Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham
Abstract
1. Introduction
2. Results
2.1. Effect of Bioprotective Agents on P. nordicum Growth in Dry-Cured Ham
2.2. Influence of Bioprotective Agents on OTA Production by P. nordicum
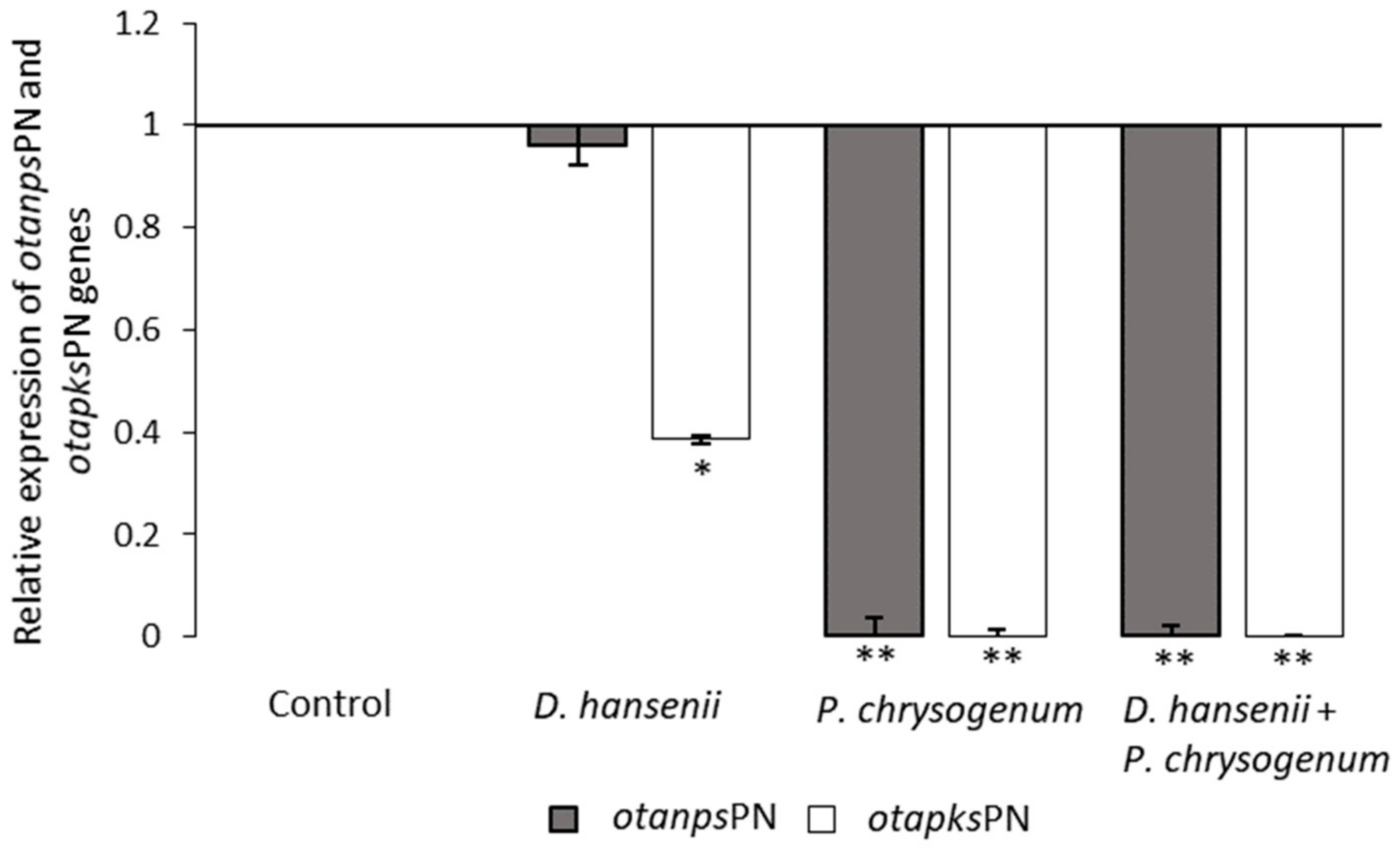
2.3. Effect of Bioprotective Agents in the Relative Expression of otapksPN and otanpsPN Genes in P. nordicum
2.4. Effect of Combined Protective Cultures on OTA Contamination in Dry-Cured Ham Inoculated with P. nordicum in Small Scale Manufacture
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Preparation of Mould and Yeast Inocula
4.3. Preparation of the Dry-Cured Ham Portions
4.4. Experimental Setting
4.5. Evaluation of Mould and Yeast Growth
4.6. Extraction and Quantification of Ochratoxin A
4.6.1. OTA Extraction
4.6.2. OTA Quantification
4.7. Relative Gene Expression
4.7.1. RNA Extraction
4.7.2. Reverse Transcription Reaction
4.7.3. RT-qPCR
Primers Used
RT-qPCR Methods
4.8. Effect of Combined Protective Culture on OTA Contamination During Ripening of Dry-Cured Ham in Small Scale Manufacture.
