Dietary Modulation of the Enteric Nervous System: From Molecular Mechanisms to Therapeutic Applications
Abstract
1. Introduction
2. Enteric Nervous System
2.1. Anatomy and Structure of the Enteric Nervous System
2.2. Cellular Composition of the ENS
2.2.1. Enteric Neurons
2.2.2. EGCs
2.3. Core Physiological Functions of the ENS
2.3.1. Regulation of GI Motility
2.3.2. Balance of Intestinal Secretion and Absorption
2.3.3. Maintenance of Intestinal Immune Homeostasis
2.3.4. Modulating Gut Microbiota Homeostasis
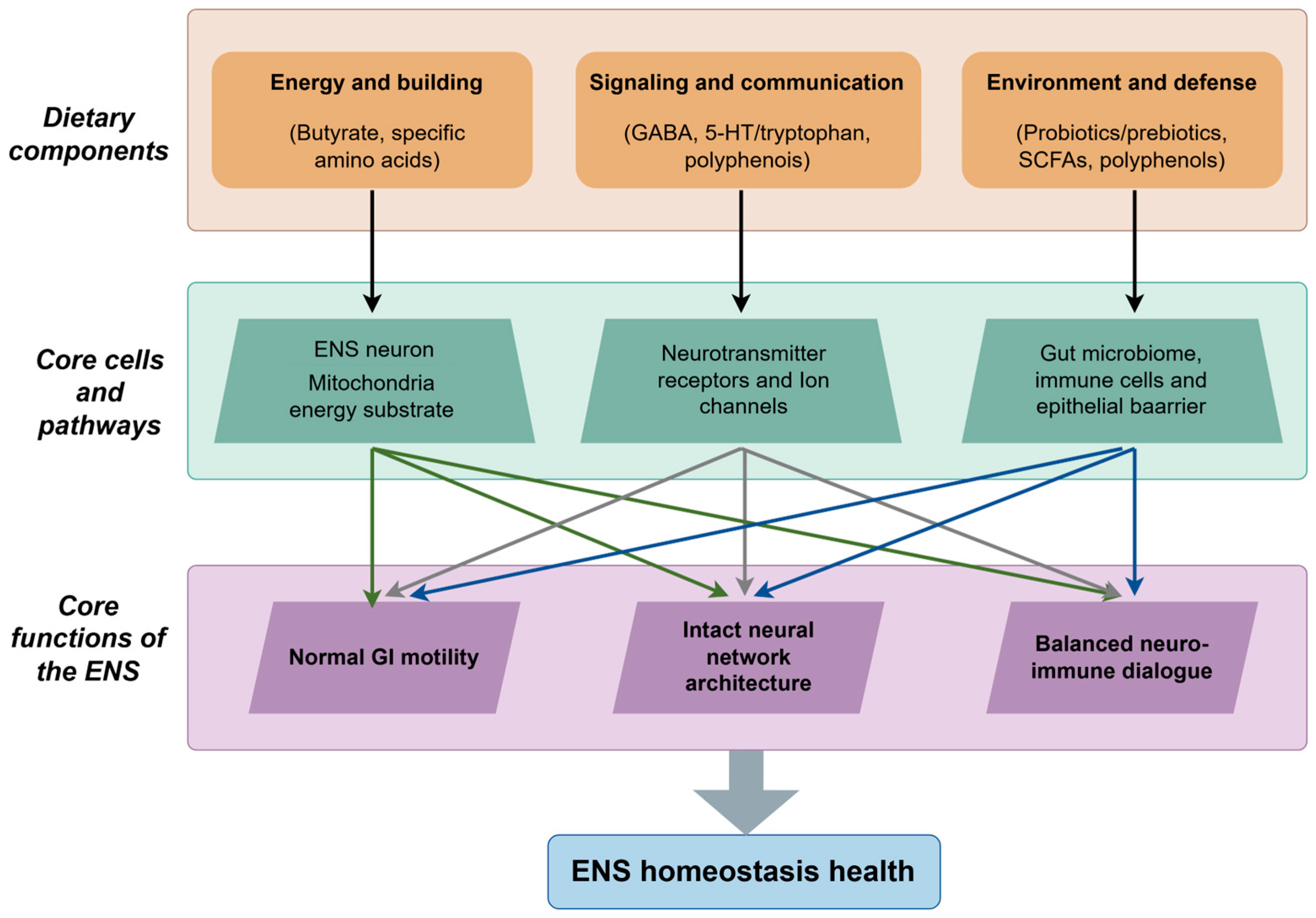
3. Key Dietary Components for ENS Modulation and Their Properties
3.1. Polyphenols
3.2. SCFAs
3.3. Amino Acids and Their Derivatives
3.3.1. Tryptophan
3.3.2. GABA
3.4. Prebiotics and Probiotics
3.4.1. Prebiotics
3.4.2. Probiotics
3.5. Synergistic and Integrated Modulation of the ENS by Dietary Components
4. Modulatory Effects of Dietary Components on ENS Functions
4.1. Regulation of Intestinal Motility
4.1.1. Regulation of Smooth Muscle Contractility
4.1.2. Maintenance of Peristaltic Frequency
4.2. Maintenance of ENS Neuronal Survival and Network Integrity
4.2.1. Provision of Metabolic Energy for Neurons
4.2.2. Inhibition of Inflammatory Damage
4.2.3. Maintenance of Plexus Architecture
4.3. Modulation of Neuro-Immune Interactions
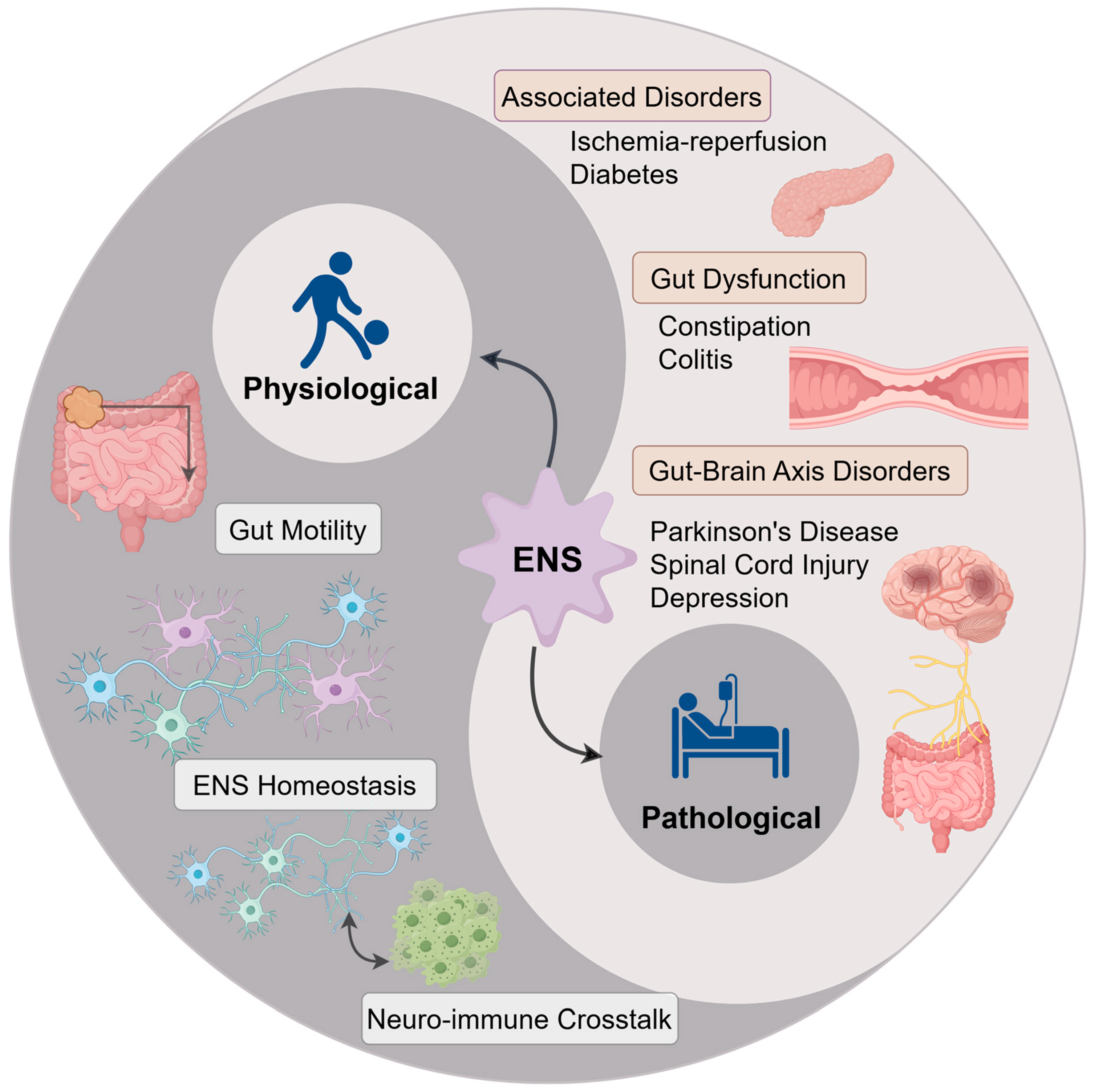
5. Therapeutic Potential of Dietary Components in ENS-Related Disorders
5.1. Gut-Related Disorders
5.1.1. Functional Constipation
5.1.2. Colitis
5.2. Gut–Brain Axis-Related Disorders
5.2.1. PD
5.2.2. Spinal Cord Injury
5.2.3. Depression
5.3. Other Related Disorders
5.3.1. Diabetes
5.3.2. Intestinal Ischemia–Reperfusion (I/R)
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The Enteric Nervous System. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef]
