Ultrasensitive Stress Biomarker Detection Using Polypyrrole Nanotube Coupled to a Field-Effect Transistor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
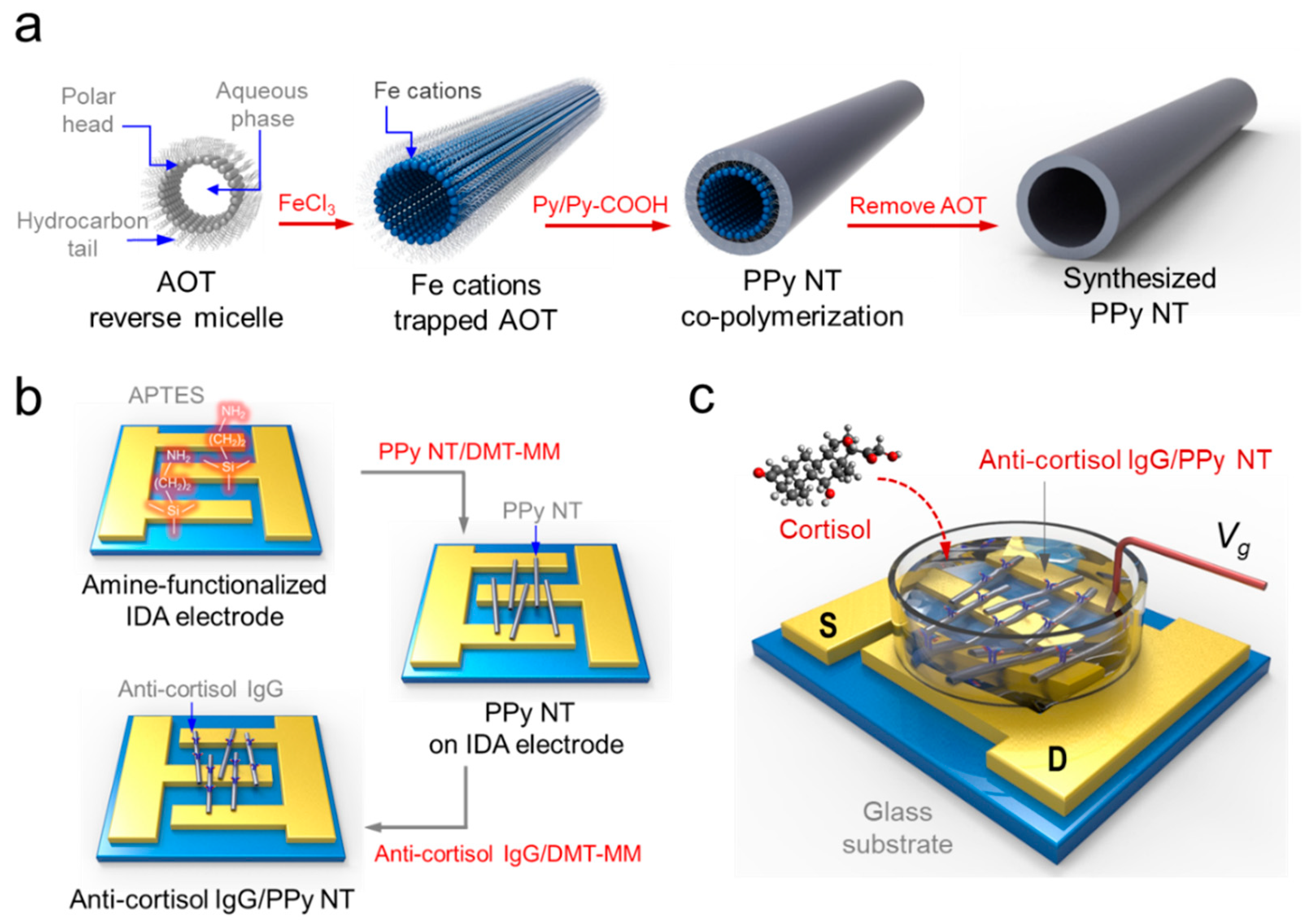
2.2. Synthesis of One-Dimensional PPy NT
2.3. Fabrication of PPyNT FET Sensor
2.4. Anti-Cortisol IgG/PPy NT/FET Based Cortisol Detection
3. Results
3.1. Fabrication of Anti-Cortisol IgG/PPy NT FET Sensor
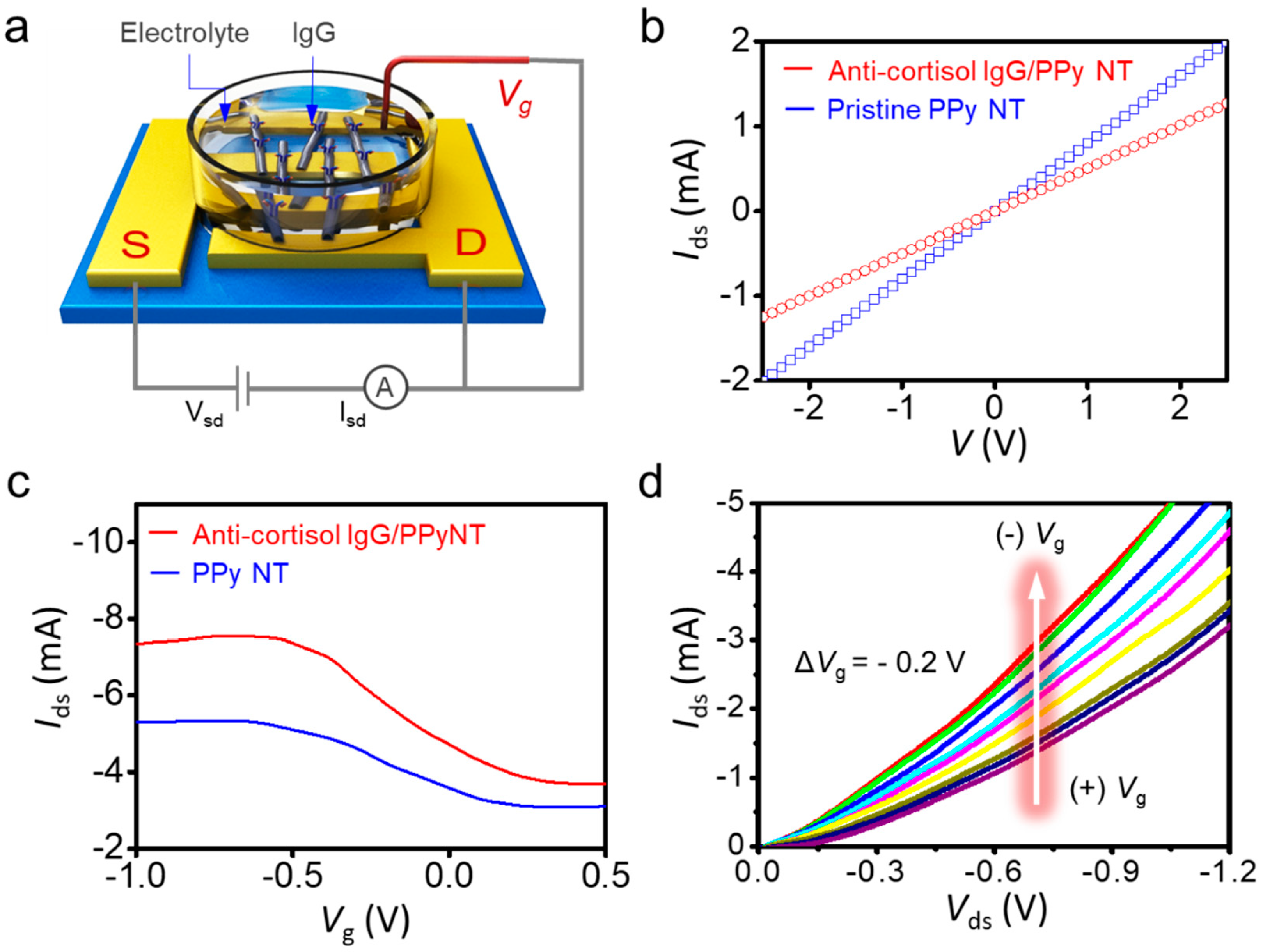
3.2. Characterization of PPy NT FET Transducer
3.3. Sensing Behavior of Liquid-Ion Gated Anti-Cortisol IgG/PPy NT FET
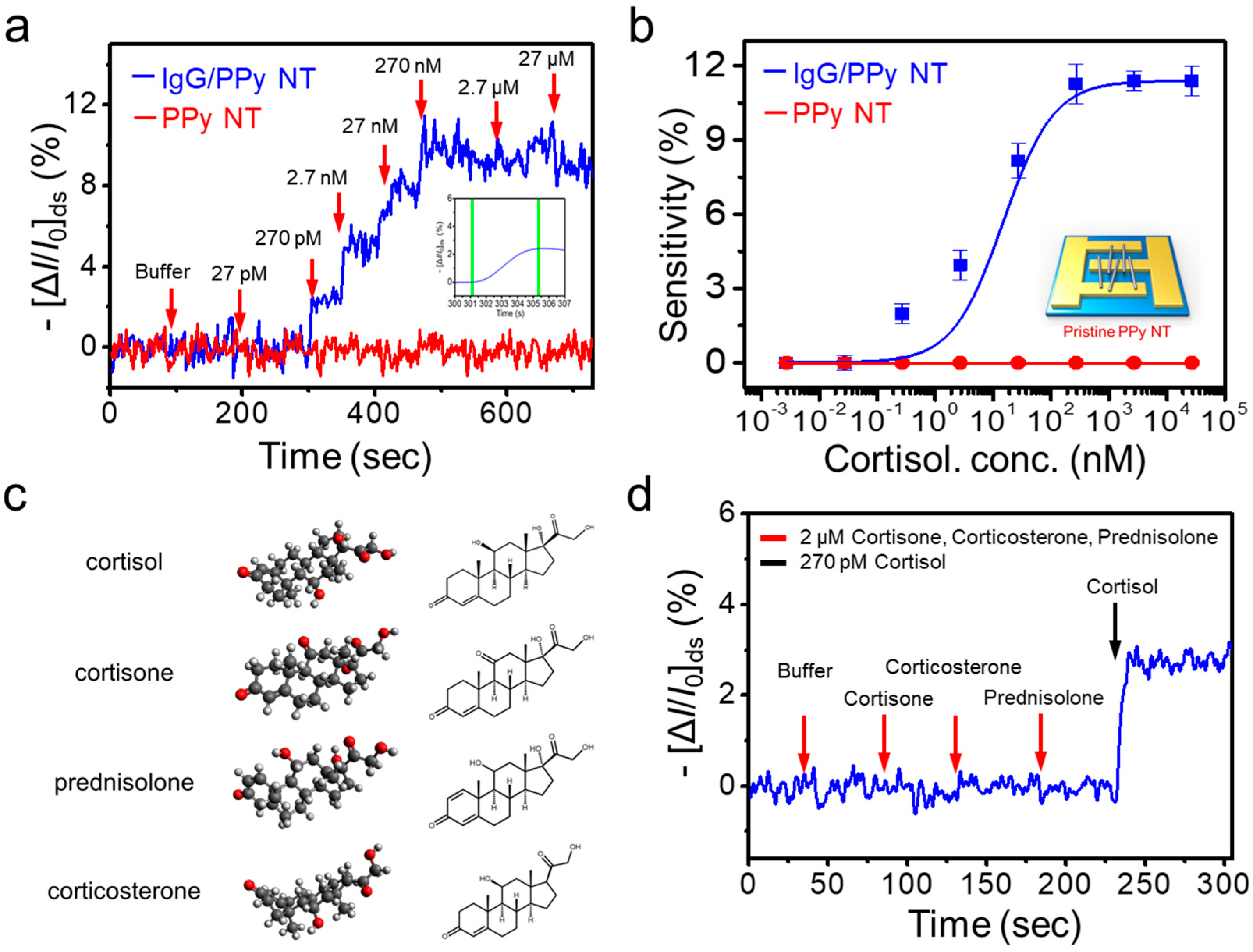
3.4. Real-Time Response to Cortisol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ray, P.; Steckl, A.J. Label-Free Optical Detection of Multiple Biomarkers in Sweat, Plasma, Urine, and Saliva. ACS Sens. 2019, 4, 1346–1357. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, S.; Song, J.; Cho, B.; Han, E.J.; Kondapavulur, S.; Kim, D.; Lee, L.P. Microphysiological Analysis Platform of Pancreatic Islet β-Cell Spheroids. Adv. Heal. Mater. 2017, 7, 1701111. [Google Scholar] [CrossRef]
- Turpeinen, U.; Hämäläinen, E. Determination of cortisol in serum, saliva and urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 795–801. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.-M.; Kim, B.N.; Kwon, O.S.; Rho, W.-Y.; Jun, B.-H. Emerging ultrafast nucleic acid amplification technologies for next-generation molecular diagnostics. Biosens. Bioelectron. 2019, 141, 111448. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, J.; Cho, B.; Hong, S.; Hoxha, O.; Kang, T.; Kim, D.; Lee, L.P. Bubble-free rapid microfluidic PCR. Biosens. Bioelectron. 2019, 126, 725–733. [Google Scholar] [CrossRef]
