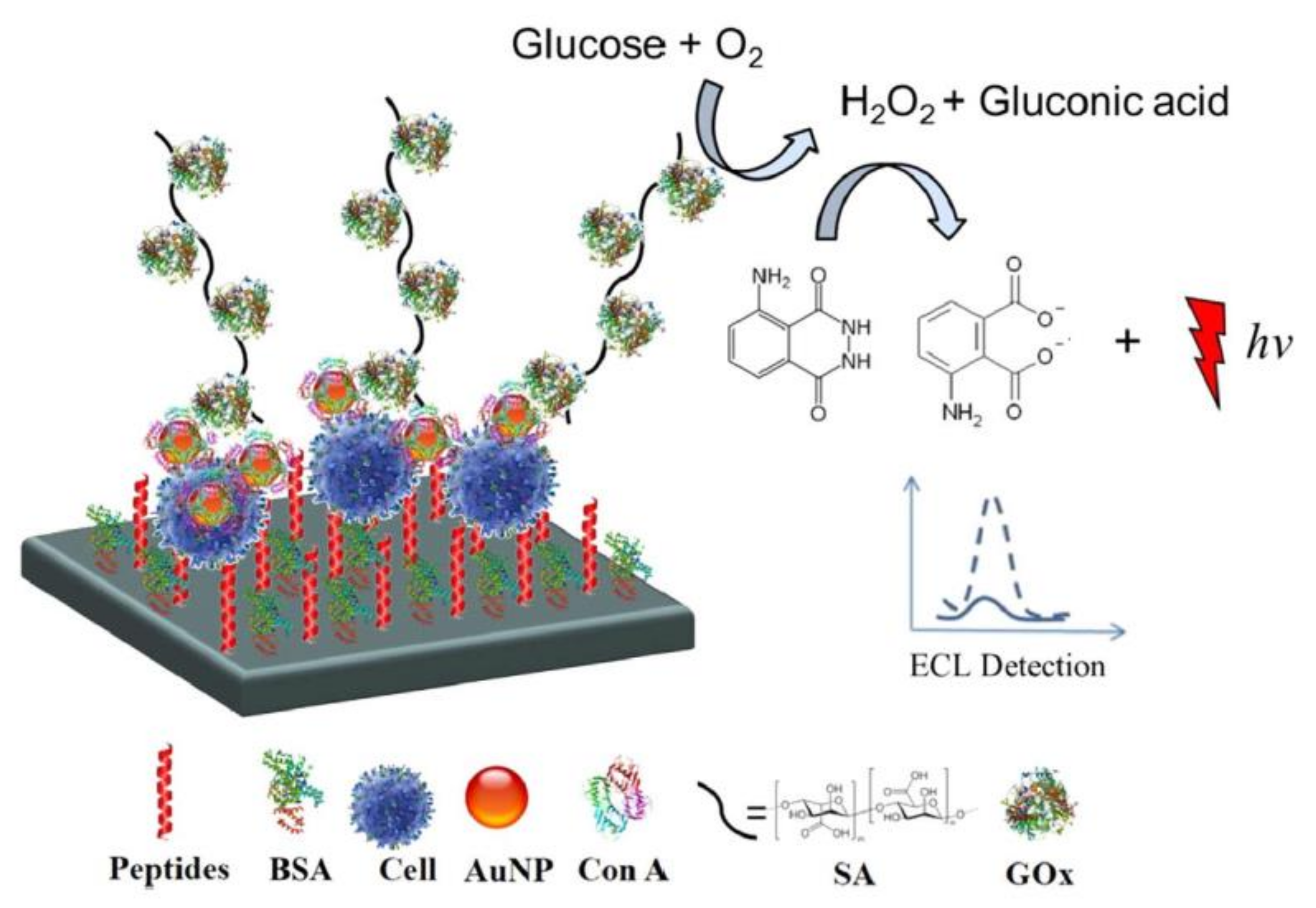
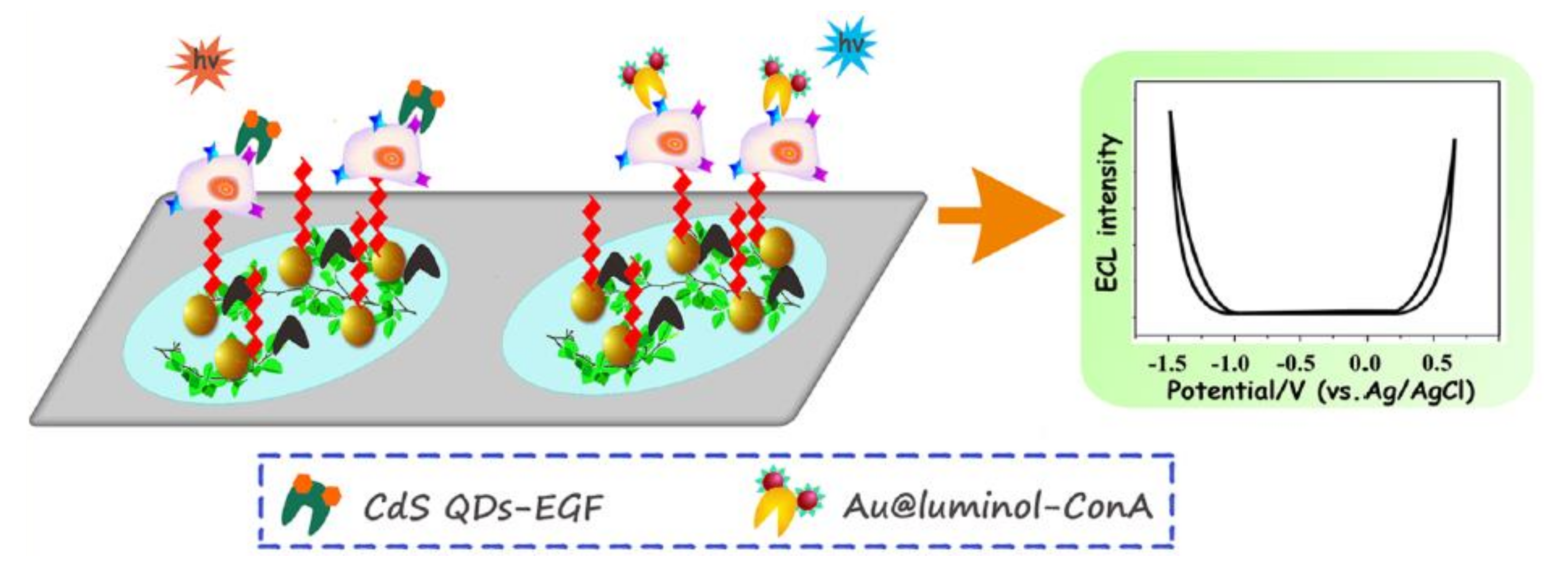
4.2. ECL Detection of Biomolecules on Cells
4.3. Applied ECL Sensing Techniques
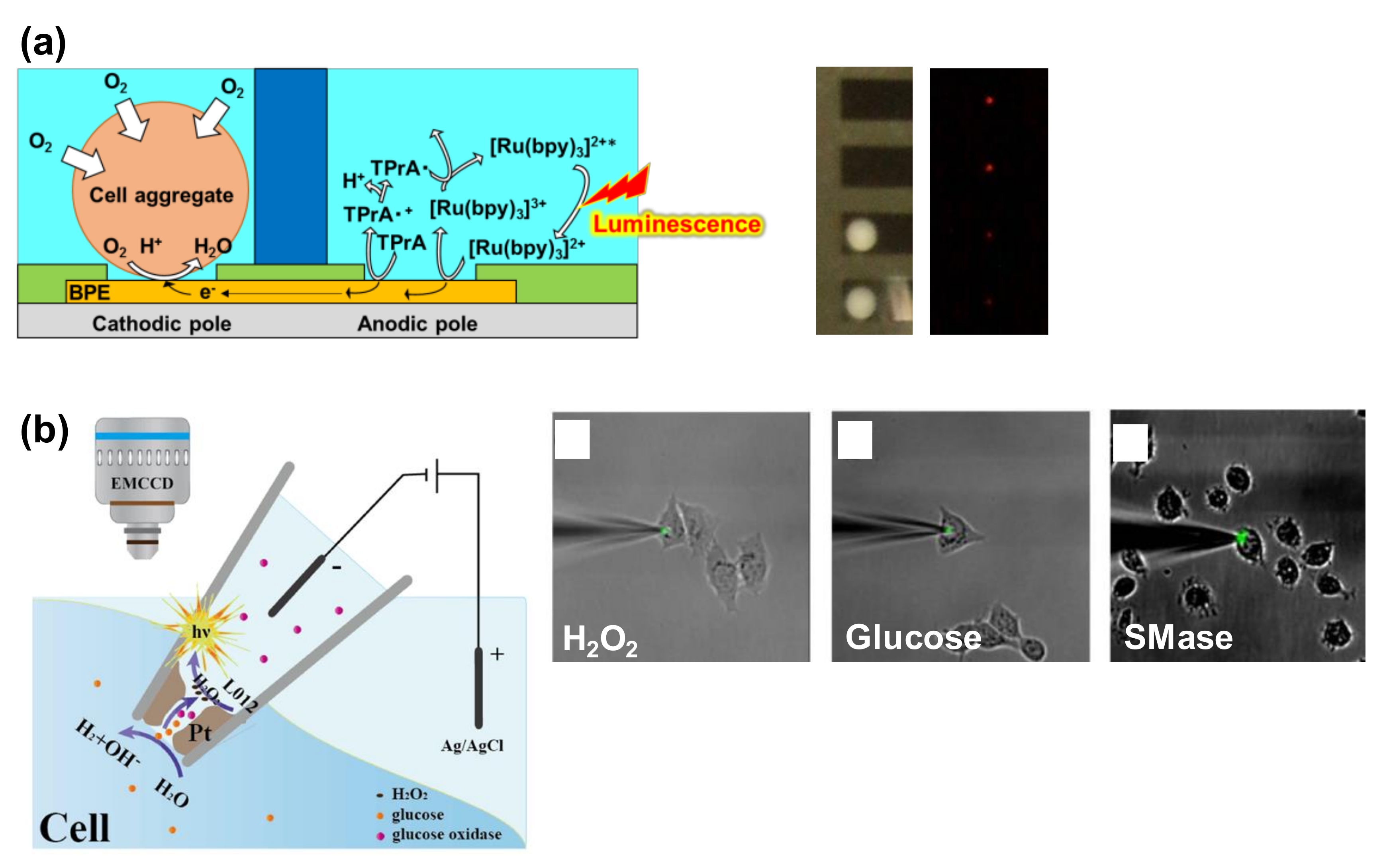
5. Bipolar Electrode (BPE) Devices for ECL-Based Cell Analysis
5.1. Open BPE ECL Devices
5.2. Closed BPE ECL Devices
5.3. BPE Probe Devices
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Chen, Y.; Zhu, J.J. Recent advances in electrochemiluminescence analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Arbault, S.; Sojic, N.; Jiang, D. Electrochemiluminescence imaging for bioanalysis. Annu. Rev. Anal. Chem. 2019, 12, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Zanut, A.; Fiorani, A.; Rebeccani, S.; Kesarkar, S.; Valenti, G. Electrochemiluminescence as emerging microscopy techniques. Anal. Bioanal. Chem. 2019, 411, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Kishi, H.; Tokimitsu, Y.; Kondo, S.; Honda, R.; Rao, S.R.; Omori, M.; Tamiya, E.; Muraguchi, A. Single-cell microarray for analyzing cellular response. Anal. Chem. 2005, 77, 8050–8056. [Google Scholar] [CrossRef]
- Inagi, S. Fabrication of gradient polymer surfaces using bipolar electrochemistry. Polym. J. 2016, 48, 39–44. [Google Scholar] [CrossRef]
- Shida, N.; Zhou, Y.; Inagi, S. Bipolar electrochemistry: A powerful tool for electrifying functional material synthesis. Accounts Chem. Res. 2019, 52, 2598–2608. [Google Scholar] [CrossRef]
- Ino, K.; Matsumoto, T.; Taira, N.; Kumagai, T.; Nashimoto, Y.; Shiku, H. Hydrogel electrodeposition based on bipolar electrochemistry. Lab Chip 2018, 18, 2425–2432. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Su, B. Electrochemiluminescence single cell analysis: Intensity- and imaging-based methods. ChemPlusChem 2020, 85, 725–733. [Google Scholar] [CrossRef]
- Shen, J.J.; Zhou, T.; Huang, R. Recent advances in electrochemiluminescence sensors for pathogenic bacteria detection. Micromachines 2019, 10, 532. [Google Scholar] [CrossRef]
- Fiorani, A.; Valenti, G.; Iurlo, M.; Marcaccio, M.; Paolucci, F. Electrogenerated chemiluminescence: A molecular electrochemistry point of view. Curr. Opin. Electrochem. 2018, 8, 31–38. [Google Scholar] [CrossRef]
- Fiorani, A.; Valenti, G.; Villani, E.; Marcaccio, M.; Rampazzo, E.; Prodi, L.; Paolucci, F. Electrochemically driven luminescence in organometallic and inorganic systems. Lumin. Electrochem. 2017, 293–326. [Google Scholar]
- Fiorani, A.; Merino, J.P.; Zanut, A.; Criado, A.; Valenti, G.; Prato, M.; Paolucci, F. Advanced carbon nanomaterials for electrochemiluminescent biosensor applications. Curr. Opin. Electrochem. 2019, 16, 66–74. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ugo, P. Recent advances in electrochemiluminescence with quantum dots and arrays of nanoelectrodes. ChemElectroChem 2017, 4, 1663–1676. [Google Scholar] [CrossRef]
- Valenti, G.; Rampazzo, E.; Kesarkar, S.; Genovese, D.; Fiorani, A.; Zanut, A.; Palomba, F.; Marcaccio, M.; Paolucci, F.; Prodi, L. Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications. Coord. Chem. Rev. 2018, 367, 65–81. [Google Scholar] [CrossRef]
- Tokel, N.E.; Bard, A.J. Electrogenerated chemiluminescence.9. Electrochemistry and emission from systems containing tris(2, 2’-bipyridine)ruthenium(II) dichloride. J. Am. Chem. Soc. 1972, 94, 2862–2863. [Google Scholar] [CrossRef]
