Template Stripping Method-Based Au Nanoarray for Surface-Enhanced Raman Scattering Detection of Antiepileptic Drug
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Fabrication of Polymer-Based Template
2.4. Fabrication of Template Stripping Method-Based PC Using Polymer-Based Template
2.5. Simulation Analysis of Electric Field Distribution Using PC
2.6. SERS Using Template Stripping Method-Based PC by Detection of Phenobarbital
3. Results and Discussion
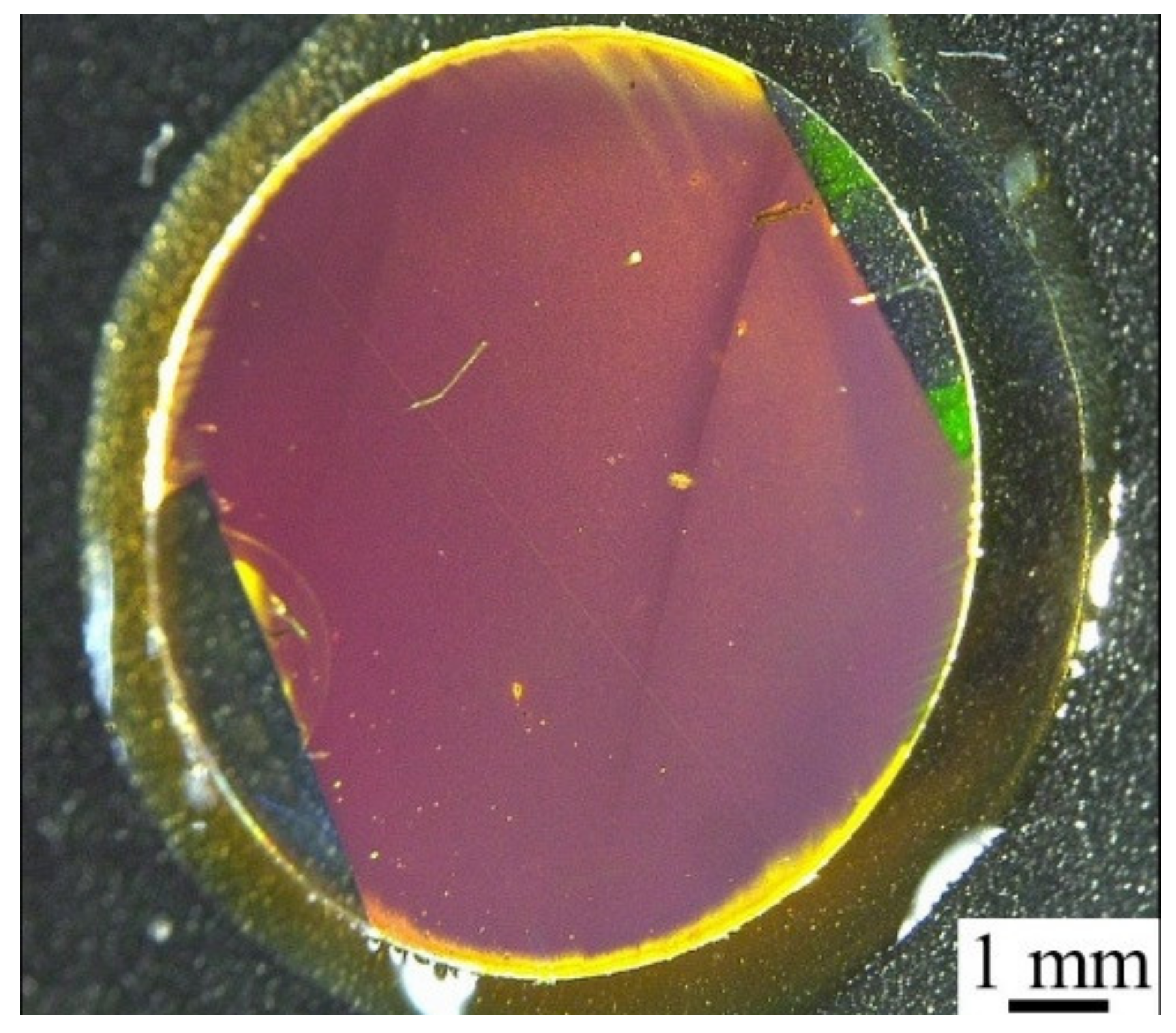
3.1. Fabrication of Template Stripping Method-Based PC Using Polymer-Based Template
3.2. Simulation Analysis of Electric Field Distribution Using PC
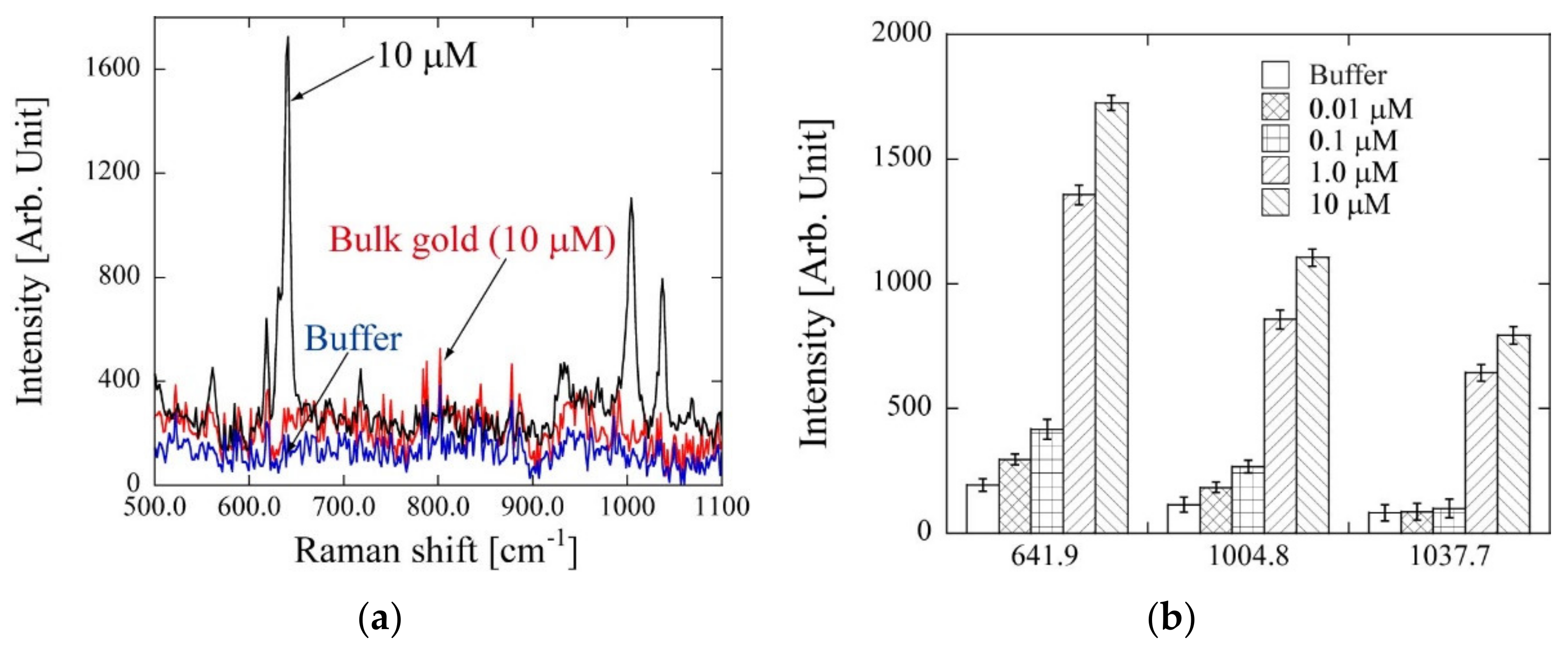
3.3. SERS Using Template Stripping Method-Based PC by Detection of Phenobarbital
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domon, B.; Aebersold, R. Mass spectrometry and protein analysis. Science 2006, 312, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Kauffmann, S.; Geoffroy, P.; Fritig, B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc. Natl. Acad. Sci. USA 1987, 84, 6750–6754. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, K.; Kinoshita, M.; Hayase, S.; Oda, Y. Capillary electrophoresis of N-acetylneuraminic acid polymers and hyaluronic acid: Correlation between migration order reversal and biological functions. Anal. Chem. 1999, 71, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Huh, W.; Falvo, V.J.; Gerke, C.L.; Carroll, S.A.; Hoson, W.R.; Weissman, S.J.; O’shea, K.E. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar] [CrossRef]
- Mazzoni, A.; Bronte, V.; Visintin, A.; Spitzer, H.J.; Apolloni, E.; Serafini, P.; Zanovello, P.; Segal, M.D. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002, 168, 689–695. [Google Scholar] [CrossRef]
- Kranz, J.K.; Schaik-Hihi, C. Protein thermal shifts to identify low molecular weight fragments. Methods Enzymol. 2011, 493, 277–298. [Google Scholar]
- Osypova, A.; Thakar, D.; Dejeu, J.; Bonnet, H.; Van der Heyden, A.; Dubacheva, G.V.; Richter, R.P.; Defrancq, E.; Spinelli, N.; Coche-Guérente, L.; et al. Sensor based on aptamer folding to detect low-molecular weight analytes. Anal. Chem. 2015, 87, 7566–7574. [Google Scholar] [CrossRef]