Sampling, Extraction and Quantification of OTA
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Núñez, F.; Rodríguez, M.M.; Bermúdez, M.E.; Córdoba, J.J.; Asensio, M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 32, 185–197. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007, 70, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Núñez, F.; Benito, M.J.; Asensio, M.A. Contribution of a selected fungal population to proteolysis on dry-cured ham. Int. J. Food Microbiol. 2004, 94, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Rodriguez, A.; Magistà, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27. [Google Scholar] [CrossRef]
- Núñez, F.; Westphal, C.D.; Bermúdez, E.; Asensio, M.A. Production of secondary metabolites by some terverticillate penicillia on carbohydrate-rich and meat substrates. J. Food Prot. 2007, 70, 2829–2836. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Delgado, J.; Córdoba, J.J. Development of a multiplex real-time PCR to quantify aflatoxin, ochratoxin A and patulin producing molds in foods. Int. J. Food Microbiol. 2012, 155, 10–18. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Chen, C.; Palumbo, J.D.; Bianchini, A.; Cappozzo, J.; Stratton, J.; Ryu, D.; Wu, F. A risk assessment of dietary ochratoxin A in the United States. Food Chem. Toxicol. 2017, 100, 265–273. [Google Scholar] [CrossRef]
- Sirot, V.; Fremy, J.-M.; Leblanc, J.-C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Bezerra da Rocha, M.E.; Oliveira Freire, F.C.; Feitosa Maia, E.F.; Florindo Guedes, M.I.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, E191. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). OchratoxinA Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In Monographs on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 1993; Volume 56, pp. 489–521. [Google Scholar]
- Vipotnik, Z.; Rodríguez, A.; Rodrigues, P. Aspergillus westerdijkiae as a major ochratoxin A risk in dry-cured ham based-media. Int. J. Food Microbiol. 2017, 241, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orlic, S.; Comi, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Delgado, J.; Córdoba, J.J. Presence of ochratoxin A on the surface of dry-cured Iberian ham after initial fungal growth in the drying stage. Meat Sci. 2012, 92, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montero, L.; Córdoba, J.J.; Peromingo, B.; Álvarez, M.; Núñez, F. Effects of environmental conditions and substrate on growth and ochratoxin A production by Penicillium verrucosum and Penicillium nordicum: Relative risk assessment of OTA in dry-cured meat products. Food Res. Int. 2019, 121, 604–611. [Google Scholar] [CrossRef]
- Sonjak, S.; Ličen, M.; Frisvad, J.C.; Gunde-Cimerman, N. Salting of dry-cured meat. A potential cause of contamination with the ochratoxin A-producing species Penicillium nordicum. Food Microbiol. 2011, 28, 1111–1116. [Google Scholar] [CrossRef]
- Delgado, J.; da Cruz Cabral, L.; Rodríguez, M.; Rodríguez, A. Influence of ochratoxin A on adaptation of Penicillium nordicum on a NaCl-rich dry-cured ham-based medium. Int. J. Food Microbiol. 2018, 272, 22–28. [Google Scholar] [CrossRef]
