The Effectiveness of Zinc-Biofortified Wheat Flour Intake on the Growth and Morbidity Outcomes of Rural Pakistani Children and Adolescent Girls: A Cluster-Randomised, Double-Blind, Controlled Trial
Highlights
- This is the first randomised controlled trial to evaluate the impact of zinc-biofortified wheat consumption on growth and morbidity outcomes in adolescent girls.
- Modest improvements in head circumference were observed in children aged 1–5 years, a secondary study population who consumed zinc-biofortified wheat.
- No significant effects were found on growth and lung function in adolescent girls.
- The longitudinal prevalence of morbidities showed no consistent differences between the biofortified and control arms, although reduced incidences of respiratory tract infections were observed in both children and adolescent girls at specific time points.
- Findings contribute to global evidence on the effectiveness of biofortification for improving health outcomes in zinc-deficient, resource-limited settings.
Abstract
1. Introduction
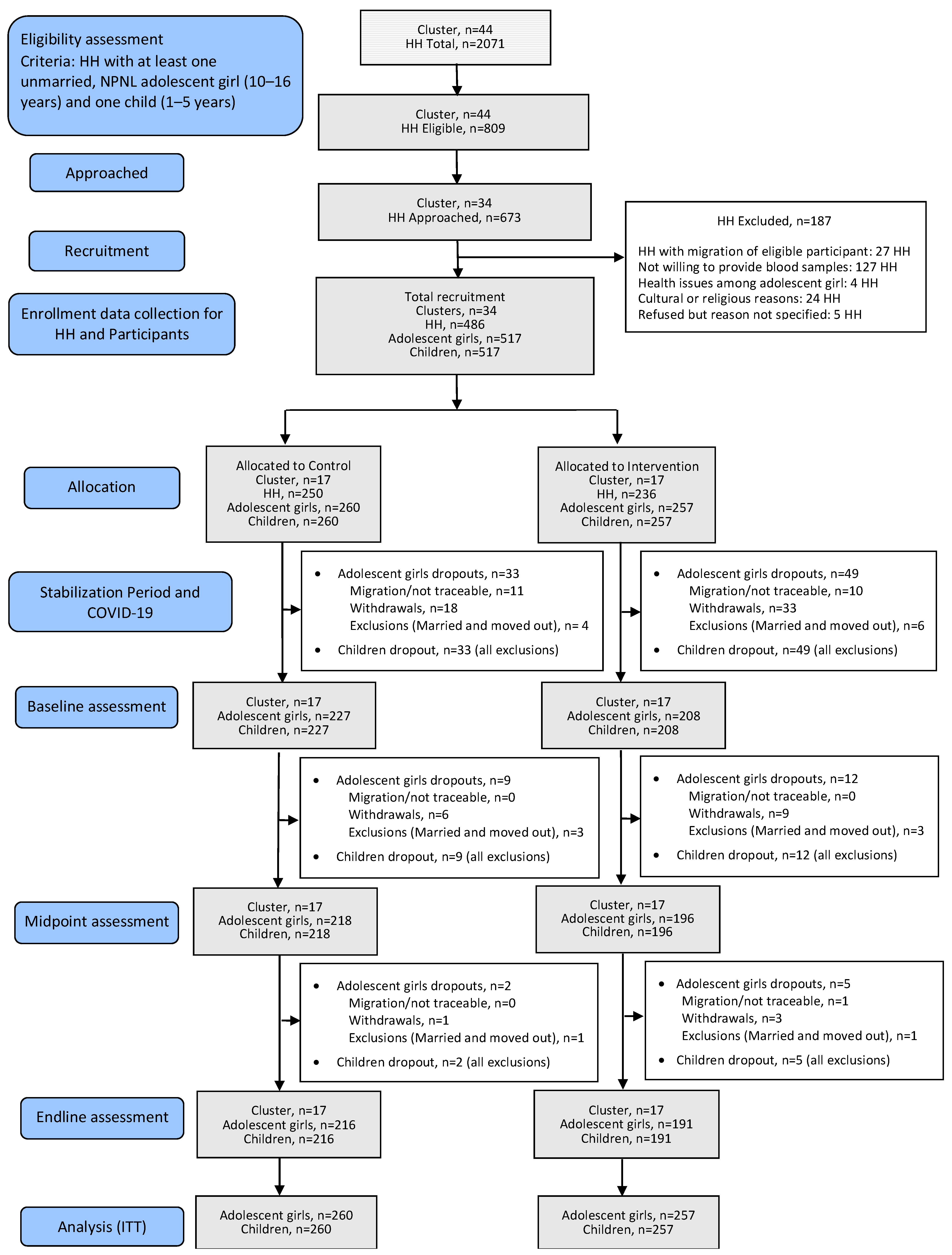
2. Materials and Methods
2.1. Study Setting and Design
2.2. Field Procedures
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.1.1. Adolescent Girls
3.1.2. Children
3.2. Impact of Intervention
3.2.1. Anthropometry
Adolescent Girls
Children
3.2.2. Morbidity
Respiratory Tract Infection
Diarrhoea
3.2.3. Lung Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M.; Addo, O.Y.; Adu-Afarwuah, S.; Alayón, S.; Bhutta, Z.; Brown, K.H.; et al. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global estimation of dietary micronutrient inadequacies: A modelling analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378s–1383s. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Stammers, A.L.; Warthon-Medina, M.; Moran, V.H.; Iglesia-Altaba, I.; Hermoso, M.; Moreno, L.A.; Lowe, N.M. Factors that affect zinc bioavailability and losses in adult and elderly populations. Nutr. Rev. 2014, 72, 334–352. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Martinez-Estevez, N.S.; Alvarez-Guevara, A.N.; Rodriguez-Martinez, C.E. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergol. Immunopathol. 2016, 44, 368–375. [Google Scholar] [CrossRef]
- Shah, U.H.; Abu-Shaheen, A.K.; Malik, M.A.; Alam, S.; Riaz, M.; Al-Tannir, M.A. The efficacy of zinc supplementation in young children with acute lower respiratory infections: A randomized double-blind controlled trial. Clin. Nutr. 2013, 32, 193–199. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sentz, J.; Miller, M.A. Role of Zinc Administration in Prevention of Childhood Diarrhea and Respiratory Illnesses: A Meta-analysis. Pediatrics 2007, 119, 1120–1130. [Google Scholar] [CrossRef]
- Fischer Walker, C.L.; Ezzati, M.; Black, R.E. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur. J. Clin. Nutr. 2009, 63, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Government of Pakistan; UNICEF. National Nutrition Survey 2018. Full Report (3 Volumes) & Key Findings Report. Available online: https://www.unicef.org/pakistan/reports/national-nutrition-survey-2018-full-report-3-volumes-key-findings-report (accessed on 6 February 2025).