- Rubinstein, I.; Bard, A.J. Electrogenerated chemi-luminescence. 37. aqueous ECL systems based on Ru(2,2’-bipyridine)32+ and oxalate or organic-acids. J. Am. Chem. Soc. 1981, 103, 512–516. [Google Scholar] [CrossRef]
- White, H.S.; Bard, A.J. Electrogenerated chemi-luminescence. 41. Electrogenerated chemi-luminescence and chemi-luminescence of the Ru(2,2‘-bpy)32+-S2O82- system in acetonitrile water solutions. J. Am. Chem. Soc. 1982, 104, 6891–6895. [Google Scholar] [CrossRef]
- Leland, J.K.; Powell, M.J. Electrogenerated chemiluminescence—an oxidative-reduction type ECL reaction sequence using tripropyl amine. J. Electrochem. Soc. 1990, 137, 3127–3131. [Google Scholar] [CrossRef]
- Miao, W.J.; Choi, J.P.; Bard, A.J. Electrogenerated chemiluminescence 69: The tris(2, 2‘-bipyridine)ruthenium(II), (Ru(bpy)32+)/tri-n-propylamine (TPrA) system revisited—A new route involving TPrA•+ cation radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Su, B. Imaging cell-matrix adhesions and collective migration of living cells by electrochemiluminescence microscopy. Angew. Chem. Int. Edit. 2020, 59, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Y.; Sun, X. Determination of glutathione in individual Ramos cells by capillary electrophoresis with electrochemiluminescence detection. Anal. Methods 2013, 5, 1542–1547. [Google Scholar] [CrossRef]
- Wu, M.-S.; Qian, G.-s.; Xu, J.-J.; Chen, H.-Y. Sensitive electrochemiluminescence detection of c-Myc mRNA in breast cancer cells on a wireless bipolar electrode. Anal. Chem. 2012, 84, 5407–5414. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.T.; Wang, Y.L.; Zhang, J.J.; Dong, Y.X.; Liu, F.R.; Ren, S.W.; Liu, Y.M. Immuno-electrochemiluminescent imaging of a single cell based on functional nanoprobes of heterogeneous Ru(bpy)32+@SiO2/Au Nanoparticles. Anal. Chem. 2018, 90, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bi, S.; Jia, X.Q.; He, P. Aptamer-conjugated bio-bar-code Au-Fe3O4 nanoparticles as amplification station for electrochemiluminescence detection of tumor cells. Anal. Chim. Acta 2014, 837, 44–51. [Google Scholar] [CrossRef]
- Chen, Z.H.; Liu, Y.; Wang, Y.Z.; Zhao, X.; Li, J.H. Dynamic evaluation of cell surface N-glycan expression via an electrogenerated chemiluminescence biosensor based on concanavalin A-integrating gold-nanoparticle-modified Ru(bpy)32+-doped silica nanoprobe. Anal. Chem. 2013, 85, 4431–4438. [Google Scholar] [CrossRef]
- Wu, M.-S.; Shi, H.-W.; Xu, J.-J.; Chen, H.-Y. CdS quantum dots/Ru(bpy)32+ electrochemiluminescence resonance energy transfer system for sensitive cytosensing. Chem. Commun. 2011, 47, 7752–7754. [Google Scholar] [CrossRef]
- Ding, C.F.; Wei, S.; Liu, H.T. Electrochemiluminescent determination of cancer cells based on aptamers, nanoparticles, and magnetic beads. Chem.-Eur. J. 2012, 18, 7263–7268. [Google Scholar] [CrossRef]
- Ding, C.F.; Ge, Y.; Zhang, S.S. Electrochemical and electrochemiluminescence determination of cancer cells based on aptamers and magnetic beads. Chem.-Eur. J. 2010, 16, 10707–10714. [Google Scholar] [CrossRef]
- Harvey, N. Luminescence during electrolysis. J. Phys. Chem. 1929, 33, 1456–1459. [Google Scholar] [CrossRef]
- Sakura, S. Electrochemiluminescence of hydrogen-peroxide luminol at a carbon electrode. Anal. Chim. Acta 1992, 262, 49–57. [Google Scholar] [CrossRef]