- Ember, J.I.K.; Hoeve, A.M.; McAughtrie, L.S.; Bergholt, S.M.; Dwyer, J.B.; Stevens, M.M.; Faulds, K.; Forbes, J.S.; Campbell, J.C. Raman spectroscopy and regenerative medicine: A review. NPJ Regen. Med. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Albro, M.B.; Stevens, M.M. Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 2017, 140, 128–137. [Google Scholar] [CrossRef]
- Pavlicek, R.L.; Crane, N.J.; Ghebremedhin, M.; Cilwa, K.E.; Elster, E.A. Diagnostic bacteriology: Raman spectroscopy. Methods Mol. Biol. 2017, 1616, 249–261. [Google Scholar] [PubMed]
- Bumbrah, S.G.; Sharma, M.R. Raman spectroscopy-basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Efemov, V.E.; Ariese, F.; Gooijer, C. Achievements in resonance Raman spectroscopy: Review of a technique with a distinct analytical chemistry potential. Anal. Chim. Acta 2008, 606, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.A.; Bezares, F.J.; Pacardo, D.B.; Ukagbu, M.; Hosten, C.; Ligler, F.S. Small-molecule detection in thiol–yne nanocomposites via surface-enhanced raman spectroscopy. Anal. Chem. 2014, 86, 12315–12320. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.B.; Lee, S.J.; Laurence, T.; Fera, N.; Fabris, L.; Bazan, G.C.; Moskovits, M.; Reich, N.O. Generalized approach to SERS-active nanomaterials via controlled nanoparticle linking, polymer encapsulation, and small-molecule infusion. J. Phys. Chem. 2009, 113, 13622–13629. [Google Scholar] [CrossRef]
- Song, J.; Duan, B.; Wang, C.; Zhou, J.; Pu, L.; Fang, Z.; Wang, P.; Lim, T.T.; Duan, H. SERS-encoded nanogapped plasmonic nanoparticles: Growth of metallic nanoshell by templating redox-active polymer brushes. J. Am. Chem. Soc. 2014, 136, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Muehhlethaler, C.; Leona, M.; Lombardi, R.J. Review of surface enhanced Raman scattering applications in forensic science. Anal. Chem. 2016, 88, 152–169. [Google Scholar] [CrossRef]
- Laing, S.; Jamieson, E.L.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. 2017, 1, 1–19. [Google Scholar] [CrossRef]
- Freeman, R.G.; Grabar, K.C.; Allison, K.J.; Bright, R.M.; Davis, J.A.; Guthrie, A.P.; Hommer, M.B.; Jackson, M.A.; Smith, P.C.; Walter, D.G.; et al. Self-assembled metal colloid monolayers: An approach to SERS substrates. Science 1995, 267, 1629–1632. [Google Scholar] [CrossRef]
- Chung, J.A.; Huh, S.Y.; Erickson, D. Large area flexible SERS active substrates using engineered nanostructures. Nanoscale 2011, 3, 2903–2908. [Google Scholar] [CrossRef]