- Kaiser, U.B. Cushing’s disease: towards precision medicine. Cell Res. 2015, 25, 649–650. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Meeran, K. Glucocorticoid replacement in Addison disease. Nat. Rev. Endocrinol. 2018, 14, 562. [Google Scholar] [CrossRef]
- Raul, J.-S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef]
- Anfossi, L.; Tozzi, C.; Giovannoli, C.; Baggiani, C.; Giraudi, G. Development of a non-competitive immunoassay for cortisol and its application to the analysis of saliva. Anal. Chim. Acta 2002, 468, 315–321. [Google Scholar] [CrossRef]
- Moore, T.J.; Sharma, B. Direct Surface Enhanced Raman Spectroscopic Detection of Cortisol at Physiological Concentrations. Anal. Chem. 2019, 92, 2052–2057. [Google Scholar] [CrossRef]
- Khan, M.S.; Misra, S.K.; Wang, Z.; Daza, E.; Schwartz-Duval, A.S.; Kus, J.M.; Pan, D.; Pan, D. Paper-Based Analytical Biosensor Chip Designed from Graphene-Nanoplatelet-Amphiphilic-diblock-co-Polymer Composite for Cortisol Detection in Human Saliva. Anal. Chem. 2017, 89, 2107–2115. [Google Scholar] [CrossRef]
- Ueno, Y.; Furukawa, K.; Hayashi, K.; Takamura, M.; Hibino, H.; Tamechika, E. Graphene-modified interdigitated array electrode: fabrication, characterization, and electrochemical immunoassay application. Anal. Sci. 2013, 29, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Ohnuki, H.; Murata, M.; Endo, H. Flow immunosensor system with an electrode replacement unit for continuous cortisol monitoring for fish. Sens. Bio-Sens. Res. 2017, 13, 122–127. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015, 63, 124–130. [Google Scholar] [CrossRef]