- Hazelton, S.G.; Zheng, X.; Zhao, J.X.; Pierce, D.T. Developments and Applications of Electrogenerated Chemiluminescence Sensors Based on Micro- and Nanomaterials. Sensors 2008, 8, 5942–5960. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wang, H.J.; Yuan, R.; Chai, Y.Q. Functional three-dimensional porous conductive polymer hydrogels for sensitive electrochemiluminescence in situ detection of H2O2 released from live cells. Anal. Chem. 2018, 90, 8462–8469. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wang, Z.L.; Wang, H.J.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. A novel metal-organic framework loaded with abundant N-(aminobutyl)-N-(ethylisoluminol) as a high-efficiency electrochemiluminescence indicator for sensitive detection of mucin1 on cancer cells. Chem. Commun. 2017, 53, 9705–9708. [Google Scholar] [CrossRef] [PubMed]
- Kai, T.H.; Zoski, C.G.; Bard, A.J. Scanning electrochemical microscopy at the nanometer level. Chem. Commun. 2018, 54, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Sen, M.; Shiku, H.; Matsue, T. Micro/nanoelectrochemical probe and chip devices for evaluation of three-dimensional cultured cells. Analyst 2017, 142, 4343–4354. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Shiku, H.; Matsue, T. Bioelectrochemical applications of microelectrode arrays in cell analysis and engineering. Curr. Opin. Electrochem. 2017, 5, 146–151. [Google Scholar] [CrossRef]
- Ino, K.; Kanno, Y.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Shiku, H.; Matsue, T. Electrochemical motion tracking of microorganisms using a large-scale-integration-based amperometric device. Angew. Chem. Int. Ed. 2017, 56, 6818–6822. [Google Scholar] [CrossRef]
- Valenti, G.; Scarabino, S.; Goudeau, B.; Lesch, A.; Jović, M.; Villani, E.; Sentic, M.; Rapino, S.; Arbault, S.; Paolucci, F.; et al. Single cell electrochemiluminescence imaging: From the proof-of-concept to disposable device-based analysis. J. Am. Chem. Soc. 2017, 139, 16830–16837. [Google Scholar] [CrossRef]
- Voci, S.; Goudeau, B.; Valenti, G.; Lesch, A.; Jović, M.; Rapino, S.; Paolucci, F.; Arbault, S.; Sojic, N. Surface-confined electrochemiluminescence microscopy of cell membranes. J. Am. Chem. Soc. 2018, 140, 14753–14760. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, R.; Jiang, D.; Chen, H.-Y. Electrochemiluminescence-based capacitance microscopy for label-free imaging of antigens on the cellular plasma membrane. J. Am. Chem. Soc. 2019, 141, 10294–10299. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, B.K.; Ma, C.; Chen, Z.X.; Zhu, J.J. Potential-resolved electrochemiluminescence nanoprobes for visual apoptosis evaluation at single-cell level. Anal. Chem. 2019, 91, 6363–6370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, G.; Chen, Y.; Fang, D.; Jiang, D.; Chen, H.-y. Electrochemiluminescence imaging for parallel single-cell analysis of active membrane cholesterol. Anal. Chem. 2015, 87, 8138–8143. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, C.; Jin, B.-K.; Chen, Z.; Zhu, J.-J. Direct electrochemiluminescence imaging of a single cell on a chitosan film modified electrode. Anal. Chem. 2018, 90, 4801–4806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ding, H.; Zhao, S.Y.; Jiang, D.C.; Chen, H.Y. Confined electrochemiluminescence in vertically ordered silica mesochannels for the imaging of hydrogen peroxide released from single cells. Electrochem. Commun. 2019, 98, 38–42. [Google Scholar] [CrossRef]
- Li, L.Y.; Liu, K.; Fang, D.J. Single cell electrochemiluminescence analysis of cholesterol in plasma membrane during testosterone treatment. Electroanalysis 2020, 32, 958–963. [Google Scholar] [CrossRef]
