Simulations of Benzene and Hydrogen-Sulfide Gas Detector Based on Single-Walled Carbon Nanotube over Intrinsic 4H-SiC Substrate
Abstract
1. Introduction
2. Materials and Methods
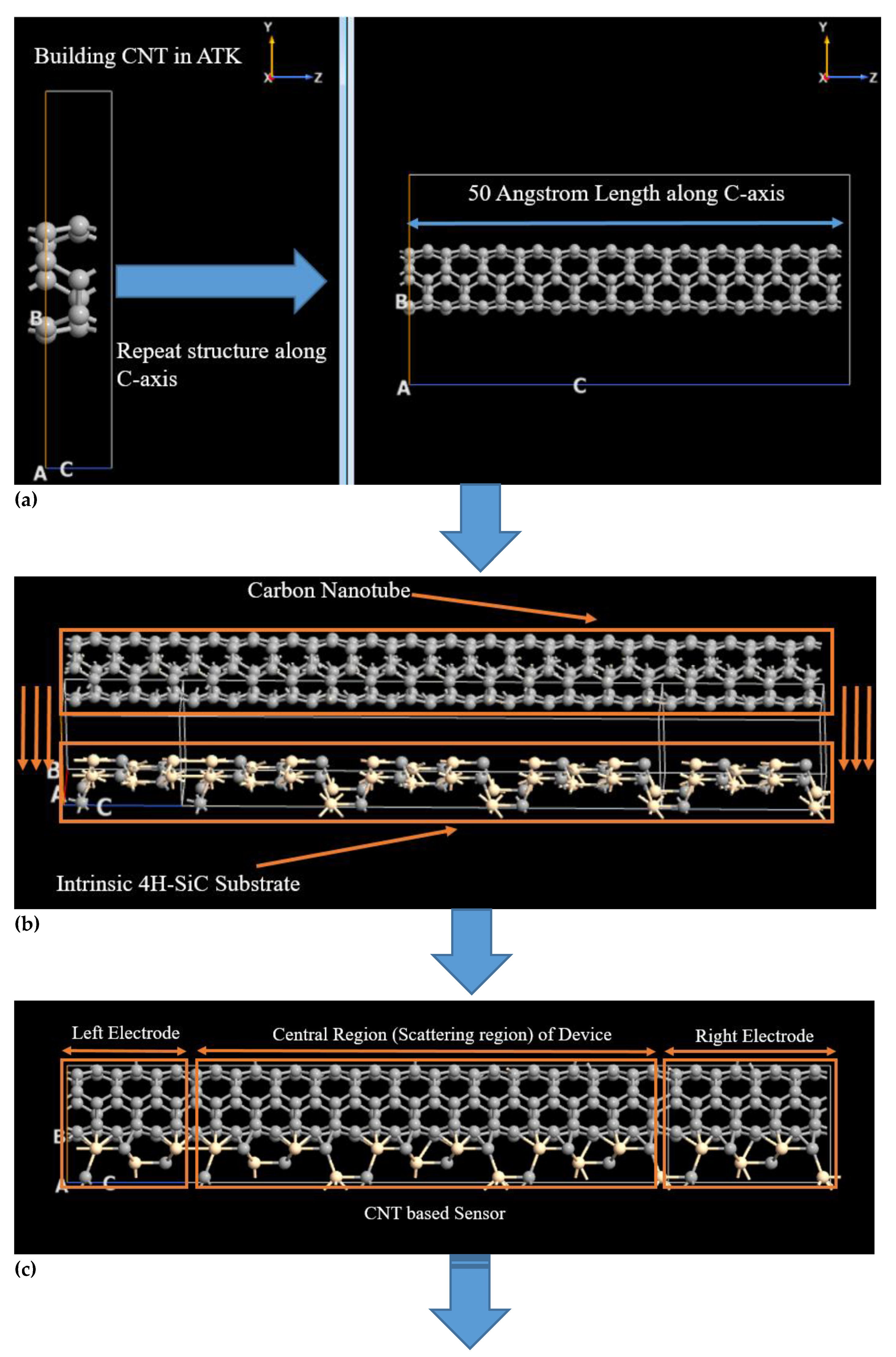
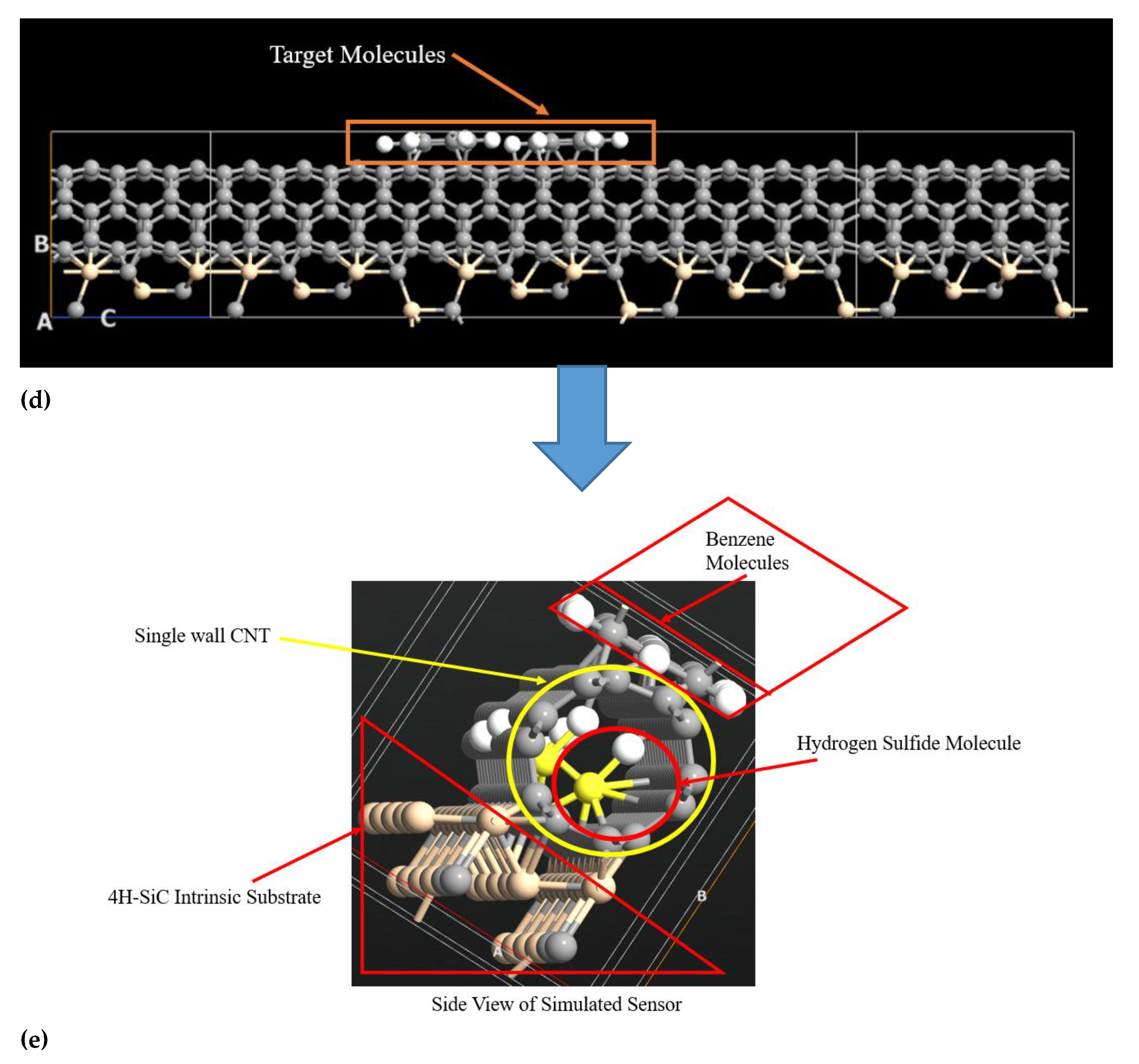
2.1. Carbon Nanotube over Intrinsic 4H-SiC Substrate for the Detection of Hydrogen Sulfide and Benzene Molecules
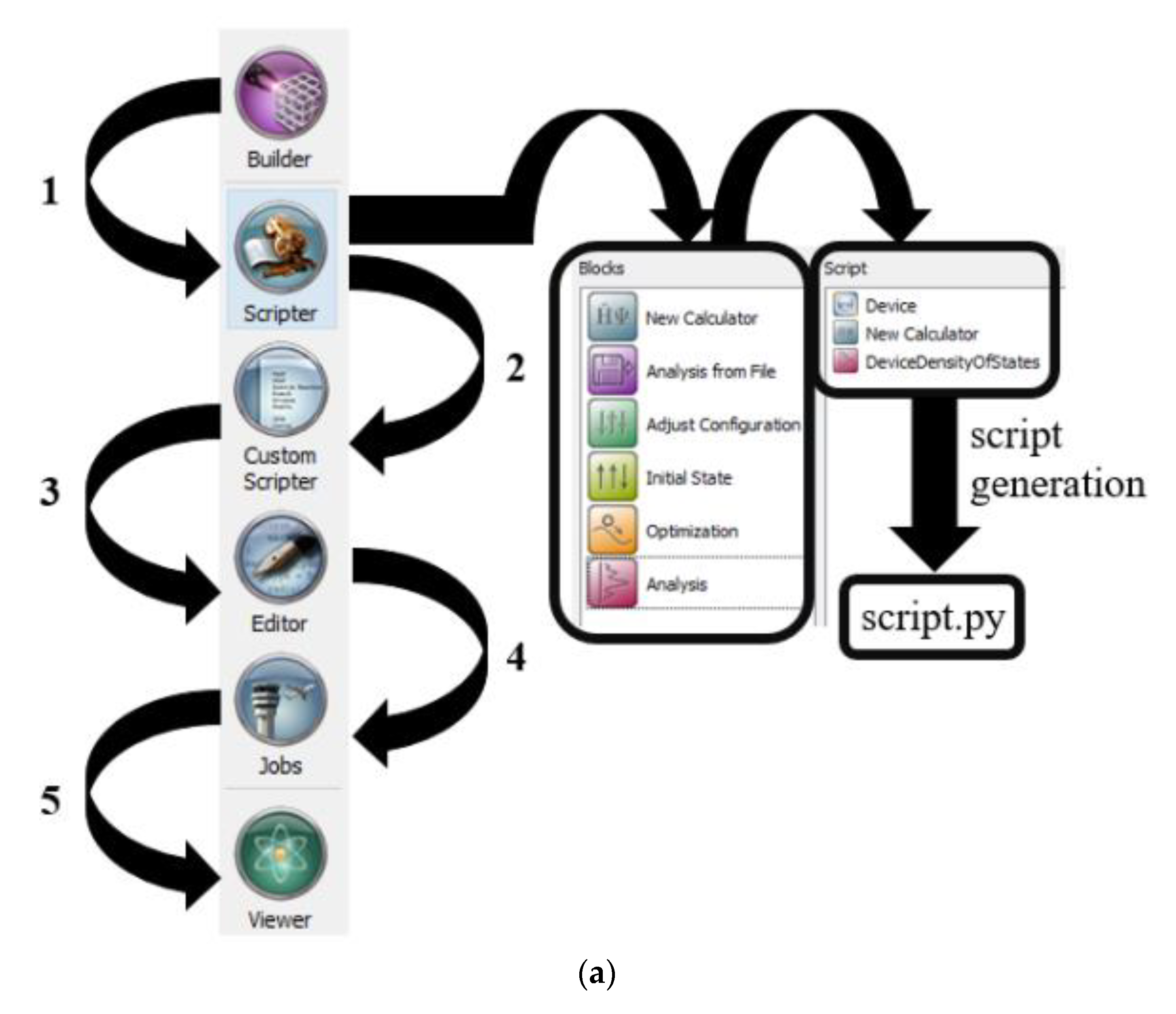
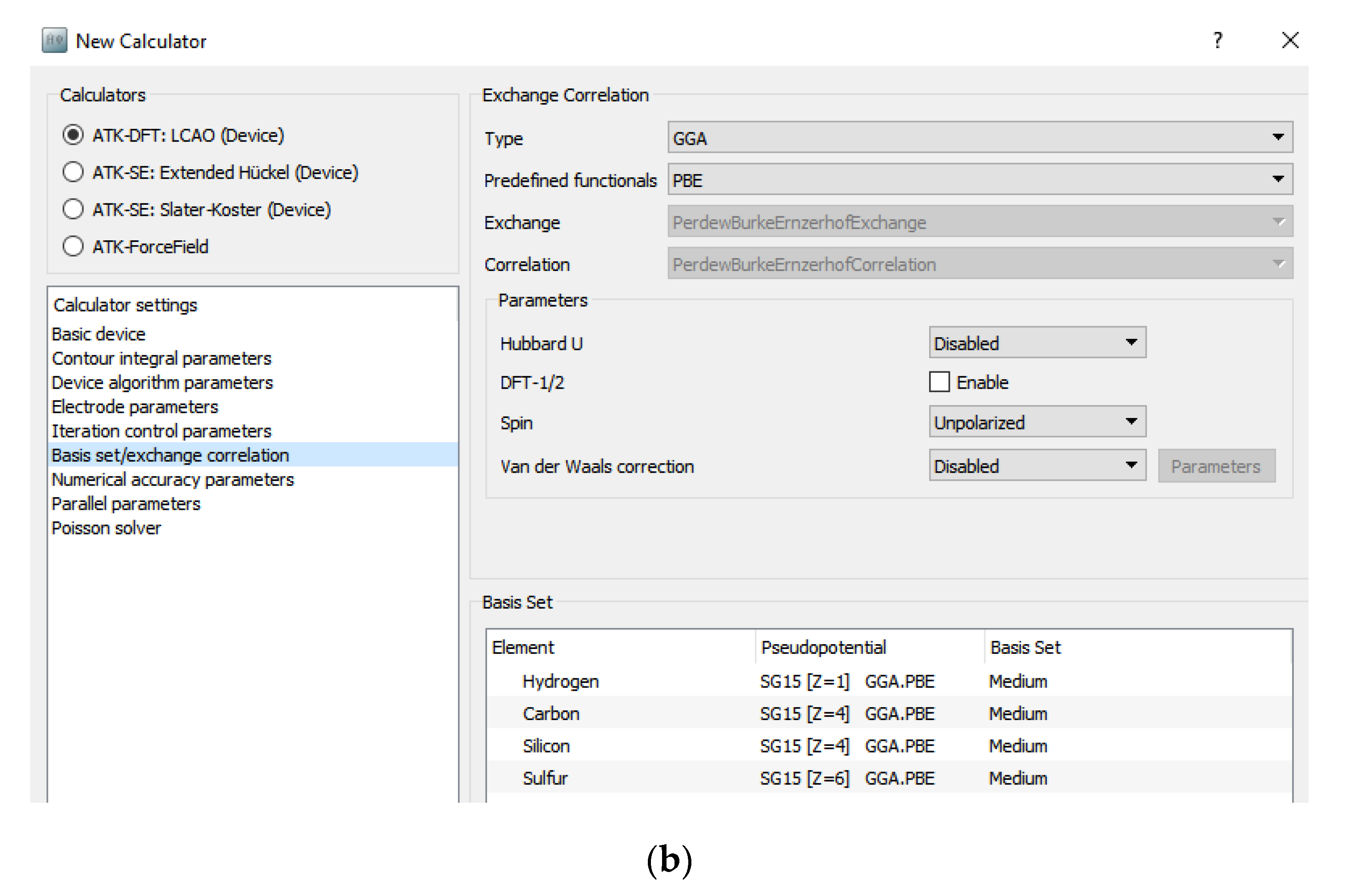
2.2. Methodology for Simulated Carbon Nanotube Based Benzene and Hydrogen Sulfide Sensor
3. Results and Discussions
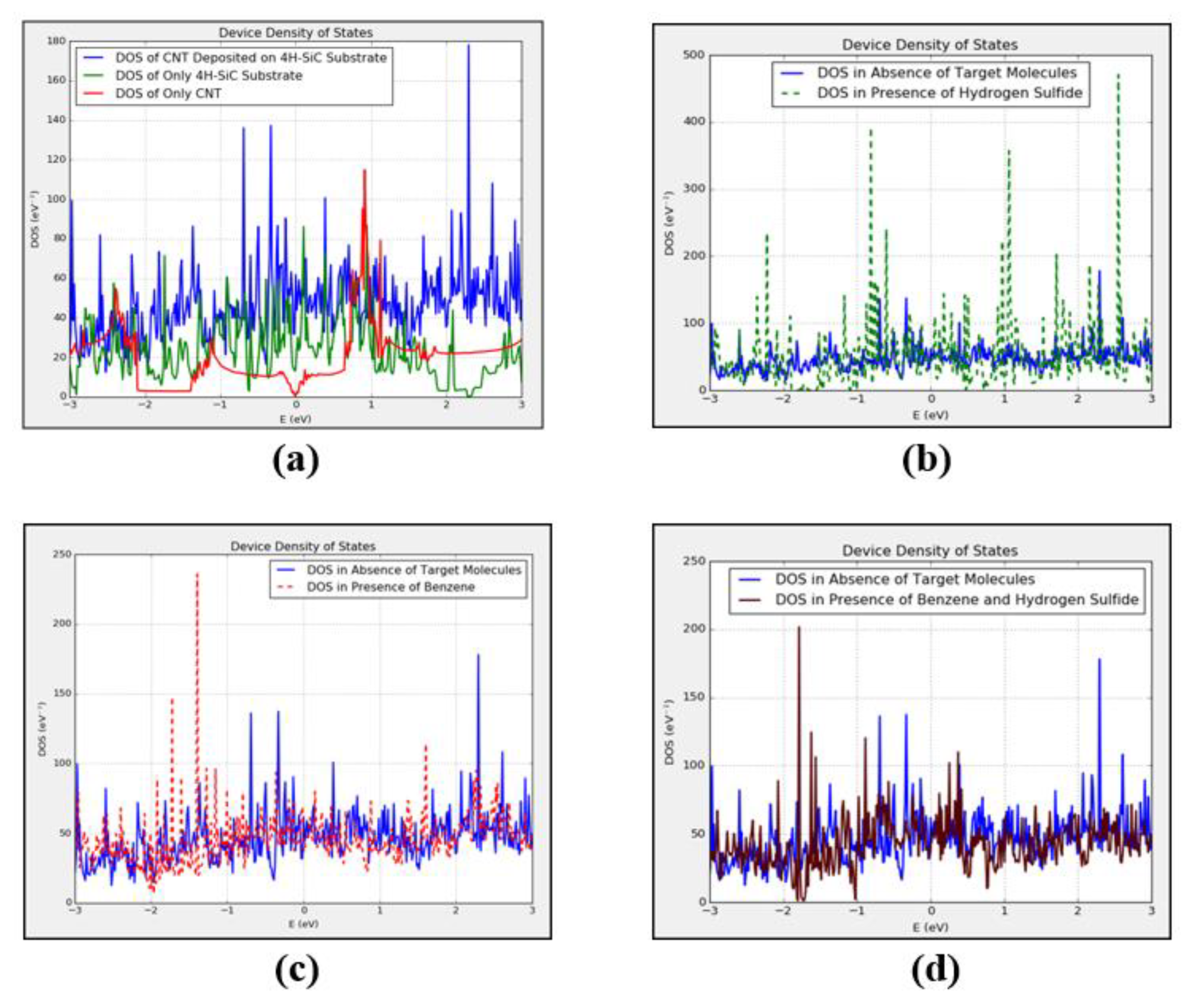
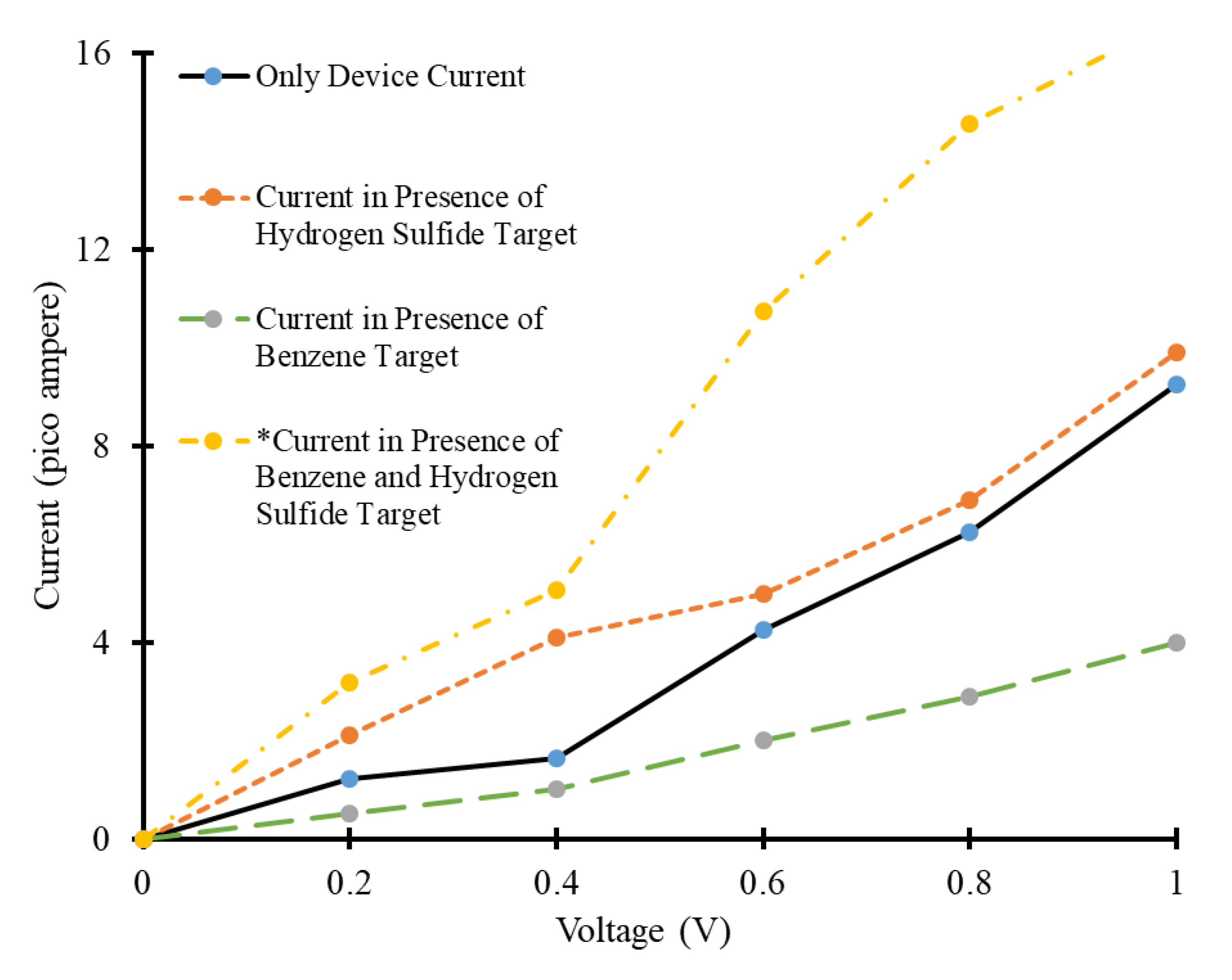
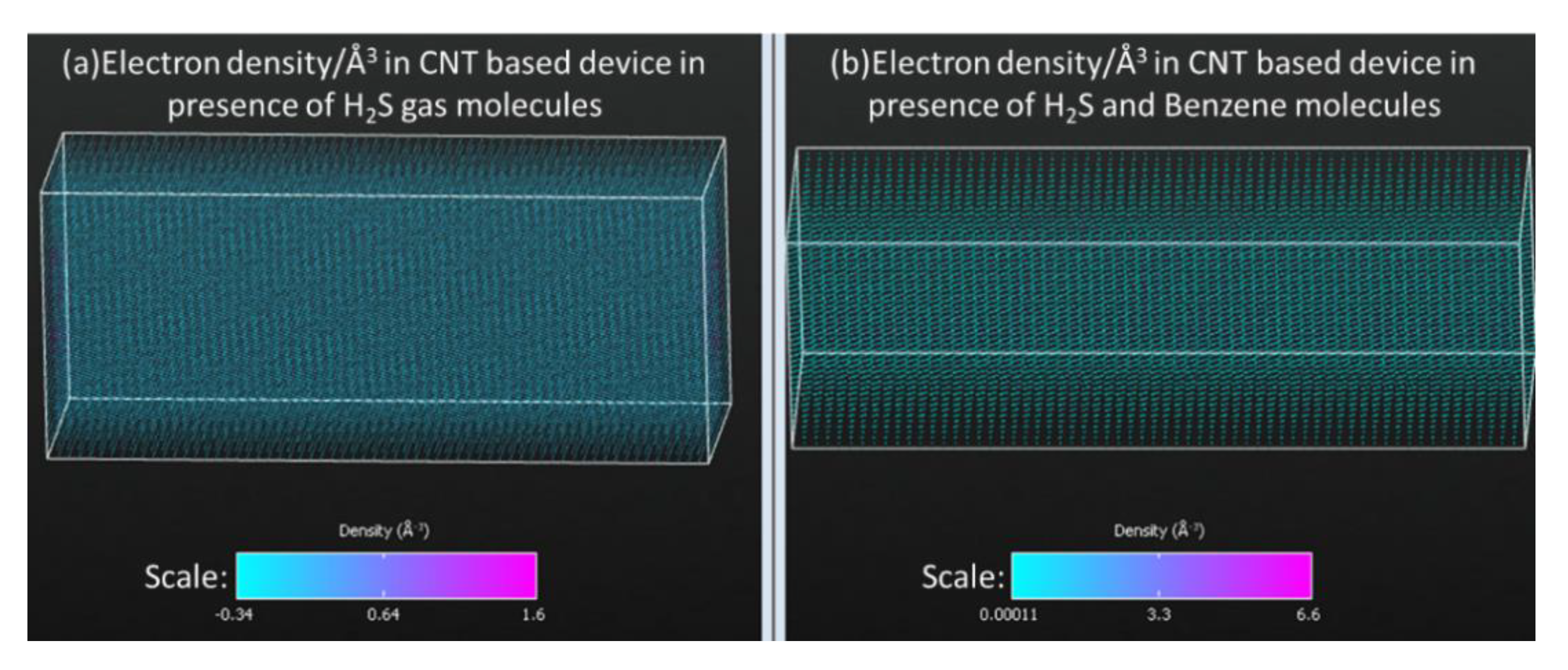
3.1. Density of States and Current-Voltage Characteristics Analysis of CNT-Based Device