- Ederth, T. Template-stripped gold surfaces with 0.4-nm rms roughness suitable for force measurements: Application to the Casimir force in the 20-100-nm range. Phys. Rev. A 2000, 62, 62104. [Google Scholar] [CrossRef]
- Atmaja, B.; Frommer, J.; Scott, J.C. Atomically flat gold on elastomeric substrate. Langmuir 2006, 22, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, J.J.; Li, Z.; Freeman, R.M.; Stewart, R.D. Ultra-flat platinum surfaces from template-stripping of sputter deposited films. Surf. Sci. 2003, 546, 87–96. [Google Scholar] [CrossRef]
- Lee, K.L.; Chen, P.W.; Wu, S.H.; Hyang, J.B.; Yang, S.Y.; Wei, P.K. Enhancing surface plasmon detection using template-stripped gold nanoslit arrays on plastic films. ACS Nano 2012, 6, 2931–2939. [Google Scholar] [CrossRef]
- Zhou, W.; Odom, T.W. Tunable subradiant lattice plasmons by out-of-plane dipolar interactions. Nat. Nanotechnol. 2011, 6, 423–427. [Google Scholar] [CrossRef]
- Endo, T.; Kajita, H.; Kawaguchi, Y.; Kosaka, T.; Himi, T. Label-free optical detection of C-reactive protein by nanoimprint lithography-based 2D-photonic crystal film. Biotechnol. J. 2016, 11, 831–837. [Google Scholar] [CrossRef]
- Aki, S.; Endo, T.; Sueyoshi, K.; Hisamoto, H. Plasticized poly(vinyl chloride)-based photonic crystal for ion sensing. Anal. Chem. 2014, 86, 11986–11991. [Google Scholar] [CrossRef]
- Hashimoto, W.; Endo, T.; Sueyoshi, K.; Hisamoto, H. Development of novel protease assay device using a nanoimprinted two dimensional photonic crystal. Chem. Lett. 2014, 43, 1728–1730. [Google Scholar] [CrossRef]
- Endo, T.; Yamamoto, K.; Sueyoshi, K.; Hisamoto, H. Development of microchip electrophoresis-integrated nanoimprinted photonic crystal. Sens. Mater. 2015, 27, 425–433. [Google Scholar] [CrossRef]
- Endo, T.; Kajita, H. Label-free optical detection of fibrinogen in visible region using nanoimprint lithography-based two-dimensional photonic crystal. IEICE Trans. Electron. 2017, E100, 166–170. [Google Scholar] [CrossRef]
- Endo, T.; Ueda, C.; Kajita, H.; Okuda, N.; Tanaka, S.; Hisamoto, H. Enhancement of the fluorescence intensity of DNA intercalators using nano-imprinted 2-dimensional photonic crystal. Microchim. Acta 2013, 180, 929–934. [Google Scholar] [CrossRef]