- Brazier, A.K.M.; Lowe, N.M.; Zaman, M.; Shahzad, B.; Ohly, H.; McArdle, H.J.; Ullah, U.; Broadley, M.R.; Bailey, E.H.; Young, S.D.; et al. Micronutrient Status and Dietary Diversity of Women of Reproductive Age in Rural Pakistan. Nutrients 2020, 12, 3407. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zaman, M.; Fatima, S.; Shahzad, B.; Brazier, A.K.M.; Moran, V.H.; Broadley, M.R.; Zia, M.H.; Bailey, E.H.; Wilson, L.; et al. The Impact of Consuming Zinc-Biofortified Wheat Flour on Haematological Indices of Zinc and Iron Status in Adolescent Girls in Rural Pakistan: A Cluster-Randomised, Double-Blind, Controlled Effectiveness Trial. Nutrients 2022, 14, 1657. [Google Scholar] [CrossRef]
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Ansari, N.; Mehmood, R.; Gazdar, H. Going against the grain of optimism: Flour fortification in Pakistan. IDS Bull. 2018, 49, 57–71. [Google Scholar] [CrossRef]
- e-Pact Consortium. Evaluation of the Supporting Nutrition in Pakistan Food Fortification Programme Endline Evaluation Report. 2021. Available online: https://iati.fcdo.gov.uk/iati_documents/90000152.pdf (accessed on 5 February 2025).
- Sazawal, S.; Dhingra, U.; Dhingra, P.; Dutta, A.; Deb, S.; Kumar, J.; Devi, P.; Prakash, A. Efficacy of high zinc biofortified wheat in improvement of micronutrient status, and prevention of morbidity among preschool children and women—A double masked, randomized, controlled trial. Nutr. J. 2018, 17, 86. [Google Scholar] [CrossRef]
- Rosado, J.L.; Hambidge, K.M.; Miller, L.V.; Garcia, O.P.; Westcott, J.; Gonzalez, K.; Conde, J.; Hotz, C.; Pfeiffer, W.; Ortiz-Monasterio, I.; et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J. Nutr. 2009, 139, 1920–1925. [Google Scholar] [CrossRef]
- Signorell, C.; Zimmermann, M.B.; Cakmak, I.; Wegmüller, R.; Zeder, C.; Hurrell, R.; Aciksoz, S.B.; Boy, E.; Tay, F.; Frossard, E.; et al. Zinc Absorption from Agronomically Biofortified Wheat Is Similar to Post-Harvest Fortified Wheat and Is a Substantial Source of Bioavailable Zinc in Humans. J. Nutr. 2019, 149, 840–846. [Google Scholar] [CrossRef]
- Signorell, C.; Kurpad, A.V.; Pauline, M.; Shenvi, S.; Mukhopadhyay, A.; King, J.C.; Zimmermann, M.B.; Moretti, D. The Effect of Zinc Biofortified Wheat Produced via Foliar Application on Zinc Status: A Randomized, Controlled Trial in Indian Children. J. Nutr. 2023, 153, 3092–3100. [Google Scholar] [CrossRef]
- Lowe, N.M.; Zaman, M.; Khan, M.J.; Brazier, A.K.M.; Shahzad, B.; Ullah, U.; Khobana, G.; Ohly, H.; Broadley, M.R.; Zia, M.H.; et al. Biofortified Wheat Increases Dietary Zinc Intake: A Randomised Controlled Efficacy Study of Zincol-2016 in Rural Pakistan. Front. Nutr. 2021, 8, 809783. [Google Scholar] [CrossRef]
- Chomba, E.; Westcott, C.M.; Westcott, J.E.; Mpabalwani, E.M.; Krebs, N.F.; Patinkin, Z.W.; Palacios, N.; Hambidge, K.M. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J. Nutr. 2015, 145, 514–519. [Google Scholar] [CrossRef]
- Jongstra, R.; Hossain, M.M.; Galetti, V.; Hall, A.G.; Holt, R.R.; Cercamondi, C.I.; Rashid, S.F.; Zimmermann, M.B.; Mridha, M.K.; Wegmueller, R. The effect of zinc-biofortified rice on zinc status of Bangladeshi preschool children: A randomized, double-masked, household-based, controlled trial. Am. J. Clin. Nutr. 2022, 115, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Khan, M.J.; Broadley, M.R.; Zia, M.H.; McArdle, H.J.; Joy, E.J.M.; Ohly, H.; Shahzad, B.; Ullah, U.; Kabana, G.; et al. Examining the effectiveness of consuming flour made from agronomically biofortified wheat (Zincol-2016/NR-421) for improving Zn status in women in a low-resource setting in Pakistan: Study protocol for a randomised, double-blind, controlled cross-over trial (BiZiFED). BMJ Open 2018, 8, e021364. [Google Scholar] [CrossRef]
- Lowe, N.M.; Zaman, M.; Moran, V.H.; Ohly, H.; Sinclair, J.; Fatima, S.; Broadley, M.R.; Joy, E.J.M.; Mahboob, U.; Lark, R.M.; et al. Biofortification of wheat with zinc for eliminating deficiency in Pakistan: Study protocol for a cluster-randomised, double-blind, controlled effectiveness study (BIZIFED2). BMJ Open 2020, 10, e039231. [Google Scholar] [CrossRef]
- World Health Organisation; Multicentre Growth Reference Study Group. WHO Child Growth Standards. Length, Height for-Age, Weightfor-Age, Weight-for-Length and Body Mass Index-for Age. Methods and Development. 2006. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 10 January 2025).