- Kang, Y.-N.; Fung, C.; Vanden Berghe, P. Gut Innervation and Enteric Nervous System Development: A Spatial, Temporal and Molecular Tour de Force. Development 2021, 148, dev182543. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Baumann, P.; Tüscher, O.; Schick, S.; Endres, K. The Aging Enteric Nervous System. Int. J. Mol. Sci. 2023, 24, 9471. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Hotta, R.; Guyer, R.A.; Picard, N.; Rahman, A.A.; Omer, M.; Soos, A.; Szocs, E.; Mueller, J.; Goldstein, A.M.; et al. A Distinct Transcriptome Characterizes Neural Crest-Derived Cells at the Migratory Wavefront during Enteric Nervous System Development. Development 2023, 150, dev201090. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.I.; Heuckeroth, R.O. Enteric Nervous System Development: Migration, Differentiation, and Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G1–G24. [Google Scholar] [CrossRef]
- Rahman, A.A.; Ohkura, T.; Bhave, S.; Pan, W.; Ohishi, K.; Ott, L.; Han, C.; Leavitt, A.; Stavely, R.; Burns, A.J.; et al. Enteric Neural Stem Cell Transplant Restores Gut Motility in Mice with Hirschsprung Disease. JCI Insight 2024, 9, e179755. [Google Scholar] [CrossRef]
- Burns, A.J.; Thapar, N. Neural Stem Cell Therapies for Enteric Nervous System Disorders. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 317–328. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The Role of the Gut Microbiota in Development, Function and Disorders of the Central Nervous System and the Enteric Nervous System. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Joly, A.; Leulier, F.; De Vadder, F. Microbial Modulation of the Development and Physiology of the Enteric Nervous System. Trends Microbiol. 2021, 29, 686–699. [Google Scholar] [CrossRef]