- Ma, G.Z.; Zhou, J.Y.; Tian, C.X.; Jiang, D.C.; Fang, D.J.; Chen, H.Y. Luminol electrochemiluminescence for the analysis of active cholesterol at the plasma membrane in single mammalian cells. Anal. Chem. 2013, 85, 3912–3917. [Google Scholar] [CrossRef]
- Zuo, H.Z.; Wang, R.; Jiang, D.C.; Fang, D.J. Determining the composition of active cholesterol in the plasma membrane of single cells by using electrochemiluminescence. ChemEectroChem 2017, 4, 1677–1680. [Google Scholar] [CrossRef]
- Xu, J.J.; Jiang, D.P.; Qin, Y.L.; Xia, J.; Jiang, D.C.; Chen, H.Y. C3N4 nanosheet modified microwell array with enhanced electrochemiluminescence for total analysis of cholesterol at single cells. Anal. Chem. 2017, 89, 2216–2220. [Google Scholar] [CrossRef]
- Cui, C.; Chen, Y.; Jiang, D.C.; Chen, H.Y.; Zhang, J.R.; Zhu, J.J. Steady-state electrochemiluminescence at single senniconductive titanium dioxide nanoparticles for local sensing of single cells. Anal. Chem. 2019, 91, 1121–1125. [Google Scholar] [CrossRef]
- Ino, K.; Nashimoto, Y.; Taira, N.; Azcon, J.R.; Shiku, H. Intracellular electrochemical sensing. Electroanalysis 2018, 30, 2195–2209. [Google Scholar] [CrossRef]
- Xu, J.; Huang, P.; Qin, Y.; Jiang, D.; Chen, H.-y. Analysis of intracellular glucose at single cells using electrochemiluminescence imaging. Anal. Chem. 2016, 88, 4609–4612. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhou, J.; Zhang, R.; Jiang, D.; Jiang, D. Gold-coated polydimethylsiloxane microwells for high-throughput electrochemiluminescence analysis of intracellular glucose at single cells. Anal. Bioanal. Chem. 2018, 410, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, B.; Sun, Z.; Zhou, H.; Zhang, S. Integration of intracellular telomerase monitoring by electrochemiluminescence technology and targeted cancer therapy by reactive oxygen species. Chem. Sci. 2017, 8, 8025–8029. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, W.; Liu, Y.; Sun, Y.; Jiang, Y.; Zhang, S. Electrochemiluminescence-microscopy for microRNA imaging in single cancer cell combined with chemotherapy-photothermal therapy. Anal. Chem. 2019, 91, 12581–12586. [Google Scholar] [CrossRef]
- He, R.; Tang, H.; Jiang, D.; Chen, H.-y. Electrochemical visualization of intracellular hydrogen peroxide at single cells. Anal. Chem. 2016, 88, 2006–2009. [Google Scholar] [CrossRef]
- Han, E.; Ding, L.; Jin, S.; Ju, H. Electrochemiluminescent biosensing of carbohydrate-functionalized CdS nanocomposites for in situ label-free analysis of cell surface carbohydrate. Biosens. Bioelectron. 2011, 26, 2500–2505. [Google Scholar] [CrossRef]
- Jie, G.; Wang, L.; Yuan, J.; Zhang, S. Versatile electrochemiluminescence assays for cancer cells based on dendrimer/CdSe–ZnS–quantum dot nanoclusters. Anal. Chem. 2011, 83, 3873–3880. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Wen, Q.; Huang, H.; Yang, P. A sensitive electrochemiluminescence cytosensor for quantitative evaluation of epidermal growth factor receptor expressed on cell surfaces. Anal. Chim. Acta 2015, 881, 148–154. [Google Scholar] [CrossRef]
- Liu, F.; Ge, S.; Su, M.; Song, X.; Yan, M.; Yu, J. Electrochemiluminescence device for in-situ and accurate determination of CA153 at the MCF-7 cell surface based on graphene quantum dots loaded surface villous Au nanocage. Biosens. Bioelectron. 2015, 71, 286–293. [Google Scholar] [CrossRef]