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO AnthroPlus Software (Version 1.0.4). Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools (accessed on 10 January 2025).
- World Health Organization. WHO Anthro (Version 3.2.2, January 2011). Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 10 January 2025).
- Eslami, M.; Pourghazi, F.; Khazdouz, M.; Tian, J.; Pourrostami, K.; Esmaeili-Abdar, Z.; Ejtahed, H.-S.; Qorbani, M. Optimal cut-off value of waist circumference-to-height ratio to predict central obesity in children and adolescents: A systematic review and meta-analysis of diagnostic studies. Front. Nutr. 2023, 9, 985319. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Lum, S.; Bountziouka, V.; Quanjer, P.; Sonnappa, S.; Wade, A.; Beardsmore, C.; Chhabra, S.K.; Chudasama, R.K.; Cook, D.G.; Harding, S.; et al. Challenges in Collating Spirometry Reference Data for South-Asian Children: An Observational Study. PLoS ONE 2016, 11, e0154336. [Google Scholar] [CrossRef]
- Global Lung Function Initiative. Global Lung Function Initiative Calculators for Spirometry, TLCO and Lung Volume. Available online: https://gli-calculator.ersnet.org/index.html (accessed on 10 January 2025).
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Moore Heslin, A.; McNulty, B. Adolescent nutrition and health: Characteristics, risk factors and opportunities of an overlooked life stage. Proc. Nutr. Soc. 2023, 82, 142–156. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858s–885s. [Google Scholar] [CrossRef]
- Black, R.E.; Sazawal, S. Zinc and childhood infectious disease morbidity and mortality. Br. J. Nutr. 2001, 85 (Suppl. S2), S125–S129. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Pimpin, L.; Shulkin, M.; Kranz, S.; Duggan, C.P.; Mozaffarian, D.; Fawzi, W.W. Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients 2018, 10, 377. [Google Scholar] [CrossRef]
- de Benoist, B.; Darnton-Hill, I.; Davidsson, L.; Fontaine, O.; Hotz, C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food Nutr. Bull. 2007, 28, S480–S484. [Google Scholar] [CrossRef]
- Konda, V.; Palika, R.; Rajendran, A.; Neeraja, C.N.; Sundaram, R.M.; Pullakhandam, R. Zinc-Biofortified Rice Improves Growth in Zinc-Deficient Rats. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Mehta, S.; Huey, S.L.; Ghugre, P.S.; Potdar, R.D.; Venkatramanan, S.; Krisher, J.T.; Ruth, C.J.; Chopra, H.V.; Thorat, A.; Thakker, V.; et al. A randomized trial of iron- and zinc-biofortified pearl millet-based complementary feeding in children aged 12 to 18 months living in urban slums. Clin. Nutr. 2022, 41, 937–947. [Google Scholar] [CrossRef]
- Surkan, P.J.; Shankar, M.; Katz, J.; Siegel, E.H.; LeClerq, S.C.; Khatry, S.K.; Stoltzfus, R.J.; Tielsch, J.M. Beneficial effects of zinc supplementation on head circumference of Nepalese infants and toddlers: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 836–842. [Google Scholar] [CrossRef]
- Gera, T.; Shah, D.; Sachdev, H.S. Zinc Supplementation for Promoting Growth in Children Under 5 years of age in Low- and Middle-income Countries: A Systematic Review. Indian Pediatr. 2019, 56, 391–406. [Google Scholar]
- Mishra, N.; Salvi, S.; Lyngdoh, T.; Agrawal, A. Low lung function in the developing world is analogous to stunting: A review of the evidence. Wellcome Open Res. 2020, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Luzak, A.; Fuertes, E.; Flexeder, C.; Standl, M.; von Berg, A.; Berdel, D.; Koletzko, S.; Heinrich, J.; Nowak, D.; Schulz, H. Which early life events or current environmental and lifestyle factors influence lung function in adolescents?—Results from the GINIplus & LISAplus studies. Respir. Res. 2017, 18, 138. [Google Scholar] [CrossRef]
- Subhanullah, M.; Ullah, S.; Javed, M.F.; Ullah, R.; Akbar, T.A.; Ullah, W.; Baig, S.A.; Aziz, M.; Mohamed, A.; Sajjad, R.U. Assessment and Impacts of Air Pollution from Brick Kilns on Public Health in Northern Pakistan. Atmosphere 2022, 13, 1231. [Google Scholar] [CrossRef]
- Nicolaou, L.; Sylvies, F.; Veloso, I.; Lord, K.; Chandyo, R.K.; Sharma, A.K.; Shrestha, L.P.; Parker, D.L.; Thygerson, S.M.; DeCarlo, P.F.; et al. Brick kiln pollution and its impact on health: A systematic review and meta-analysis. Environ. Res. 2024, 257, 119220. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.; Ruel, M.T.; Salm, L.; Sinclair, B.; Branca, F. Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet 2020, 395, 142–155. [Google Scholar] [CrossRef]
- Shahzad, B.; Holt, R.R.; Gupta, S.; Zaman, M.; Shahzad, M.; Lowe, N.M.; Hall, A.G. Effects of Zinc-Biofortified Wheat Intake on Plasma Markers of Fatty Acid Metabolism and Oxidative Stress Among Adolescents. Nutrients 2024, 16, 4265. [Google Scholar] [CrossRef]
- HarvestPlus. The Journey of Scaling in Pakistan: Zinc-Enriched Biofortified Wheat. 2022. Available online: https://www.harvestplus.org/wp-content/uploads/2022/03/The-Journey-of-Scaling-in-Pakistan.pdf (accessed on 2 February 2025).
