Combining E-Nose and Lateral Flow Immunoassays (LFIAs) for Rapid Occurrence/Co-Occurrence Aflatoxin and Fumonisin Detection in Maize
Abstract
:1. Introduction
2. Results
2.1. Lateral Flow Immunoassay
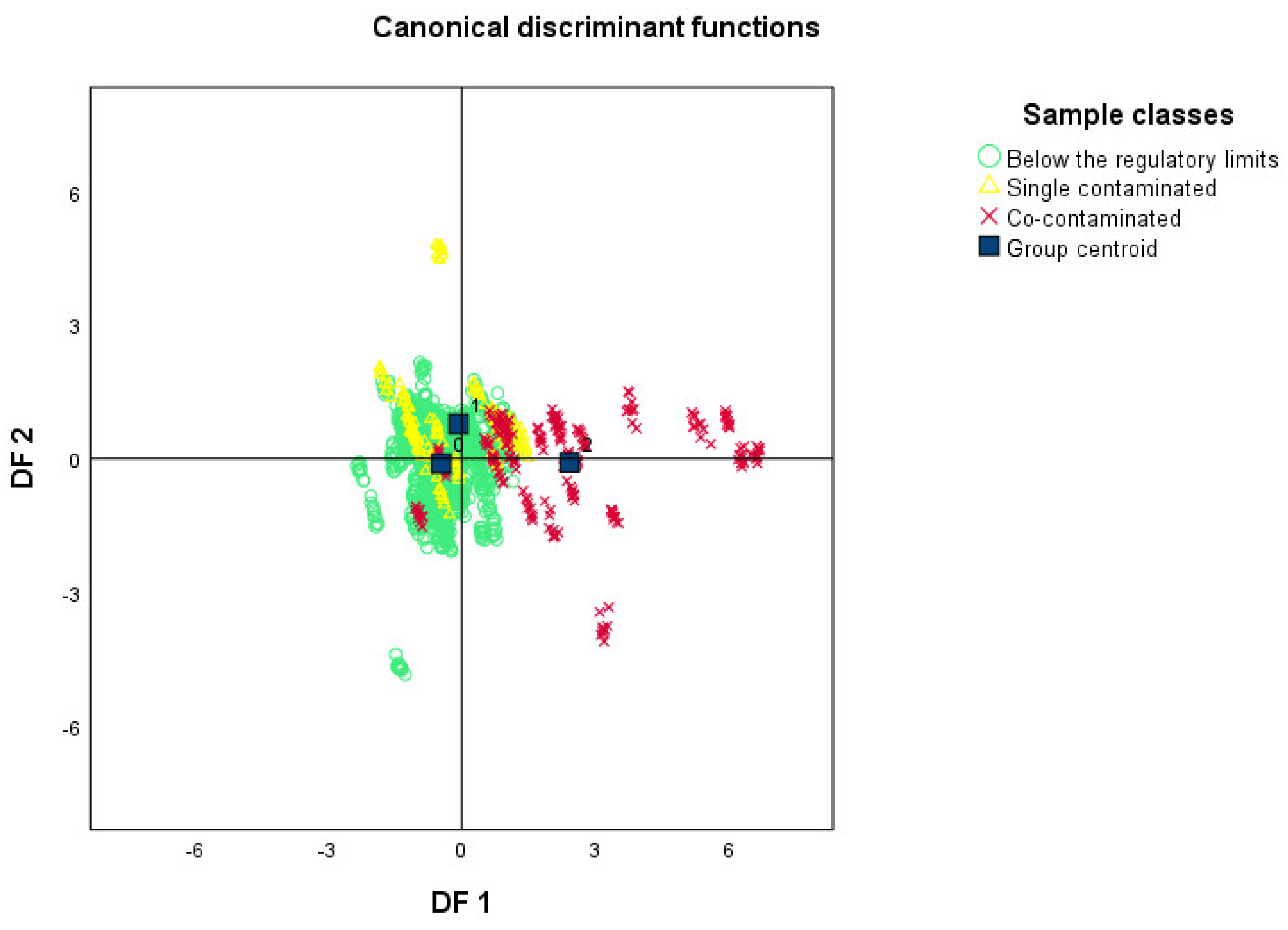
2.2. Electronic Nose
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Samples Collection and Analysis
5.2. Lateral Flow Immuno Assay
5.3. Electronic Nose Analysis
5.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cheli, F.; Campagnoli, A.; Pinotti, L.; Savoini, G.; Dell’Orto, V. Electronic nose for determination of aflatoxins in maize. Biotechnol. Agron. Soc. Environ. 2009, 13, 39–43. [Google Scholar]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916–4023. [Google Scholar]
- FAO—Food and Agriculture Organization. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. In FAO Food and Nutrition Paper 81; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; Available online: http://www.fao.org/docrep/007/y5499e/y5499e00.htm (accessed on 13 September 2017).