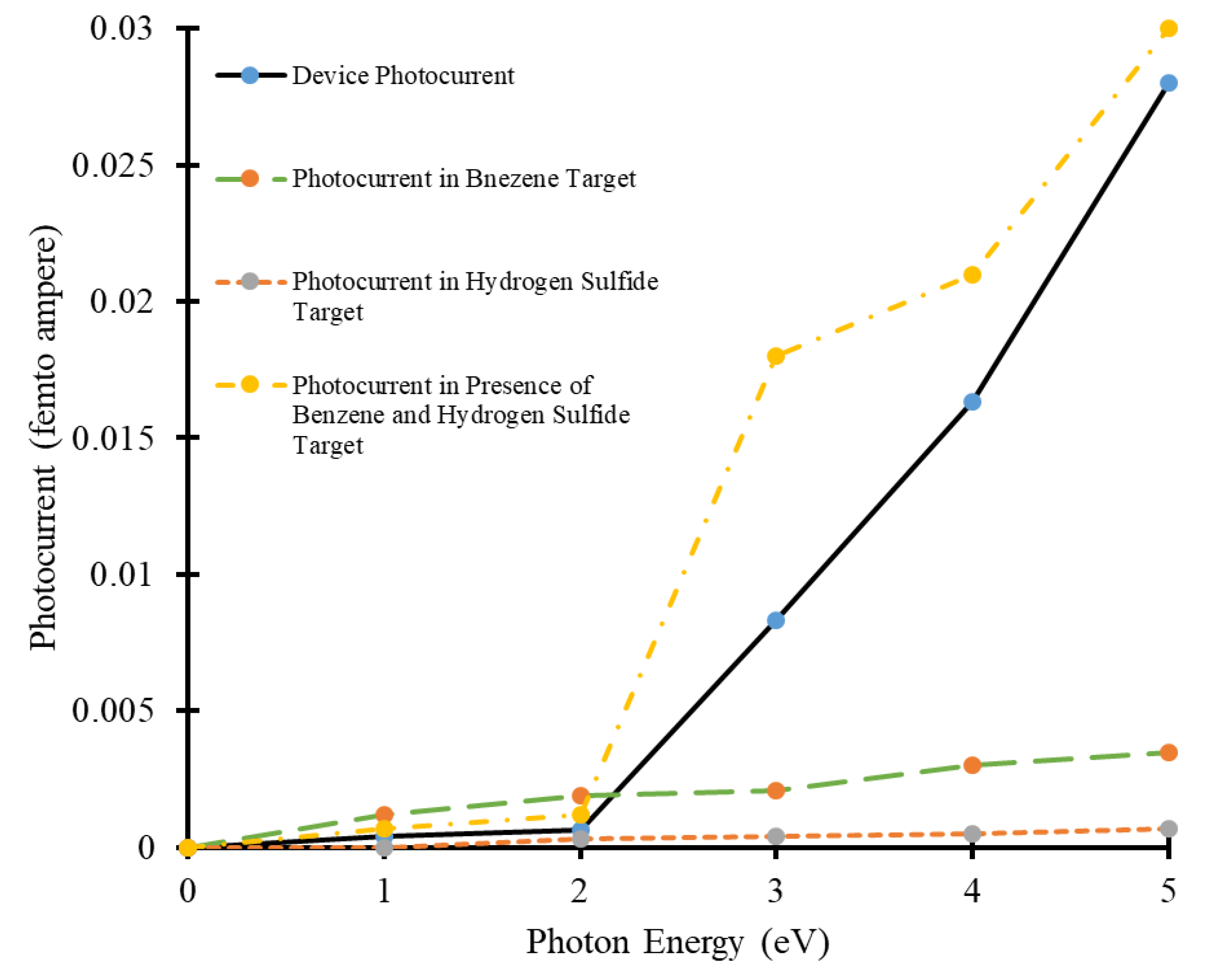
3.2. Photocurrent Analysis of Carbon Nanotube Based Benzene and Hydrogen Sulfide Detector
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef]
- Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Ajayan, P.M.; Zhou, O.Z. Applications of carbon nanotubes. In Carbon Nanotubes Topics in Applied Physics; Springer: Berlin, Germany, 2001; Volume 80, pp. 391–425. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A.D. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dai, H. Carbon nanotubes: Continued innovations and challenges. Adv. Carbon Nanotub. 2004, 29, 237–243. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Tran, T.Q.; Lee, J.K.Y.