| n | Overall | n | Control | n | Intervention | |
|---|---|---|---|---|---|---|
| General | ||||||
| Age (years) | 517 | 12.11 ± 1.71 | 260 | 12.16 ± 1.69 | 257 | 12.07 ± 1.73 |
| Anthropometry | ||||||
| Weight (kg) | 433 | 42.13 ± 10.32 | 227 | 42.71 ± 9.83 | 206 | 41.49 ± 10.82 |
| Height (cm) | 430 | 148.34 ± 8.93 | 226 | 149.26 ± 8.75 | 204 | 147.31 ± 9.04 |
| HAZ | 430 | −0.74 ± 1.12 | 226 | −0.62 ± 1.19 | 204 | −0.86 ± 1.02 |
| BMI (kg/m2) | 429 | 18.91 ± 3.46 | 226 | 18.96 ± 3.32 | 203 | 18.85 ± 3.61 |
| BAZ | 429 | −0.09 ± 1.11 | 226 | −0.06 ± 1.09 | 203 | −0.12 ± 1.14 |
| MUAC (cm) | 433 | 22.22 ± 3.31 | 227 | 22.30 ± 3.18 | 206 | 22.13 ± 3.46 |
| Waist circumference (cm) | 431 | 63.54 ± 7.98 | 226 | 63.53 ± 7.49 | 205 | 63.55 ± 8.50 |
| Hip circumference (cm) | 430 | 79.57 ± 9.06 | 226 | 80.19 ± 9.08 | 204 | 78.89 ± 9.02 |
| WHR | 427 | 0.80 ± 0.06 | 225 | 0.79 ± 0.05 | 202 | 0.81 ± 0.06 |
| WHtR | 427 | 0.43 ± 0.05 | 225 | 0.43 ± 0.04 | 202 | 0.43 ± 0.05 |
| Stunted, HAZ < −2 | 430 | 43 (10.0) | 226 | 21 (9.3) | 204 | 22 (10.8) |
| Thinness, BAZ < −2 | 429 | 12 (2.8) | 226 | 7 (3.1) | 203 | 5 (2.5) |
| Overweight or obesity, BAZ > 1 | 429 | 68 (15.9) | 226 | 37 (16.4) | 203 | 31 (15.3) |
| Central obesity, WHtR > 0.49 | 427 | 41 (9.6) | 225 | 16 (7.1) | 202 | 25 (12.4) |
| Lung function | ||||||
| FEV1 (% Pred, GLI-O) | 414 | 97.21 ± 15.75 | 220 | 97.94 ± 16.49 | 194 | 96.39 ± 14.85 |
| zFEV1 | 414 | −0.22 ± 1.33 | 220 | −0.16 ± 1.39 | 194 | −0.29 ± 1.25 |
| FVC (% Pred, GLI-O) | 414 | 100.04 ± 15.49 | 220 | 101.07 ± 15.95 | 194 | 98.87 ± 14.91 |
| zFVC | 414 | −0.01 ± 1.37 | 220 | 0.07 ± 1.41 | 194 | −0.12 ± 1.32 |
| FEV1/FVC (% Pred, GLI-O) | 414 | 96.87 ± 5.39 | 220 | 96.52 ± 5.43 | 194 | 97.26 ± 5.33 |
| zFEV1/FVC | 414 | −0.46 ± 0.75 | 220 | −0.51 ± 0.74 | 194 | −0.39 ± 0.76 |
| FEV1 < −1.64 Z-score, GLI-O | 414 | 51 (12.3) | 220 | 24 (10.9) | 194 | 27 (13.9) |
| FVC < −1.64 Z-score, GLI-O | 414 | 40 (9.7) | 220 | 17 (7.7) | 194 | 23 (11.9) |
| FEV1/FVC < −1.64 Z-score, GLI-O | 414 | 27 (6.5) | 220 | 16 (7.3) | 194 | 11 (5.7) |
| Morbidity | ||||||
| Longitudinal prevalence #, RTI | 434 | 6.8 (5.6, 8.0) | 226 | 8.2 (6.24, 10.26) | 208 | 5.19 (3.90, 6.48) |
| n | Total | n | Control | n | Intervention | |
|---|---|---|---|---|---|---|
| General | ||||||
| Age (years) | 517 | 3.05 ± 1.03 | 260 | 3.04 ± 1.02 | 257 | 3.06 ± 1.03 |
| Gender | 517 | 260 | 257 | |||
| Male | 267 (51.6) | 125 (48.1) | 142 (55.3) | |||
| Female | 250 (48.4) | 135 (51.9) | 115 (44.7) | |||
| Ever breastfed | 505 | 254 | 251 | |||
| Yes | 489 (96.8) | 247 (97.2) | 242 (96.4) | |||
| No | 16 (3.2) | 7 (2.8) | 9 (3.6) | |||
| Currently breastfeeding | 480 | 239 | 241 | |||
| Yes | 88 (18.3) | 46 (19.2) | 42 (17.4) | |||
| No | 392 (81.7) | 193 (80.8) | 199 (82.6) | |||
| Attending school | 509 | 256 | 253 | |||
| Yes | 22 (4.3) | 10 (3.9) | 12 (4.7) | |||
| No | 487 (95.7) | 246 (96.1) | 241 (95.3) | |||
| Anthropometry | ||||||
| Weight (kg) | 425 | 14.29 ± 2.84 | 220 | 14.25 ± 2.94 | 205 | 14.33 ± 2.73 |
| Height (cm) | 414 | 95.46 ± 10.25 | 216 | 95.58 ± 10.46 | 198 | 95.34 ± 10.01 |
| BMI (kg/m2) | 407 | 15.76 ± 2.16 | 211 | 15.74 ± 2.28 | 196 | 15.79 ± 2.04 |
| MUAC (cm) | 430 | 14.85 ± 1.08 | 225 | 14.86 ± 1.04 | 205 | 14.85 ± 1.12 |