- Cheli, F.; Gallo, R.; Battaglia, D.; Dell’Orto, V. EU legislation on feed related issues: An update. Ital. J. Anim. Sci. 2013, 12, 295–312. [Google Scholar] [CrossRef]
- Cheli, F.; Battaglia, D.; Gallo, R.; Dell’Orto, V. EU legislation on cereal safety: An update with a focus on mycotoxins. Food Control 2014, 37, 315–325. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2014, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Food Sci. Nutr. 2016, 57, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Dagnac, T.; Latorre, A.; Fernández Lorenzo, B.; Llompart, M. Validation and application of a liquid chromatography-tandem mass spectrometry based method for the assessment of the co-occurrence of mycotoxins in maize silages from dairy farms in NW Spain. Food Addit. Contam. 2016, 33, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Brown, M.C. Antibodies: Key to a Robust Lateral Flow Immunoassay. In LFIA Book; Wong, R.C., Tse, H.Y., Eds.; Springer: Berlin, Germnay, 2009; pp. 59–74. [Google Scholar]
- Feast, S. Potential application of electronic noses in cereals. Cereal Food World 2011, 46, 159–161. [Google Scholar]
- Orina, I.; Manley, M.; Williams, P.J. Non-destructive techniques for the detection of fungal infection in cereal grains. Food Res. Int. 2017, 100, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Borjesson, T.; Lundstedt, T.; Schnuerer, J. Detection and quantification of ochratoxin and deoxinivalenol in barley grain by GC-MS and electronic nose. Int. J. Food Microbiol. 2002, 72, 203–214. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Zawirska-Wojtasiak, R.; Wiewiórowska, M.; Wasowicz, E. Determination of geosmin, 2-methylisoborneol, and a musty-earthy odor in wheat grain by SPME-GC-MS, profiling volatiles, and sensory analysis. J. Agric. Food Chem. 2003, 51, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Evans, P. Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J. Stored Prod. 2000, 36, 319–340. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Cervellieri, S.; Damascelli, A.; Visconti, A. Screening of deoxynivalenol contamination in durum wheat by MOS-based electronic nose and identification of the relevant pattern of volatile compounds. Food Control 2014, 37, 263–271. [Google Scholar] [CrossRef]
- Keshri, G.; Magan, N. Detection and differentiation between mycotoxigenic and non-mycotoxigenic strains of two Fusarium spp. using volatile production profiles and hydrolytic enzymes. J. Appl. Mirobiol. 2000, 89, 825–833. [Google Scholar] [CrossRef]