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Ji, D.; Kumar, V.V.; Ramakrishna, S. Strong, lightweight, and highly conductive CNT/Au/Cu wires from sputtering and electroplating methods. J. Mater. Sci. Tech. 2020, 40, 99–106. [Google Scholar] [CrossRef]
- Duong, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Post-spinning treatment to carbon nano fibers. In Carbon Nanotubes Fibers and Yarn; WOODHEAD Publishing: Cambridge, UK, 2020. [Google Scholar]
- Meyyappan, M. Carbon Nanotubes: Science and Applications; CRC Press: Washington, DC, USA, 2004. [Google Scholar]
- Harris, P.J.F. Carbon Naotube Science; Cambridge university Press: Cambridge, UK, 2010. [Google Scholar]
- Antisari, M.V.; Marazzi, R.; Krsmanovic, R. Synthesis of multiwall carbon nanotubes by electric arc discharge in liquid environments. Carbon 2003, 41, 2393–2401. [Google Scholar] [CrossRef]
- Arepalli, S. Laser ablation process for single-walled carbon nanotube production. Nanosci. Nanotechnol. 2004, 4, 317–325. [Google Scholar] [CrossRef]
- Braidy, N.; El Khakani, M.A.; Botton, G.A. Carbon nanotubular structures synthesis by means of ultraviolet laser ablation. J. Mater. Res. 2002, 17, 2189–2192. [Google Scholar] [CrossRef]
- Choi, Y.C.; Bae, D.J.; Lee, Y.H.; Lee, B.S. Growth of carbon nanotubes by microwave plasma-enhanced chemical vapor deposition at low temperature. J. Vac. Sci. Technol. 2000, 18, 1862–1868. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Wang, F.; Wang, T.; Sun, L.; Wang, Z. Chirality dependence of the thermal conductivity of carbon nanotubes. Nanotechnology 2004, 15, 936–939. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar] [CrossRef]
- Martel, R.; Schmidt, T.; Shea, H.R.; Hertel, T.; Avouris, P. Single- and multi-wall carbon nanotube field-effect transistors. Appl. Physc. Lett. 1998, 73, 2447–2449. [Google Scholar] [CrossRef]
- Choi, K.I.; Kim, H.J.; Kang, Y.C.; Lee, J.H. Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sens. Actuators B Chem. 2014, 194, 371–376. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Extra-oral halitosis: An overview. J. Breath Res. 2010, 4, 017003. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef]
- Exposure to Benzene: A Major Public Health Concern (World Health Organization). Available online: https://www.who.int/ipcs/features/benzene.pdf (accessed on 17 April 2020).
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Collins, J.J.; Lineker, G.A. A review and meta-analysis of formaldehyde exposure and leukemia. Regul. Toxicol. Pharmacol. 2004, 40, 81–91. [Google Scholar] [CrossRef]
- Seo, H.; Jung, S.; Jeon, S. Detection of formaldehyde vapor using mercaptophenol-coated piezoresistive cantilevers. Sens. Actuators B Chem. 2007, 126, 522–526. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lin, Y.T.; Chen, J.L.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens. Actuators B Chem. 2014, 190, 171–176. [Google Scholar] [CrossRef]
- Gu, C.; Xu, X.; Huang, J.; Wang, W.; Sun, Y.; Liu, J. Porous flower-like SnO2 nanostructures as sensitive gas sensors for volatile organic compounds detection. Sens. Actuators B Chem. 2012, 174, 31–38. [Google Scholar] [CrossRef]
- Xu, K.; Zeng, D.; Tian, S.; Zhang, S.; Xie, C. Hierarchical porous SnO2 micro-rods topologically transferred from tin oxalate for fast response sensors to trace formaldehyde. Sens. Actuators B Chem. 2014, 190, 585–592. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Barkaline, V.A.; Chashynski, A.S. Adsorption properties of carbon nanotubes from molecular dynamic viewpoint. Rev. Adv. Mater. Sci. 2009, 20, 21–27. [Google Scholar]
- Atomic-Scale Modelling for Semiconductor & Materials Research. Available online: https://www.synopsys.com/silicon/quantumatk.html (accessed on 1 November 2019).