- Jin, H.J.; Lee, S.H.; Kim, T.H.; Park, J.; Song, H.S.; Park, T.H.; Hong, S. Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens. Bioelectron. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.H.; Seo, S.E.; Kim, M.I.; Park, S.J.; Kwon, O.S. Cytochrome C-decorated graphene field-effect transistor for highly sensitive hydrogen peroxide detection. J. Ind. Eng. Chem. 2020, 83, 29–34. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole Nanotubes Conjugated with Human Olfactory Receptors: High-Performance Transducers for FET-Type Bioelectronic Noses. Angew. Chem. 2009, 121, 2793–2796. [Google Scholar] [CrossRef]
- Park, J.W.; Park, S.J.; Kwon, O.S.; Lee, C.; Jang, J. Polypyrrole Nanotube Embedded Reduced Graphene Oxide Transducer for Field-Effect Transistor-Type H2O2 Biosensor. Anal. Chem. 2014, 86, 1822–1828. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Formation Mechanism of Conducting Polypyrrole Nanotubes in Reverse Micelle Systems. Langmuir 2005, 21, 11484–11489. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile fabrication of polypyrrole nanotubes using reverse microemulsion polymerization. Chem. Commun. 2003, 6, 720–721. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.H.; Yang, H.; Park, C.S.; Lee, C.-S.; Kwon, O.S.; Park, T.H.; Jang, J. Human Dopamine Receptor-Conjugated Multidimensional Conducting Polymer Nanofiber Membrane for Dopamine Detection. ACS Appl. Mater. Interfaces 2016, 8, 28897–28903. [Google Scholar] [CrossRef]
- Ovando-Medina, V.; Peralta, R.D.; Mendizabal, E.; Martínez-Gutiérrez, H.; Lara-Ceniceros, T.E.; Ledezma-Rodríguez, R. Synthesis of polypyrrole nanoparticles by oil-in-water microemulsion polymerization with narrow size distribution. Colloid Polym. Sci. 2011, 289, 759–765. [Google Scholar] [CrossRef]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.-Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and Selective Recognition of Peptide Hormone Using Close-Packed Arrays of hPTHR-Conjugated Polymer Nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.; Seo, S.E.; Kim, K.H.; Park, C.S.; Lee, S.H.; Ban, H.S.; Lee, B.D.; Song, H.S.; Kim, J.; et al. High-Performance Conducting Polymer Nanotube-based Liquid-Ion Gated Field-Effect Transistor Aptasensor for Dopamine Exocytosis. Sci. Rep. 2020, 10, 3772. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.-S.; Yoon, H. Carboxylic Acid-Functionalized Conducting-Polymer Nanotubes as Highly Sensitive Nerve-Agent Chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef]
- Brince Paul, K.; Panigrahi, A.K.; Singh, V.; Singh, S.G. Nonlithographic Fabrication of Plastic-Based Nanofibers Integrated Microfluidic Biochip for Sensitive Detection of Infectious Biomarker. ACS Appl. Mater. Interfaces 2017, 9, 39994–40005. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrnata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef] [Green Version]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Kengne-Momo, R.P.; Daniel, P.; Lagarde, F.; Jeyachandran, Y.L.; Pilard, J.F.; Durand-Thouand, M.J.; Thouand, G. Protein Interactions Investigated by the Raman Spectroscopy for Biosensor Applications. Int. J. Spectrosc. 2012, 2012, 462901. [Google Scholar] [CrossRef]
- Marquart, M.; Deisenhofer, J.; Huber, R.; Palm, W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 Å and 1.9 Å resolution. J. Mol. Boil. 1980, 141, 369–391. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Ishpal; Kaur, A. Spectroscopic investigations of ammonia gas sensing mechanism in polypyrrole nanotubes/nanorods. J. Appl. Phys. 2013, 113, 094504. [Google Scholar] [CrossRef]