- Long, D.; Shang, Y.; Qiu, Y.; Zhou, B.; Yang, P. A single-cell analysis platform for electrochemiluminescent detection of platelets adhesion to endothelial cells based on Au@DL-ZnCQDs nanoprobes. Biosens. Bioelectron. 2018, 102, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, B.; Yang, X.; Long, D.; Hao, Y.; Yang, P. Novel single-cell analysis platform based on a solid-state zinc-coadsorbed carbon quantum dots electrochemiluminescence probe for the evaluation of CD44 expression on breast cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 16848–16856. [Google Scholar] [CrossRef]
- Long, D.; Chen, C.; Cui, C.; Yao, Z.; Yang, P. A high precision MUA-spaced single-cell sensor for cellular receptor assay based on bifunctional Au@Cu-PbCQD nanoprobes. Nanoscale 2018, 10, 18597–18605. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, J.; Guo, L.; Qiu, B.; Lin, Z. A surface-enhanced electrochemiluminescence sensor based on Au-SiO2 core–shell nanocomposites doped with Ru(bpy)32+ for the ultrasensitive detection of prostate-specific antigen in human serum. Analyst 2020, 145, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.L.; Deng, X.; Gu, X.X.; Jia, X.F.; Lou, B.H.; Zhang, X.W.; Li, J.; Wang, E.K. Stabilized, superparamagnetic functionalized graphene/Fe3O4@Au nanocomposites for a magnetically-controlled solid-state electrochemiluminescence biosensing application. Anal. Chem. 2015, 87, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; He, Y.; Zhang, Y.Y.; Liu, M.L.; Liu, Y.; Li, J.H. Ultrasensitive detection of cancer cells and glycan expression profiling based on a multivalent recognition and alkaline phosphatase-responsive electrogenerated chemiluminescence biosensor. Nanoscale 2014, 6, 11196–11203. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tian, Q.; Liu, Y.; Li, J. Multienzyme decorated polysaccharide amplified electrogenerated chemiluminescence biosensor for cytosensing and cell surface carbohydrate profiling. Biosens. Bioelectron. 2017, 89, 1013–1019. [Google Scholar] [CrossRef]
- Liang, W.; Zhuo, Y.; Xiong, C.; Zheng, Y.; Chai, Y.; Yuan, R. Ultrasensitive cytosensor based on self-enhanced electrochemiluminescent ruthenium-silica composite nanoparticles for efficient drug screening with cell apoptosis monitoring. Anal. Chem. 2015, 87, 12363–12371. [Google Scholar] [CrossRef]
- Cui, C.; Chen, Y.; Jiang, D.; Zhu, J.-J.; Chen, H.-Y. Attomole antigen detection using self-electrochemiluminous graphene oxide-capped Au@L012 nanocomposite. Anal. Chem. 2017, 89, 2418–2423. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Sun, Y.; Su, J.; Jin, B.; Geng, L.; Song, Y.-Y.; Huang, X.; Yang, M. Signal-on electrochemiluminescence of self-ordered molybdenum oxynitride nanotube arrays for label-free cytosensing. Anal. Chem. 2018, 90, 10858–10864. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Liu, Y.; Hou, Z.; Gu, Y.; Zhao, W. An electrochemiluminescence cytosensor for sensitive detection of HeLa cells based on a signal amplification strategy of Au-NaYF4:Yb,Er nanocomposites. Analyst 2018, 143, 4199–4205. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ma, Q. Nanoparticle-based electrochemiluminescence cytosensors for single cell level detection. Trac-Trends Anal. Chem. 2019, 110, 277–292. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wu, M.S.; Xu, J.J.; Chen, H.Y. Signal-on dual-potential electrochemiluminescence based on luminol-gold bifunctional nanoparticles for telomerase detection. Anal. Chem. 2014, 86, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Han, F.F.; Jiang, H.; Fang, D.J.; Jiang, D.C. Potential-resolved electrochemiluminescence for determination of two antigens at the cell surface. Anal. Chem. 2014, 86, 6896–6902. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sun, F.; Chen, L.; Lei, J.; Ju, H. Ratiometric electrochemiluminescence detection of circulating tumor cells and cell-surface glycans. J. Electroanal. Chem. 2016, 781, 48–55. [Google Scholar] [CrossRef]
- Zhou, B.; Qiu, Y.; Wen, Q.; Zhu, M.; Yang, P. Dual electrochemiluminescence signal system for In situ and simultaneous evaluation of multiple cell-surface receptors. ACS Appl. Mater. Interfaces 2017, 9, 2074–2082. [Google Scholar] [CrossRef]
- Ding, C.; Li, Y.; Wang, L.; Luo, X. Ratiometric electrogenerated chemiluminescence cytosensor based on conducting polymer hydrogel Loaded with Internal standard molecules. Anal. Chem. 2019, 91, 983–989. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Chen, L.; Yan, M.; Ge, L.; Ge, S.; Yu, J. A disposable electrochemiluminescence device for ultrasensitive monitoring of K562 leukemia cells based on aptamers and ZnO@carbon quantum dots. Biosens. Bioelectron. 2013, 49, 79–85. [Google Scholar] [CrossRef]
- Su, M.; Liu, H.; Ge, S.; Ren, N.; Ding, L.; Yu, J.; Song, X. An electrochemiluminescence lab-on-paper device for sensitive detection of two antigens at the MCF-7 cell surface based on porous bimetallic AuPd nanoparticles. RSC Adv. 2016, 6, 16500–16506. [Google Scholar] [CrossRef]
- Lyu, Z.-M.; Zhou, X.-L.; Wang, X.-N.; Li, P.; Xu, L.; Liu, E.H. Miniaturized electrochemiluminescent biochip prepared on gold nanoparticles-loaded mesoporous silica film for visual detection of hydrogen peroxide released from living cells. Sens. Actuator B Chem. 2019, 284, 437–443. [Google Scholar] [CrossRef]
- Chow, K.F.; Mavre, F.; Crooks, J.A.; Chang, B.Y.; Crooks, R.M. A large-scale, wireless electrochemical bipolar electrode microarray. J. Am. Chem. Soc. 2009, 131, 8364–8365. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Yuan, D.-J.; Xu, J.-J.; Chen, H.-Y. Sensitive electrochemiluminescence biosensor based on Au-ITO hybrid bipolar electrode amplification system for cell surface protein detection. Anal. Chem. 2013, 85, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Xu, B.-Y.; Shi, H.-W.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence analysis of folate receptors on cell membrane with on-chip bipolar electrode. Lab Chip 2011, 11, 2720–2724. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Liu, Z.; Xu, J.-J.; Chen, H.-Y. Highly specific electrochemiluminescence detection of cancer cells with a closed bipolar electrode. ChemElectroChem 2016, 3, 429–435. [Google Scholar] [CrossRef]
- Hiramoto, K.; Pai, H.J.; Ino, K.; Nashimoto, Y.; Shiku, H. Electrochemical measurement of respiratory activity for evaluation of fibroblast spheroids containing endothelial cell networks. Electrochim. Acta 2020, 340, 135979. [Google Scholar] [CrossRef]
- Ino, K.; Onodera, T.; Fukuda, M.T.; Nashimoto, Y.; Shiku, H. Combination of double-mediator system with large-scale integration-based amperometric devices for detecting NAD(P)H:quinone oxidoreductase 1 activity of cancer cell aggregates. ACS Sens. 2019, 4, 1619–1625. [Google Scholar] [CrossRef]