- Rodríguez, A.; Medina, Á.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Ministerio Della Sanità. Circolare 09/06/1999, n. 10. Direttive in materia di controllo ufficiale sui prodotti alimentari: Valori massimi ammissibili di micotossine nelle derrate alimentari di origine nazionale, comunitaria e Paesi terzi. Gazz. Uff. della Repubb. Ital. 1999, 135, 52–57. [Google Scholar]
- Asensio, M.A.; Núñez, F.; Delgado, J.; Bermúdez, E. Control of toxigenic molds in food processing. In Microbial Food Safety and Preservation Techniques; Rai, V.R., Bai, A.J., Eds.; CRC Press (Taylor and Francis): Boca Raton, FL, USA, 2014; pp. 329–357. [Google Scholar]
- Álvarez, M.; Rodríguez, A.; Peromingo, B.; Núñez, F.; Rodríguez, M. Enterococcus faecium: A promising protective culture to control growth of ochratoxigenic moulds and mycotoxin production in dry-fermented sausages. Mycotoxin Res. 2019. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, E.; Núñez, F.; Alía, A.; Bermúdez, E.; Rodríguez, M. Effect of Staphylococcus xylosus on the growth of toxigenic moulds in meat substrates. Report of the IVth Workshop of the Spanish National Network on Mycotoxins and Toxigenic Fungi and Their Decontamination Processes (MICOFOOD), Pamplona, Spain, 29–31 May 2019. Toxins 2019, 11, 415. [Google Scholar] [CrossRef]
- Delgado, J.; Peromingo, B.; Rodríguez, A.; Rodríguez, M. Biocontrol of Penicillium griseofulvum to reduce cyclopiazonic acid contamination in dry-fermented sausages. Int. J. Food Microbiol. 2019, 293, 1–6. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Andyanto, D.; Comi, G. Biocontrol of ochratoxigenic moulds (Aspergillus ochraceus and Penicillium nordicum) by Debaryomyces hansenii and Saccharomycopsis fibuligera during speck production. Food Microbiol. 2017, 62, 188–195. [Google Scholar] [CrossRef]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef]
- Peromingo, B.; Andrade, M.J.; Delgado, J.; Sánchez-Montero, L.; Núñez, F. Biocontrol of aflatoxigenic Aspergillus parasiticus by native Debaryomyces hansenii in dry-cured meat products. Food Microbiol. 2019, 82, 269–276. [Google Scholar] [CrossRef]
- Peromingo, B.; Núñez, F.; Rodríguez, A.; Alía, A.; Andrade, M.J. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018, 268, 73–80. [Google Scholar] [CrossRef]
- Simoncini, N.; Virgili, R.; Spadola, G.; Battilani, P. Autochthonous yeasts as potential biocontrol agents in dry-cured meat products. Food Control 2014, 46, 160–167. [Google Scholar] [CrossRef]
- Virgili, R.; Simoncini, N.; Toscani, T.; Camardo Leggieri, M.; Formenti, S.; Battilani, P. Biocontrol of Penicillium nordicum growth and ochratoxin A production by native yeasts of dry cured ham. Toxins 2012, 4, 68–82. [Google Scholar] [CrossRef]
- Andrade, M.J.; Córdoba, J.J.; Casado, E.M.; Córdoba, M.G.; Rodríguez, M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichón”. Meat Sci. 2010, 85, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Cano-García, L.; Belloch, C.; Flores, M. Impact of Debaryomyces hansenii strains inoculation on the quality of slow dry-cured fermented sausages. Meat Sci. 2014, 96, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.; Rodríguez, M.M.; Córdoba, J.J.; Bermúdez, M.E.; Asensio, M.A. Yeast population during ripening of dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 29, 271–280. [Google Scholar] [CrossRef]