| HC (cm) | 416 | 48.16 ± 2.24 | 220 | 48.12 ± 2.20 | 196 | 48.20 ± 2.29 |
| WHZ | 354 | 0.07 ± 1.46 | 188 | 0.00 ± 1.53 | 166 | 0.15 ± 1.37 |
| HAZ | 414 | −1.28 ± 1.67 | 216 | −1.19 ± 1.76 | 198 | −1.39 ± 1.57 |
| WAZ | 425 | −0.77 ± 1.26 | 220 | −0.76 ± 1.33 | 205 | −0.80 ± 1.19 |
| BAZ | 407 | 0.13 ± 1.46 | 211 | 0.09 ± 1.53 | 196 | 0.16 ± 1.37 |
| MUACZ | 381 | −0.93 ± 0.82 | 205 | −0.91 ± 0.82 | 176 | −0.95 ± 0.82 |
| HCZ | 370 | −0.95 ± 1.37 | 200 | −0.95 ± 1.35 | 170 | −0.94 ± 1.39 |
| Stunting, HAZ < −2 | 414 | 140 (33.8) | 216 | 74 (34.3) | 198 | 66 (33.3) |
| Underweight, WAZ<−2 | 425 | 70 (16.5) | 220 | 37 (16.8) | 205 | 33 (16.1) |
| Wasting, WHZ < −2 SD | 427 | 19 (4.4) | 214 | 12 (5.6) | 213 | 7 (3.3) |
| Overweight or obesity, WHZ > 2 | 427 | 50 (11.7) | 214 | 25 (11.7) | 213 | 25 (11.7) |
| Morbidity | ||||||
| Longitudinal prevalence #, Diarrhoea | 434 | 5.5 (4.6, 6.4) | 226 | 5.8 (4.5, 7.2) | 208 | 5.1 (4.0, 6.3) |
| Longitudinal prevalence #, RTI | 434 | 8.9 (7.6, 10.1) | 226 | 8.6 (6.9, 10.4) | 208 | 9.1 (7.4, 10.9) |
| Time Points | n | Control | n | Intervention | β (95% CI) * | X2 | t | p | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Baseline | 227 | 42.71 ± 9.83 | 206 | 41.49 ± 10.82 | ||||
| Midpoint | 217 | 43.85 ± 9.84 | 194 | 42.39 ± 10.55 | −0.191 (−0.548, 0.167) | −1.086 | 0.285 | ||
| Endline | 215 | 45.47 ± 9.73 | 191 | 43.43 ± 10.15 | −0.0480 (−0.981, 0.020) | −1.952 | 0.059 | ||
| Height (cm) | Baseline | 226 | 149.26 ± 8.75 | 204 | 147.31 ± 9.04 | ||||
| Midpoint | 216 | 149.81 ± 8.68 | 191 | 148.00 ± 8.60 | −0.028 (−0.244, 0.189) | −0.259 | 0.796 | ||
| Endline | 213 | 150.56 ± 8.52 | 189 | 148.66 ± 8.45 | −0.065 (−0.449, 0.319) | −0.339 | 0.736 | ||
| BMI (kg/m2) | Baseline | 226 | 18.96 ± 3.32 | 203 | 18.85 ± 3.61 | ||||
| Midpoint | 216 | 19.39 ± 3.35 | 191 | 19.08 ± 3.48 | −0.064 (−0.237, 0.108) | −0.759 | 0.453 | ||
| Endline | 213 | 19.93 ± 3.33 | 189 | 19.42 ± 3.32 | −0.216 (−0.501, 0.068) | −1.537 | 0.132 | ||
| MUAC (cm) | Baseline | 227 | 22.30 ± 3.18 | 206 | 22.13 ± 3.46 | ||||
| Midpoint | 216 | 22.65 ± 3.23 | 190 | 22.31 ± 3.42 | −0.023 (−0.175, 0.129) | −0.312 | 0.757 | ||
| Endline | 214 | 23.14 ± 3.31 | 187 | 22.76 ± 3.30 | −0.005 (−0.259, 0.249) | −0.040 | 0.968 | ||
| Waist circumference (cm) | Baseline | 226 | 63.53 ± 7.49 | 205 | 63.55 ± 8.50 | ||||
| Midpoint | 216 | 64.36 ± 7.39 | 191 | 63.92 ± 8.47 | −0.236 (0.622, 0.150) | −1.239 | 0.223 | ||
| Endline | 209 | 65.59 ± 7.52 | 187 | 64.85 ± 8.35 | −0.650 (1.309, 0.009) | −1.989 | 0.053 | ||
| Hip circumference (cm) | Baseline | 226 | 80.19 ± 9.08 | 204 | 78.89 ± 9.02 | ||||
| Midpoint | 217 | 81.08 ± 9.00 | 189 | 79.51 ± 9.05 | 0.052 (−0.392, −0.495) | 0.234 | 0.816 | ||
| Endline | 215 | 82.35 ± 8.86 | 186 | 80.44 ± 8.69 | −0.301 (−0.882, 0.281) | −1.044 | 0.302 | ||
| BAZ | Baseline | 226 | −0.06 ± 1.09 | 203 | −0.12 ± 1.14 | ||||
| Midpoint | 216 | 0.03 ± 1.08 | 191 | −0.08 ±1.11 | −0.013 (−0.086, 0.061) | −0.353 | 0.726 | ||
| Endline | 213 | 0.19 ± 1.00 | 189 | 0.02 ± 1.05 | −0.067 (−0.181, 0.047) | −1.196 | 0.239 | ||
| HAZ | Baseline | 226 | −0.62 ± 1.19 | 204 | −0.86 ± 1.02 | ||||
| Midpoint | 216 | −0.71 ± 1.16 | 191 | −0.90 ± 0.98 | 0.001 (−0.028, 0.031) | 0.093 | 0.926 | ||