- Ino, K.; Onodera, T.; Kanno, Y.; Suda, A.; Kunikata, R.; Matsue, T.; Shiku, H. Electrochemicolor imaging of endogenous alkaline phosphatase and respiratory activities of mesenchymal stem cell aggregates in early-stage osteodifferentiation. Electrochim. Acta 2018, 268, 554–561. [Google Scholar] [CrossRef]
- Ino, K.; Yokokawa, Y.; Taira, N.; Suda, A.; Kunikata, R.; Nashimoto, Y.; Matsue, T.; Shiku, H. Electrochemical imaging of cell activity in hydrogels embedded in grid-shaped polycaprolactone scaffolds using a large-scale integration-based amperometric device. Anal. Sci. 2019, 35, 39–43. [Google Scholar] [CrossRef]
- Kanno, Y.; Ino, K.; Abe, H.; Sakamoto, C.; Onodera, T.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Shiku, H.; et al. Electrochemicolor imaging using an LSI-based device for multiplexed cell assays. Anal. Chem. 2017, 89, 12778–12786. [Google Scholar] [CrossRef]
- Ino, K.; Yaegaki, R.; Hiramoto, K.; Nashimoto, Y.; Shiku, H. Closed bipolar electrode array for on-chip analysis of cellular respiration by cell aggregates. ACS Sens. 2020, 5, 740–745. [Google Scholar] [CrossRef]
- Ge, S.G.; Zhao, J.G.; Wang, S.P.; Lan, F.F.; Yan, M.; Yu, J.H. Ultrasensitive electrochemiluminescence assay of tumor cells and evaluation of H2O2 on a paper-based closed-bipolar electrode by in-situ hybridization chain reaction amplification. Biosens. Bioelectron. 2018, 102, 411–417. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Wang, Y.-Z.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Visual color-switch electrochemiluminescence biosensing of cancer cell based on multichannel bipolar electrode chip. Anal. Chem. 2016, 88, 2884–2890. [Google Scholar] [CrossRef]
- Shi, H.-W.; Zhao, W.; Liu, Z.; Liu, X.-C.; Xu, J.-J.; Chen, H.-Y. Temporal sensing platform based on bipolar electrode for the ultrasensitive detection of cancer cells. Anal. Chem. 2016, 88, 8795–8801. [Google Scholar] [CrossRef]
- Iwama, T.; Inoue, K.Y.; Abe, H.; Matsue, T. Chemical imaging using a closed bipolar electrode array. Chem. Lett. 2018, 47, 843–845. [Google Scholar] [CrossRef]
- Anderson, T.J.; Defnet, P.A.; Zhang, B. Electrochemiluminescence (ECL)-based electrochemical imaging using a massive array of bipolar ultramicroelectrodes. Anal. Chem. 2020, 92, 6748–6755. [Google Scholar] [CrossRef] [PubMed]
- Guerrette, J.P.; Percival, S.J.; Zhang, B. Fluorescence coupling for direct imaging of electrocatalytic heterogeneity. J. Am. Chem. Soc. 2013, 135, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Sojic, N.; Jiang, D.; Chen, H.Y. Intracellular wireless analysis of single cells by bipolar electrochemiluminescence confined in nanopipette. Angew. Chem. Int. Ed. 2020. In press. [Google Scholar]
- Chen, Y.; Liu, Y.N.; Xia, J.; Liu, J.; Jiang, D.C.; Jiang, D.P. Analysis of sphingomyelin in plasma membrane at single cells using luminol electrochemiluminescence. RSC Adv. 2016, 6, 9518–9521. [Google Scholar] [CrossRef]
- Essmann, V.; Santana Santos, C.; Tarnev, T.; Bertotti, M.; Schuhmann, W. Scanning bipolar electrochemical microscopy. Anal. Chem. 2018, 90, 6267–6274. [Google Scholar] [CrossRef]
- Santos, C.S.; Conzuelo, F.; Essmann, V.; Bertotti, M.; Schuhmann, W. Enhanced sensitivity of scanning bipolar electrochemical microscopy for O2 detection. Anal. Chim. Acta 2019, 1087, 36–43. [Google Scholar] [CrossRef]
| Device Types | References | Notes |
|---|---|---|
| Chip devices not for microscopic imaging | [74,78,79,80,91] | NA |
| BPE devices | [82,83,84,90,91,92,93,97] | NA |