- Almeida, P.P.; Tavares-Gomes, A.L.; Stockler-Pinto, M.B. Relaxing the “Second Brain”: Nutrients and Bioactive Compounds as a Therapeutic and Preventive Strategy to Alleviate Oxidative Stress in the Enteric Nervous System. Nutr. Rev. 2022, 80, 2206–2224. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-Gut Microbiota Interplay and Brain Neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Pang, S.; Ren, Z.; Ding, H.; Chan, P. Short-Chain Fatty Acids Mediate Enteric and Central Nervous System Homeostasis in Parkinson’s Disease: Innovative Therapies and Their Translation. Neural Regen. Res. 2025, 21, 938. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. Microbe-Host Interactions: Influence of the Gut Microbiota on the Enteric Nervous System. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation With Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid. Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.-P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal Biofilms in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef]
- Schneider, K.M.; Kim, J.; Bahnsen, K.; Heuckeroth, R.O.; Thaiss, C.A. Environmental Perception and Control of Gastrointestinal Immunity by the Enteric Nervous System. Trends Mol. Med. 2022, 28, 989–1005. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric Nervous System: Sensory Transduction, Neural Circuits and Gastrointestinal Motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, J.; Gao, X.; Huang, J.; Liu, Q.; Fu, L. In Vivo Imaging of Vagal-Induced Myenteric Plexus Responses in Gastrointestinal Tract with an Optical Window. Nat. Commun. 2024, 15, 8123. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The Bowel and beyond: The Enteric Nervous System in Neurological Disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Sanchini, G.; Vaes, N.; Boesmans, W. Mini-Review: Enteric Glial Cell Heterogeneity: Is It All about the Niche? Neurosci. Lett. 2023, 812, 137396. [Google Scholar] [CrossRef]