- Nishiguchi, K.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Fabrication of gold-deposited plasmonic crystal based on nanoimprint lithography for label-free biosensing application. Jpn. J. Appl. Phys. 2016, 55, 08RE02. [Google Scholar] [CrossRef]
- Humphrey, A.D.; Meinzer, N.; Starkey, T.A.; Barnes, W.L. Surface lattice resonances in plasmonic arrays of asymmetric disc dimers. ACS Photon. 2016, 3, 634–639. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, J.M. Phenobarbital for the treatment of epilepsy in the 21st century: A critical review. Epilepsia 2004, 45, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yokoyama, M.; Jeong, H.; Kido, M.; Ohno, Y. Paper-based surfaced enhanced Raman spectroscopy for drug level testing with tear fluid. Proc. SPIE 2015, 9537. [Google Scholar] [CrossRef]
- Xu, X.; Luo, J.; Liu, M.; Wang, Y.; Yi, Z.; Li, X.; Yi, Y.; Tang, Y. The influence of edge and corner evolution on plasmon properties and resonant edge effect in gold nanoplatelets. Phys. Chem. Chem. Phys. 2015, 17, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.N.; Cook, R.B.; Jankow, R. Raman spectrometry of some common barbiturates. Anal. Chem. 1972, 44, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Fazio, B.; D’Andrea, C.; Foti, A.; Messina, E.; Irrera, A.; Donato, G.M.; Villari, V.; Micali, N.; Marago, M.O.; Gucciardi, G.P. SERS detection of biomolecules at physiological pH via aggregation of gold nanorods mediated by optical forces and plasmonic heating. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, T.; Yamada, H.; Yamada, K. Template Stripping Method-Based Au Nanoarray for Surface-Enhanced Raman Scattering Detection of Antiepileptic Drug. Micromachines 2020, 11, 936. https://doi.org/10.3390/mi11100936
Endo T, Yamada H, Yamada K. Template Stripping Method-Based Au Nanoarray for Surface-Enhanced Raman Scattering Detection of Antiepileptic Drug. Micromachines. 2020; 11(10):936. https://doi.org/10.3390/mi11100936
Chicago/Turabian StyleEndo, Tatsuro, Hirotaka Yamada, and Kenji Yamada. 2020. "Template Stripping Method-Based Au Nanoarray for Surface-Enhanced Raman Scattering Detection of Antiepileptic Drug" Micromachines 11, no. 10: 936. https://doi.org/10.3390/mi11100936
APA StyleEndo, T., Yamada, H., & Yamada, K. (2020). Template Stripping Method-Based Au Nanoarray for Surface-Enhanced Raman Scattering Detection of Antiepileptic Drug. Micromachines, 11(10), 936. https://doi.org/10.3390/mi11100936