| Endline | 213 | −0.73 ± 1.14 | 189 | −0.92 ± 1.01 | 0.006 (−0.046, 0.059) | 0.247 | 0.806 | ||
| WHtR | Baseline | 225 | 0.43 ± 0.04 | 202 | 0.43 ± 0.05 | ||||
| Midpoint | 215 | 0.43 ± 0.05 | 188 | 0.43 ± 0.05 | −0.002 (−0.004, 0.001) | −1.220 | 0.232 | ||
| Endline | 207 | 0.44 ± 0.05 | 185 | 0.44 ± 0.05 | −0.004 (−0.009, 0.001) | −1.703 | 0.960 | ||
| Stunting, HAZ < −2 | Baseline | 226 | 21 (9.3) | 204 | 22 (10.8) | 0.265 | 0.607 | ||
| Midpoint | 216 | 20 (9.3) | 191 | 21 (11.0) | 0.337 | 0.562 | |||
| Endline | 213 | 23 (10.8) | 189 | 22 (11.6) | 0.071 | 0.789 | |||
| Thinness, BAZ < −2 | Baseline | 226 | 7 (3.1) | 203 | 5 (2.5) | 0.158 | 0.691 | ||
| Midpoint | 216 | 7 (3.2) | 191 | 8 (4.2) | 0.256 | 0.613 | |||
| Endline | 213 | 2 (0.9) | 189 | 5 (2.6) | 1.705 | 0.192 | |||
| Overweight or obese, BAZ > 1 | Baseline | 226 | 37 (16.4) | 203 | 31 (15.3) | 0.097 | 0.755 | ||
| Midpoint | 216 | 41 (19.0) | 191 | 30 (15.7) | 0.755 | 0.385 | |||
| Endline | 213 | 45 (21.1) | 189 | 31 (16.4) | 1.458 | 0.227 | |||
| Central obesity, WHtR > 0.49 | Baseline | 225 | 16 (7.1) | 202 | 25 (12.4) | 3.399 | 0.065 | ||
| Midpoint | 215 | 16 (7.4) | 188 | 23 (12.2) | 2.635 | 0.105 | |||
| Endline | 207 | 23 (11.1) | 185 | 24 (13.0) | 0.321 | 0.571 |
| Time Points | n | Control | n | Intervention | β (95% CI) * | X2 | t | p | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Baseline | 220 | 14.25 ± 2.94 | 205 | 14.33 ± 2.73 | ||||
| Midpoint | 208 | 15.13 ± 3.05 | 190 | 15.21 ± 2.86 | 0.051 (−0.193, 0.296) | 0.426 | 0.673 | ||
| Endline | 208 | 15.98 ± 3.09 | 186 | 15.77 ± 2.94 | −0.229 (−0.570, 0.111) | −1.356 | 0.182 | ||
| Height (cm) | Baseline | 216 | 95.58 ± 10.46 | 198 | 95.34 ± 10.01 | ||||
| Midpoint | 205 | 96.85 ± 10.59 | 188 | 96.45 ± 9.89 | 0.15 (−0.197, 0.497) | 0.858 | 0.393 | ||
| Endline | 198 | 98.65 ± 10.29 | 181 | 97.66 ± 9.87 | 0.396 (−0.160, 0.953) | 1.412 | 0.161 | ||
| BMI (kg/m2) | Baseline | 211 | 15.74 ± 2.28 | 196 | 15.79 ± 2.04 | ||||
| Midpoint | 198 | 16.26 ± 2.55 | 182 | 16.29 ± 2.04 | 1.593 (−0.253, 0.437) | 0.502 | 0.618 | ||
| Endline | 192 | 16.44 ± 2.50 | 176 | 16.37 ± 2.10 | −0.183 (−0.693, 0.326) | −0.720 | 0.475 | ||
| MUAC (cm) | Baseline | 225 | 14.86 ± 1.04 | 205 | 14.85 ± 1.12 | ||||
| Midpoint | 213 | 15.18 ± 1.12 | 194 | 15.25 ± 1.12 | 0.072 (−0.086, 0.231) | 0.919 | 0.362 | ||
| Endline | 214 | 15.56 ± 1.13 | 189 | 15.59 ± 1.18 | 0.057 (−0.168, 0.282) | 0.510 | 0.612 | ||
| HC (cm) | Baseline | 220 | 48.12 ± 2.20 | 196 | 48.20 ± 2.29 | ||||
| Midpoint | 214 | 48.26 ± 2.13 | 194 | 48.58 ± 1.92 | 0.188 (−0.038, 0.414) | 1.658 | 0.101 | ||
| Endline | 213 | 48.47 ± 2.03 | 190 | 48.76 ± 1.82 | 0.432 (0.151, 0.713) | 3.048 | 0.003 | ||
| WHZ | Baseline | 188 | 0.00 ± 1.53 | 166 | 0.15 ± 1.37 | ||||
| Midpoint | 165 | 0.46 ± 1.65 | 146 | 0.51 ± 1.33 | 0.067 (−0.174, 0.308) | 0.558 | 0.580 | ||
| Endline | 147 | 0.61 ± 1.62 | 130 | 0.55 ± 1.35 | −0.121 (−0.469, 228) | −0.695 | 0.490 | ||
| HAZ | Baseline | 216 | −1.19 ± 1.76 | 198 | −1.39 ± 1.57 | ||||
| Midpoint | 205 | −1.33 ± 1.76 | 188 | −1.53 ± 1.54 | 0.022 (−0.072, 0.116) | 0.474 | 0.637 | ||
| Endline | 198 | −1.28 ± 1.72 | 181 | −1.57 ± 1.53 | 0.091 (−0.057, 0.240) | 1.221 | 0.225 | ||