- Andrade, M.J.; Rodríguez, M.; Casado, E.M.; Bermúdez, E.; Córdoba, J.J. Differentiation of yeasts growing on dry-cured Iberian ham by mitochondrial DNA restriction analysis, RAPD-PCR and their volatile compounds production. Food Microbiol. 2009, 26, 578–586. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Patiño, B.; Cortés, L.; González-Jaén, M.T.; Vázquez, C. Mechanisms involved in reduction of ochratoxin A produced by Aspergillus westerdijkiae using Debaryomyces hansenii CYC 1244. Int. J. Food Microbiol. 2011, 151, 113–118. [Google Scholar] [CrossRef]
- Acosta, R.; Rodríguez-Martín, A.; Martín, A.; Núñez, F.; Asensio, M.A. Selection of antifungal protein-producing molds from dry-cured meat products. Int. J. Food Microbiol. 2009, 135, 39–46. [Google Scholar] [CrossRef]
- Rodríguez-Martín, A.; Acosta, R.; Liddell, S.; Núñez, F.; Benito, M.J.; Asensio, M.A. Characterization of the novel antifungal protein PgAFP and the encoding gene of Penicillium chrysogenum. Peptides 2010, 31, 541–547. [Google Scholar] [CrossRef]
- Rodríguez, A.; Bernáldez, V.; Rodríguez, M.; Andrade, M.J.; Núñez, F.; Córdoba, J.J. Effect of selected protective cultures on ochratoxin A accumulation in dry-cured Iberian ham during its ripening process. LWT—Food Sci. Technol. 2015, 60, 923–928. [Google Scholar] [CrossRef]
- Delgado, J.; Núñez, F.; Asensio, M.A.; Owens, R.A. Quantitative proteomics of Penicillium nordicum profiles and ochratoxin A repression by protective cultures. Int. J. Food Microbiol. 2019, 305, 108243. [Google Scholar] [CrossRef]
- Karolewiez, A.; Geisen, R. Cloning a part of the ochratoxin A biosynthetic gene cluster of Penicillium nordicum and characterization of the ochratoxin polyketide synthase gene. Syst. Appl. Microbiol. 2005, 28, 588–595. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Andrade, M.J.; Alía, A.; Rodríguez, A. Selection of reference genes to quantify relative expression of ochratoxin A-related genes by Penicillium nordicum in dry-cured ham. Food Microbiol. 2017, 68, 104–111. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Romero, D.; De Vicente, A.; Pérez-García, A. Analysis of β-tubulin-carbendazim interaction reveals that binding site for MBC fungicides does not include residues involved in fungicide resistance. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Bernáldez, V.; Rodríguez, A.; Martín, A.; Lozano, D.; Córdoba, J.J. Development of a multiplex qPCR method for simultaneous quantification in dry-cured ham of an antifungal-peptide Penicillium chrysogenum strain used as protective culture and aflatoxin-producing moulds. Food Control 2014, 36, 257–265. [Google Scholar] [CrossRef]
- Delgado, J.; Acosta, R.; Rodríguez-Martín, A.; Bermúdez, E.; Núñez, F.; Asensio, M.A. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int. J. Food Microbiol. 2015, 205, 23–29. [Google Scholar] [CrossRef]
- Galaverna, G.; Dall’Asta, C. Sampling Techniques for the Determination of Mycotoxins in Food Matrices. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 381–403. [Google Scholar]