- Gobbi, E.; Falasconi, M.; Torelli, E.; Sberveglieri, G. Electronic nose predicts high and low fumonisin contamination in maize cultures. Food Res. Int. 2011, 44, 992–999. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. L 2009, 54, 1–130. [Google Scholar]
| Group | Measurements, N | N (% of Sample Correctly Classify) | ||
|---|---|---|---|---|
| Aflatoxins | Below the regulatory limit (<5 ppb) | Above the regulatory limit (>5 ppm) | ||
| Below regulatory limit (<5 ppb) | 1070 | 954 (89.2) | 116 (10.8) | |
| Above regulatory limit (>5 ppb) | 360 | 112 (31.1) | 248 (68.9) | |
| Fumonisins | Below the regulatory limit (<4 ppm) | Above the regulatory limit (>4 ppm) | ||
| Below regulatory limit (<4 ppm) | 540 | 471 (87.2) | 69 (12.8) | |
| Above regulatory limit (>4 ppm) | 300 | 54 (18.0) | 246 (82.0) | |
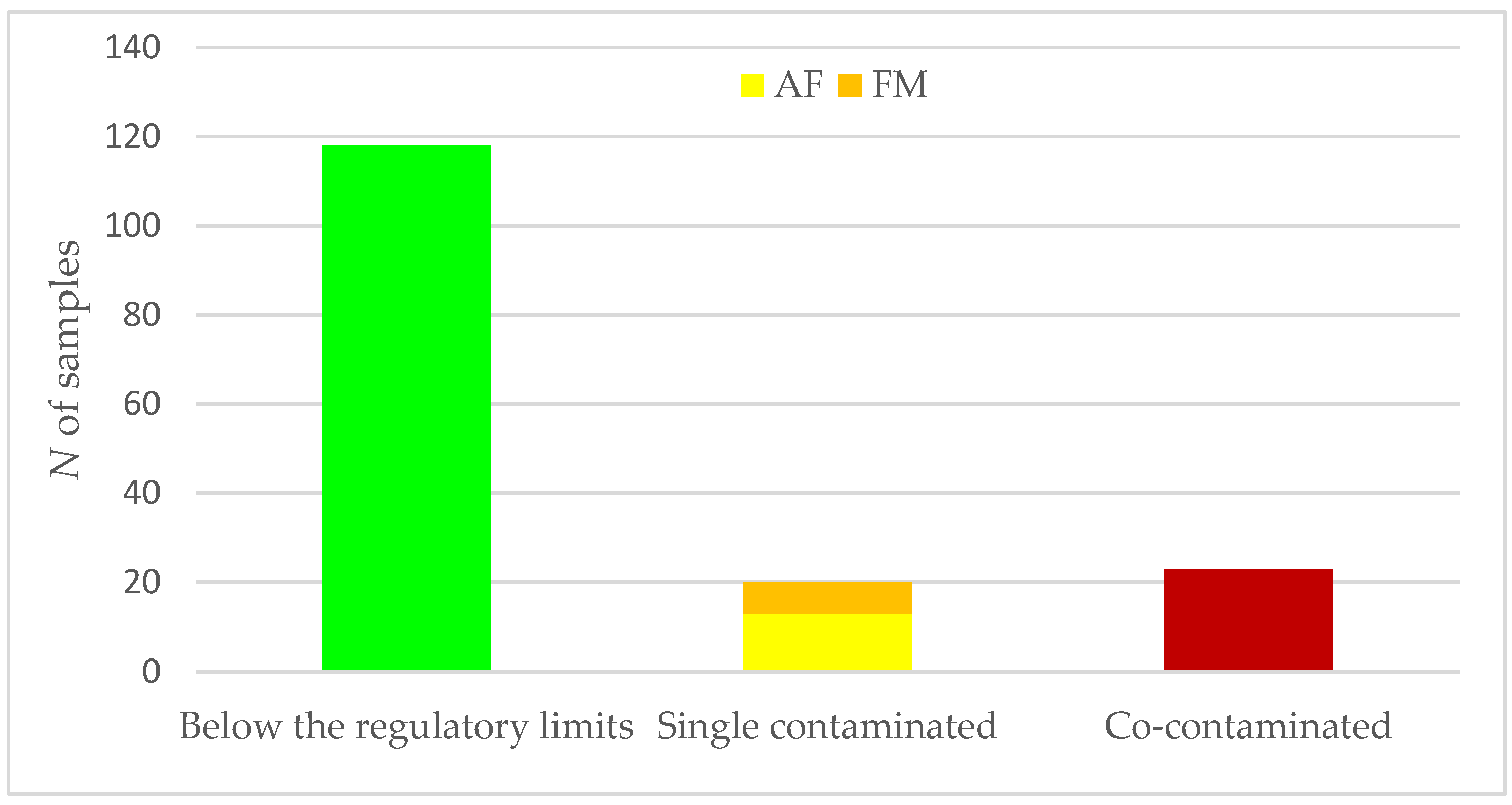
| Class assigned | Below the regulatory limit | Above the regulatory limit for 1 mycotoxin | Above the regulatory limit for 2 mycotoxin | |
| Below regulatory limit | 1180 | 772 (65.4) | 403 (34.2) | 5 (0.4) |
| Above regulatory limit for 1 mycotoxin | 200 | 64 (32.0) | 122 (61.0) | 14 (7.0) |
| Above regulatory limit for 2 mycotoxin | 230 | 26 (11.3) | 49 (21.3) | 155 (67.4) |
| Mycotoxin Detected | Weight and Dilution | Fully Wet Sample, then Mix | Clarify | Protocol | Pre-Mix as Noted, then Transfer 200 µL to Reaction Tube | Pre-Mix Sample in Blue Dilution Tube Followed by Transfer to Clear Reaction Tube | Add Reaction Tube to Incubator Set at 22 °C | Add Strip for | Read Results in |
|---|---|---|---|---|---|---|---|---|---|
| AF | 50 g sample in EB17 pouches with 150 mL water. Immediately shake vigorously for 10 s by hand | 1 min highest speed on shaker table | Centrifuge | Base Range 0–30 ppb | Pre-Mix 100 µL DB5 buffer + 100 µL extract in Reaction Tube | Pre-Mix 2.5 mL buffer + 50 µL extract. Transfer 200 µL | Acclimate tube for 2 min | 4 min | QuickScan EnvirologixTM |
| FM | 50 g sample in 250 mL water | 1 min highest speed on shaker table | Settle | Base Range 1.5 ppm to 7.0 ppm | Pre-Mix 100 µL DB5 buffer + 100 µL extract in Reaction Tube | Pre-Mix 2.5 mL buffer + 50 µL extract. Transfer 200 µL | Acclimate tube for 2 min | 5 min | QuickScan EnvirologixTM |
| Sensor Number in Array | Sensor Name | Description | Reference |
|---|---|---|---|
| W1C | Aromatic | Aromatic compound | Toluene 10 ppm |