- Park, R.S.; Shulaker, M.M.; Hills, G.; Liyanage, L.S.; Lee, S.; Tang, A.; Mitra, S.; Wong, H.-S.P. Hysteresis in Carbon Nanotube Transistors: Measurement and Analysis of Trap Density, Energy Level, and Spatial Distribution. ACS Nano 2016, 10, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, O.S.; Song, H.S.; Park, S.J.; Sung, J.H.; Jang, J.; Park, T.H. Mimicking the human smell sensing mechanism with an artificial nose platform. Biomater. 2012, 33, 1722–1729. [Google Scholar] [CrossRef]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W.C. Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer. Adv. Heal. Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, T.; Song, J.; Russell, L.; Li, H.; Dailey, J.; Searson, P.C.; Katz, H.E. Electronic Cortisol Detection Using an Antibody-Embedded Polymer Coupled to a Field-Effect Transistor. ACS Appl. Mater. Interfaces 2018, 10, 16233–16237. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Lee, S.H.; Seo, S.E.; Bae, J.; Park, S.J.; Kwon, O.S. Ultrasensitive Stress Biomarker Detection Using Polypyrrole Nanotube Coupled to a Field-Effect Transistor. Micromachines 2020, 11, 439. https://doi.org/10.3390/mi11040439
Kim KH, Lee SH, Seo SE, Bae J, Park SJ, Kwon OS. Ultrasensitive Stress Biomarker Detection Using Polypyrrole Nanotube Coupled to a Field-Effect Transistor. Micromachines. 2020; 11(4):439. https://doi.org/10.3390/mi11040439
Chicago/Turabian StyleKim, Kyung Ho, Sang Hun Lee, Sung Eun Seo, Joonwon Bae, Seon Joo Park, and Oh Seok Kwon. 2020. "Ultrasensitive Stress Biomarker Detection Using Polypyrrole Nanotube Coupled to a Field-Effect Transistor" Micromachines 11, no. 4: 439. https://doi.org/10.3390/mi11040439
APA StyleKim, K. H., Lee, S. H., Seo, S. E., Bae, J., Park, S. J., & Kwon, O. S. (2020). Ultrasensitive Stress Biomarker Detection Using Polypyrrole Nanotube Coupled to a Field-Effect Transistor. Micromachines, 11(4), 439. https://doi.org/10.3390/mi11040439