| Probe devices | [56,97] | Intracellular detection |
| ECL microscopes for single-cell imaging | [21,23,39,40,41,42,43,44,45,49,50,52,53,55] | NA |
| Target Analytes | References | Notes |
|---|---|---|
| Cellular membrane | [39,40] | NA |
| Cell adhesion | [21] | NA |
| Cellular H2O2 | [33,43,44,45,50,56,80,91,97] | NA |
| Glutathione | [22] | NA |
| Cholesterol | [43,46,47,48,49] | NA |
| Intracellular glucose | [52,53,97]. | NA |
| SMase | [97,98] | NA |
| N-glycan on cell surface | [66,75] | NA |
| Mucin 1 on cell surface | [34,76,82] | NA |
| Mannan on cell membrane | [57] | NA |
| CEA on cell membrane | [41,69,74,79] | NA |
| AFP on cell membrane | [74,79] | NA |
| PSA on LNCaP cell | [24] | BPE device |
| CA153 on breast cancer cell | [60] | NA |
| CD44 on breast cancer cell | [62,63] | NA |
| β2 Microglobulin | [27] | NA |
| E-selectin on endothelial cell | [61] | Platelet adhesion |
| Phosphatidylserine | [68] | Cell apoptosis |
| EGFR | [39,59,76] | NA |
| FA and FR | [83] | BPE device |
| Intracellular telomerase | [54,73] | NA |
| miRNA | [55] | NA |
| mRNA | [23] | NA |
| Cancer cells | [25,58,65,67,77,78,82,84,91,92,93] | NA |
| Respiration activity | [90] | BPE device |
| Apoptosis | [42] | Detection of EGFR and PS |
| ECL Systems | References | Notes |
|---|---|---|
| Ru(bpy)32+-TPrA | [24,26,39,40,66,82,83,84,90] | NA |
| Ru(bpy)2(dcbpy)-TPrA | [25] | NA |
| Ru(bpy)32+-HEPES | [21] | NA |
| Ru(bpy)32+-GSH | [22] | NA |
| Ru(dcbpy)32+-silica-NPs | [68] | Self-enhanced ECL with intracoreactant |
| Luminol-H2O2 | [46,47,55,56,65,67,80,91,93,98] | NA |
| L012-H2O2 | [42,43,44,45,48,49,50,52,53,97] | NA |
| Polyluminol-H2O2 | [54] | NA |
| L012 | [41,69] | Square wave voltage with high frequency [41] Graphene oxide-capped Au NPs [69] |
| ABEI-H2O2 | [33] | NA |
| ABEI functionalized MOFs | [34] | O2•− as a coreactant |
| g-C3N4-S2O82− | [42] | NA |
| Quantum dots | [23,57,58,59,60,61,62,63,78] | NA |
| Ru(bpy)32+-S2O82−/CdS QDs | [27] | Resonance energy transfer |
| Luminol-H2O2/CdS NCs | [73,76] | Dual ECL signal system |
| Luminol/Ru(bpy)32+-S2O82− | [74] | Dual ECL signal system |
| CNNS/Luminol-H2O2 | [75] | Dual ECL signal system |
| Luminol/CdTe-S2O82− | [77] | Dual ECL signal system |
| Luminol/CdTe-H2O2 | [79] | Dual ECL signal system |
| Luminol-H2O2/Ru(bpy)32+-TPrA | [92] | Dual ECL signal system with BPE device |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramoto, K.; Villani, E.; Iwama, T.; Komatsu, K.; Inagi, S.; Inoue, K.Y.; Nashimoto, Y.; Ino, K.; Shiku, H. Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis. Micromachines 2020, 11, 530. https://doi.org/10.3390/mi11050530
Hiramoto K, Villani E, Iwama T, Komatsu K, Inagi S, Inoue KY, Nashimoto Y, Ino K, Shiku H. Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis. Micromachines. 2020; 11(5):530. https://doi.org/10.3390/mi11050530
Chicago/Turabian StyleHiramoto, Kaoru, Elena Villani, Tomoki Iwama, Keika Komatsu, Shinsuke Inagi, Kumi Y. Inoue, Yuji Nashimoto, Kosuke Ino, and Hitoshi Shiku. 2020. "Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis" Micromachines 11, no. 5: 530. https://doi.org/10.3390/mi11050530
APA StyleHiramoto, K., Villani, E., Iwama, T., Komatsu, K., Inagi, S., Inoue, K. Y., Nashimoto, Y., Ino, K., & Shiku, H. (2020). Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis. Micromachines, 11(5), 530. https://doi.org/10.3390/mi11050530