| W5S | Broadrange | Broad range sensitivity reacts to nitrogen oxides and ozone, very sensitive with negative signal | NO2 10 ppm |
| W3C | Aromatic | Ammonia, used as sensor for aromatic compounds | Benzene 10 ppm |
| W6S | Hydrogen | Mainly hydrogen, selective (breath gases) | H2 100 ppm |
| W5C | Arom-aliph | Alkanes, aromatic compounds, less polar compounds | Propane 1 ppm |
| W1S | Broad-methane | Sensitive to methane (environment) 10 ppm. Broad range | CH4 100 ppm |
| W1W | Sulphur-organic | Reacts on sulphur compounds. Sensitive to many terpenes and sulphur organic compounds, which are important for smell, limonene, pyrazine | H2S 0.1 ppm |
| W2S | Broad-alcohol | Detects alcohol, partially aromatic compounds, broad range | CO 100 ppm |
| W2W | Sulph-clor | Aromatic compounds, sulphur organic compounds | H2S 1 ppm |
| W3S | Methane-aliph | Reacts on high concentration >100 ppm, sometimes very selective (methane) | CH4 10 ppm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottoboni, M.; Pinotti, L.; Tretola, M.; Giromini, C.; Fusi, E.; Rebucci, R.; Grillo, M.; Tassoni, L.; Foresta, S.; Gastaldello, S.; et al. Combining E-Nose and Lateral Flow Immunoassays (LFIAs) for Rapid Occurrence/Co-Occurrence Aflatoxin and Fumonisin Detection in Maize. Toxins 2018, 10, 416. https://doi.org/10.3390/toxins10100416
Ottoboni M, Pinotti L, Tretola M, Giromini C, Fusi E, Rebucci R, Grillo M, Tassoni L, Foresta S, Gastaldello S, et al. Combining E-Nose and Lateral Flow Immunoassays (LFIAs) for Rapid Occurrence/Co-Occurrence Aflatoxin and Fumonisin Detection in Maize. Toxins. 2018; 10(10):416. https://doi.org/10.3390/toxins10100416
Chicago/Turabian StyleOttoboni, Matteo, Luciano Pinotti, Marco Tretola, Carlotta Giromini, Eleonora Fusi, Raffaella Rebucci, Maria Grillo, Luca Tassoni, Silvia Foresta, Silvia Gastaldello, and et al. 2018. "Combining E-Nose and Lateral Flow Immunoassays (LFIAs) for Rapid Occurrence/Co-Occurrence Aflatoxin and Fumonisin Detection in Maize" Toxins 10, no. 10: 416. https://doi.org/10.3390/toxins10100416
APA StyleOttoboni, M., Pinotti, L., Tretola, M., Giromini, C., Fusi, E., Rebucci, R., Grillo, M., Tassoni, L., Foresta, S., Gastaldello, S., Furlan, V., Maran, C., Dell’Orto, V., & Cheli, F. (2018). Combining E-Nose and Lateral Flow Immunoassays (LFIAs) for Rapid Occurrence/Co-Occurrence Aflatoxin and Fumonisin Detection in Maize. Toxins, 10(10), 416. https://doi.org/10.3390/toxins10100416