- Bernáldez, V.; Córdoba, J.J.; Rodríguez, M.; Cordero, M.; Polo, L.; Rodríguez, A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “salchichón”. Food Control 2013, 32, 69–76. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Patiño, B.; Cortés, L.; González-Jaén, M.T.; Vázquez, C. Aspergillus steynii and Aspergillus westerdijkiae as potential risk of OTA contamination in food products in warm climates. Food Microbiol. 2014, 46, 168–175. [Google Scholar] [CrossRef]
- Fasoyin, O.E.; Wang, B.; Qiu, M.; Han, X.; Chung, K.R.; Wang, S. Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 2018, 115, 41–51. [Google Scholar] [CrossRef]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT—Food Sci. Technol. 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Luque, M.I.; Justesen, A.F.; Córdoba, J.J. Quantification of ochratoxin A-producing molds in food products by SYBR Green and TaqMan real-time PCR methods. Int. J. Food Microbiol. 2011, 149, 226–235. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−∆∆CT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Batches | P. nordicum | P. chrysogenum | D. hansenii |
|---|---|---|---|
| Control | 7.02 ± 0.14 | - | - |
| D. hansenii | 7.41 ± 0.38 | - | 8.28 ± 0.18 |
| P. chrysogenum | 7.25 ± 0.42 | 6.50 ± 0.18 | - |
| D. hansenii + P. chrysogenum | 6.71 ± 0.36* | 7.19 ± 0.26 | 8.36 ± 0.03 |
| Batches | OTA Concentration (µg/kg)* |
|---|---|
| Control | 15.48 ± 8.87a |
| D. hansenii | 2.78 ± 2.18b |
| P. chrysogenum | 0.44 ± 0.63bc |
| D. hansenii + P. chrysogenum | 0.23 ± 0.52c |
| Batches | Sample Reference of Dry-Cured Ham | Average OTA Concentration (µg/kg) |
|---|---|---|
| P. nordicum | 1 | 0.39 |
| 2 | 7.71 | |
| 3 | 1226.92 | |
| 4 | - | |
| 5 | 1275.55 | |
| 6 | 176.86 | |
| 7 | - | |
| 8 | - | |
| 9 | 0.58 | |
| 10 | 284.13 | |
| 11 | 1620.00 | |
| 12 | 0.67 | |
| P. nordicum + P. chrysogenum + D. hansenii | 13 | 11.73 |
| 14 | 3.67 | |
| 15 | - | |
| 16 | - | |
| 17 | 9.68 | |
| 18 | - | |
| 19 | - | |
| 20 | - | |
| 21 | - | |
| 22 | - | |
| 23 | - | |
| 24 | 6.48 |
| Genes | Primers | Nucleotide Sequences (5’-3’) | Product Size (pb) | Positions |
|---|---|---|---|---|
| β-tubulin | β-tubF1 | GCCAGCGGTGACAAGTACGT | 93 | 279 a |
| β-tubR1 | TACCGGGCTCCAAATCGA | 54 a | ||
| otapksPN | otapksF3 | CGCCGCTGCGGTTACT | 80 | 1816 b |
| otapksR3 | GGTAACAATCAACGCTCCCTCTT | 1873 b | ||
| otanpsPN | F-npstr | GCCGCCCTCTGTCATTCCAAG | 113 | 5090 b |
| R-npstr | GCCATCTCCAAACTCAAGCGTG | 5181 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián, E.; Rodríguez, M.; Peromingo, B.; Bermúdez, E.; Núñez, F. Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham. Toxins 2019, 11, 710. https://doi.org/10.3390/toxins11120710
Cebrián E, Rodríguez M, Peromingo B, Bermúdez E, Núñez F. Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham. Toxins. 2019; 11(12):710. https://doi.org/10.3390/toxins11120710
Chicago/Turabian StyleCebrián, Eva, Mar Rodríguez, Belén Peromingo, Elena Bermúdez, and Félix Núñez. 2019. "Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham" Toxins 11, no. 12: 710. https://doi.org/10.3390/toxins11120710
APA StyleCebrián, E., Rodríguez, M., Peromingo, B., Bermúdez, E., & Núñez, F. (2019). Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham. Toxins, 11(12), 710. https://doi.org/10.3390/toxins11120710