| WAZ | Baseline | 220 | −0.76 ± 1.33 | 205 | −0.80 ± 1.19 | ||||
| Midpoint | 208 | −0.56 ± 1.34 | 190 | −0.59 ± 1.17 | 0.019 (−0.109, 0.148) | 0.306 | 0.761 | ||
| Endline | 208 | −0.38 ± 1.25 | 186 | −0.53 ± 1.14 | −0.105 (−0.278, 0.068) | −1.228 | 0.226 | ||
| BAZ | Baseline | 211 | 0.09 ± 1.53 | 196 | 0.16 ± 1.37 | ||||
| Midpoint | 198 | 0.46 ± 1.65 | 182 | 0.54 ± 1.32 | 0.104 (−0.124, 0.332) | 0.920 | 0.362 | ||
| Endline | 192 | 0.60 ± 1.55 | 176 | 0.61 ± 1.34 | −0.022 (−0.337, 0.293) | −0.141 | 0.889 | ||
| HCZ | Baseline | 200 | −0.95 ± 1.35 | 170 | −0.94 ± 1.39 | ||||
| Midpoint | 183 | −0.99 ± 1.27 | 159 | −0.78 ± 1.19 | 0.169 (0.001, 0.337) | 1.998 | 0.049 | ||
| Endline | 162 | −0.91 ± 1.12 | 141 | −0.73 ± 1.08 | 0.367 (0.149, 0.586) | 3.342 | 0.001 | ||
| MUACZ | Baseline | 205 | −0.91 ± 0.82 | 176 | −0.95 ± 0.82 | ||||
| Midpoint | 182 | −0.72 ± 0.85 | 159 | −0.73 ± 0.78 | 0.067 (−0.066, 0.201) | 1.007 | 0.318 | ||
| Endline | 163 | −0.49 ± 0.84 | 140 | −0.52 ± 0.83 | 0.079 (−0.121, 0.278) | 0.792 | 0.432 | ||
| Stunting, HAZ < −2 | Baseline | 216 | 74 (34.3) | 198 | 66 (33.3) | 0.040 | 0.842 | ||
| Midpoint | 205 | 72 (35.1) | 188 | 76 (40.4) | 1.175 | 0.278 | |||
| Endline | 198 | 74 (37.4) | 181 | 71 (39.2) | 0.137 | 0.711 | |||
| Underweight, WAZ < −2 | Baseline | 220 | 37 (16.8) | 205 | 33 (16.1) | 0.040 | 0.841 | ||
| Midpoint | 208 | 24 (11.5%) | 190 | 25 (13.2) | 0.241 | 0.623 | |||
| Endline | 208 | 19 (9.1) | 186 | 18 (9.7) | 0.034 | 0.854 |
| n | Control | n | Intervention | β (95% CI) * | t | p | |
|---|---|---|---|---|---|---|---|
| RTI in adolescent girls | |||||||
| Baseline | 226 | 8.2 (6.2, 10.3) | 208 | 5.2 (3.9, 6.5) | |||
| Midpoint | 218 | 10.6 (8.6, 12.7) | 196 | 9.9 (8.0, 11.9) | −0.608 (−5.124, 3.909) | −0.270 | 0.788 |
| Endline | 215 | 8.1 (6.4, 9.8) | 191 | 6.3 (4.9, 7.7) | −2.098 (−5.851, 1.656) | −1.121 | 0.267 |
| RTI in children | |||||||
| Baseline | 226 | 8.6 (6.9, 10.4) | 208 | 9.1 (7.4, 10.9) | |||
| Midpoint | 218 | 16.5 (14.1, 18.9) | 196 | 14.8 (12.5, 17.2) | −3.425 (−9.143, 2.293) | −1.194 | 0.237 |
| Endline | 215 | 14.0 (11.5, 16.4) | 191 | 12.2 (9.9, 14.5) | −3.28 (−9.66, 3.100) | −1.020 | 0.310 |
| Diarrhoea in children | |||||||
| Baseline | 226 | 5.8 (4.5, 7.2) | 208 | 5.1 (4.0, 6.3) | |||
| Midpoint | 218 | 4.7 (3.2, 6.1) | 196 | 4.9 (3.5, 6.2) | 0.331 (−2.449, 3.110) | 0.240 | 0.811 |
| Endline | 215 | 3.2 (2.1, 4.3) | 191 | 2.9 (1.6, 4.2) | −0.255 (−2.374, 1.865) | −0.245 | 0.808 |
| n | Control | n | Intervention | β (95% CI) * | X2 | t | p | |
|---|---|---|---|---|---|---|---|---|
| FEV1 (% Pred, GLI-O) | ||||||||
| Baseline | 220 | 97.94 ± 16.49 | 194 | 96.39 ± 14.85 | ||||
| Midpoint | 214 | 92.62 ± 14.62 | 187 | 92.56 ± 14.10 | 0.733 (−1.766, 3.232) | 0.577 | 0.564 | |
| Endline | 212 | 90.89 ± 14.34 | 187 | 90.46 ± 13.83 | −0.097 (−2.694, 2.499) | −0.074 | 0.941 | |
| zFEV1 | ||||||||
| Baseline | 220 | −0.16 ± 1.39 | 194 | −0.29 ± 1.25 | ||||
| Midpoint | 214 | −0.61 ± 1.23 | 187 | −0.61 ± 1.18 | 0.062 (−0.148, 0.271) | 0.581 | 0.562 | |
| Endline | 212 | −0.75 ± 1.20 | 187 | −0.79 ± 1.15 | −0.008 (−0.225, 0.209) | −0.074 | 0.941 | |
| FVC (% Pred, GLI-O) | ||||||||
| Baseline | 220 | 101.07 ± 15.95 | 194 | 98.87 ± 14.91 | ||||