- Hu, F.-F.; Tang, H.-Y.; Tan, C.-J.; Ye, H.-Y.; Chen, X.-P.; Zhang, G.-Q. Nitrogen dioxide gas sensor based in monolayer SnS: A first principles study. IEEE Electron Device Lett. 2017, 38, 983–986. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Cui, P.; Guo, S.; Liu, W.; Wang, X. Sensitivity investigation for the dependence of monolayer and stacking graphene NH3 gas sensor. Diam. Relat. Mater. 2017, 73, 56–61. [Google Scholar] [CrossRef]
- Harada, N.; Sato, S. Electron properties of NH4 adsorbed graphene nanoribbon as a promising candidate for gas sensor. AIP Adv. 2016, 6, 055023. [Google Scholar]
- Nahlik, J.; Voves, J.; Laposa, A.; Kroutil, J. The study of graphene gas sensor. Key Eng. Mater. 2014, 605, 495–498. [Google Scholar] [CrossRef]
- Ruixue, D.; Yintang, Y.; Lianxi, L. Working of a SiC nanotube NO2 gas sensor. J. Semicond. 2009, 30, 114010. [Google Scholar] [CrossRef]
- QuantumWise User Manual, “ATK-DFT Calculator”. Available online: https://docs.quantumwise.com/v2017/manuals/ATKDFT.html (accessed on 18 November 2019).
- High Performance Computing. Available online: https://www.ttu.ee/support-structure/it-services/services-4/high-performance-computing-2/ (accessed on 5 November 2019).
- VNL Tasks and Workflow. Available online: https://docs.quantumwise.com/v2017/tutorials/vnl.html (accessed on 10 November 2019).
- Wang, Y.; Yeow, T.W. A review of carbon nanotube-based gas sensors. J. Sens. 2009, 2009, 24. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Sinha, N.; Ma, J.; Yeow, J.T. Carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2006, 6, 573–590. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory-Solar Spectra. Available online: https://www.nrel.gov/grid/solar-resource/spectra.html (accessed on 15 November 2019).
- Freitag, M.; Martin, Y.; Misewich, J.A.; Martel, R.; Avouris, P.H. Photoconductivity of single carbon nanotubes. Nano Lett. 2003, 3, 1067–1071. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Fan, Y.; Burghard, M.; Kern, K. Photoelectronic transport imaging of individual semiconducting carbon nanotubes. Appl. Phys. Lett. 2004, 8, 2400. [Google Scholar] [CrossRef]
- Pichler, T.; Knupfer, M.; Golden, M.S.; Fink, J.; Rinzler, A.; Smalley, R.E. Localized and delocalized electronic states in single-wall carbon nanotubes. Phys. Rev. Lett. 1998, 80, 4729–4732. [Google Scholar] [CrossRef]
- Ohfuchi, M.; Miyamoto, Y. Optical properties of oxidized single-wall carbon nanotubes. Carbon 2017, 114, 418–423. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.; Reboredo, F.A.; Galli, G. Structural stability and optical properties of nanomaterials with reconstructed surfaces. Phys. Rev. Lett. 2003, 91, 1–4. [Google Scholar] [CrossRef]
- Bubke, K.; Gnewuch, H.; Hempstead, M. Optical anisotropy of dispersed carbon nanotubes induced by an electric field. Appl. Phys. Lett. 1997, 71, 1906–1908. [Google Scholar] [CrossRef]
- Freitag, M.; Low, T.; Zhu, W.; Yan, H.; Xia, F.; Avouris, P. Photocurrent in graphene harnessed by tunable intrinsic plasmons. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.L.; Chiu, K.-C.; Farmer, D.B.; Cao, Q.; Tersoff, J.; Lee, Y.-S.; Avouris, P.; Han, S.-J. Coherent plasmon and phonon-plasmon resonances in carbon nanotubes. Phys. Rev. Lett. 2017, 118, 257401–257406. [Google Scholar] [CrossRef] [PubMed]
- Ojarand, J.; Min, M.; Koel, A. Multichannel electrical impedance spectroscopy analyzer with microfluidic Sensors. Sensors 2019, 19, 1891. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.H.; Koel, A.; Rang, T.; Ziko, M.H. Simulations of Benzene and Hydrogen-Sulfide Gas Detector Based on Single-Walled Carbon Nanotube over Intrinsic 4H-SiC Substrate. Micromachines 2020, 11, 453. https://doi.org/10.3390/mi11050453
Rashid MH, Koel A, Rang T, Ziko MH. Simulations of Benzene and Hydrogen-Sulfide Gas Detector Based on Single-Walled Carbon Nanotube over Intrinsic 4H-SiC Substrate. Micromachines. 2020; 11(5):453. https://doi.org/10.3390/mi11050453
Chicago/Turabian StyleRashid, Muhammad Haroon, Ants Koel, Toomas Rang, and Mehadi Hasan Ziko. 2020. "Simulations of Benzene and Hydrogen-Sulfide Gas Detector Based on Single-Walled Carbon Nanotube over Intrinsic 4H-SiC Substrate" Micromachines 11, no. 5: 453. https://doi.org/10.3390/mi11050453
APA StyleRashid, M. H., Koel, A., Rang, T., & Ziko, M. H. (2020). Simulations of Benzene and Hydrogen-Sulfide Gas Detector Based on Single-Walled Carbon Nanotube over Intrinsic 4H-SiC Substrate. Micromachines, 11(5), 453. https://doi.org/10.3390/mi11050453