| Midpoint | 214 | 97.61 ± 14.84 | 187 | 97.93 ± 13.69 | 1.240 (−1.588, 4.068) | 0.910 | 0.373 | |
| Endline | 212 | 95.21 ± 14.17 | 187 | 94.74 ± 14.05 | 0.042 (−2.616, 2.701) | 0.031 | 0.975 | |
| zFVC | ||||||||
| Baseline | 220 | 0.07 ± 1.41 | 194 | −0.12 ± 1.32 | ||||
| Midpoint | 214 | −0.23 ± 1.32 | 187 | −0.20 ± 1.22 | 0.111 (−0.140, 0.361) | 0.916 | 0.370 | |
| Endline | 212 | −0.44 ± 1.25 | 187 | −0.48 ± 1.25 | 0.002 (−0.234, 0.237) | 0.015 | 0.988 | |
| FEV1/FVC (% Pred, GLI-O) | ||||||||
| Baseline | 220 | 96.52 ± 5.43 | 194 | 97.26 ± 5.33 | ||||
| Midpoint | 214 | 94.71 ± 6.15 | 186 | 94.51 ± 7.34 | −0.782 (−2.228, 0.664) | −1.091 | 0.281 | |
| Endline | 212 | 95.29 ± 7.01 | 187 | 95.39 ± 7.12 | −0.229 (−1.927, 1.468) | −0.275 | 0.785 | |
| zFEV1/FVC | ||||||||
| Baseline | 220 | −0.51 ± 0.74 | 194 | −0.39 ± 0.76 | ||||
| Midpoint | 214 | −0.77 ± 0.93 | 186 | −0.77 ± 1.01 | −0.088 (−0.296, 0.120) | −0.855 | 0.398 | |
| Endline | 212 | −0.66 ± 0.97 | 187 | −0.64 ± 1.03 | −0.030 (−0.269, 0.209) | −0.258 | 0.798 | |
| FEV1 <−1.64 Z-score, GLI-O | ||||||||
| Baseline | 220 | 24 (10.9) | 194 | 27 (13.9) | 0.864 | 0.353 | ||
| Midpoint | 214 | 30 (14.0) | 187 | 37 (19.8) | 2.385 | 0.122 | ||
| Endline | 212 | 48 (22.6) | 187 | 36 (19.3) | 0.687 | 0.407 | ||
| FVC <−1.64 Z-score, GLI-O | ||||||||
| Baseline | 220 | 17 (7.7) | 194 | 23 (11.9) | 2.013 | 0.156 | ||
| Midpoint | 214 | 23 (10.7) | 187 | 17 (9.1) | 0.305 | 0.581 | ||
| Endline | 212 | 31 (14.6) | 187 | 30 (16.0) | 0.155 | 0.694 | ||
| FEV1/FVC <−1.64 Z-score, GLI-O | ||||||||
| Baseline | 220 | 16 (7.3) | 194 | 11 (5.7) | 0.434 | 0.510 | ||
| Midpoint | 214 | 32 (15.0) | 186 | 29 (15.6) | 0.031 | 0.859 | ||
| Endline | 212 | 23 (10.8) | 187 | 22 (11.8) | 0.083 | 0.773 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Zaman, M.; Fatima, S.; Moran, V.H.; Sinclair, J.K.; Lowe, N.M. The Effectiveness of Zinc-Biofortified Wheat Flour Intake on the Growth and Morbidity Outcomes of Rural Pakistani Children and Adolescent Girls: A Cluster-Randomised, Double-Blind, Controlled Trial. Nutrients 2025, 17, 1137. https://doi.org/10.3390/nu17071137
Gupta S, Zaman M, Fatima S, Moran VH, Sinclair JK, Lowe NM. The Effectiveness of Zinc-Biofortified Wheat Flour Intake on the Growth and Morbidity Outcomes of Rural Pakistani Children and Adolescent Girls: A Cluster-Randomised, Double-Blind, Controlled Trial. Nutrients. 2025; 17(7):1137. https://doi.org/10.3390/nu17071137
Chicago/Turabian StyleGupta, Swarnim, Mukhtiar Zaman, Sadia Fatima, Victoria H. Moran, Jonathan K. Sinclair, and Nicola M. Lowe. 2025. "The Effectiveness of Zinc-Biofortified Wheat Flour Intake on the Growth and Morbidity Outcomes of Rural Pakistani Children and Adolescent Girls: A Cluster-Randomised, Double-Blind, Controlled Trial" Nutrients 17, no. 7: 1137. https://doi.org/10.3390/nu17071137
APA StyleGupta, S., Zaman, M., Fatima, S., Moran, V. H., Sinclair, J. K., & Lowe, N. M. (2025). The Effectiveness of Zinc-Biofortified Wheat Flour Intake on the Growth and Morbidity Outcomes of Rural Pakistani Children and Adolescent Girls: A Cluster-Randomised, Double-Blind, Controlled Trial. Nutrients, 17(7), 1137. https://doi.org/10.3390/nu17071137