- Neckel, P.H. Annotated Translation of Georg Meissner’s First Description of the Submucosal Plexus. Neurogastroenterol. Motil. 2023, 35, e14480. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A. Classification of Human Enteric Neurons. Histochem. Cell Biol. 2021, 156, 95–108. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e23. [Google Scholar] [CrossRef]
- Johnson, C.D.; Barlow-Anacker, A.J.; Pierre, J.F.; Touw, K.; Erickson, C.S.; Furness, J.B.; Epstein, M.L.; Gosain, A. Deletion of Choline Acetyltransferase in Enteric Neurons Results in Postnatal Intestinal Dysmotility and Dysbiosis. FASEB J. 2018, 32, 4744–4752. [Google Scholar] [CrossRef]
- Spencer, N.J.; Keating, D.J. Role of 5-HT in the Enteric Nervous System and Enteroendocrine Cells. Br. J. Pharmacol. 2025, 182, 471–483. [Google Scholar] [CrossRef]
- Galligan, J.J. Colonic 5-HT4 Receptors Are Targets for Novel Prokinetic Drugs. Neurogastroenterol. Motil. 2021, 33, e14125. [Google Scholar] [CrossRef]
- Shajib, M.S.; Baranov, A.; Khan, W.I. Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation. ACS Chem. Neurosci. 2017, 8, 920–931. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From Bugs to Brain: Unravelling the GABA Signalling Networks in the Brain-Gut-Microbiome Axis. Brain 2025, 148, 1479–1506. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Spencer, N.J.; Brookes, S.J.H. The Role of Enteric Inhibitory Neurons in Intestinal Motility. Auton. Neurosci. 2021, 235, 102854. [Google Scholar] [CrossRef]
- Agibalova, T.; Hempel, A.; Maurer, H.C.; Ragab, M.; Ermolova, A.; Wieland, J.; Waldherr Ávila de Melo, C.; Heindl, F.; Giller, M.; Fischer, J.C.; et al. Vasoactive Intestinal Peptide Promotes Secretory Differentiation and Mitigates Radiation-Induced Intestinal Injury. Stem Cell Res. Ther. 2024, 15, 348. [Google Scholar] [CrossRef]
- Tirassa, P.; Schirinzi, T.; Raspa, M.; Ralli, M.; Greco, A.; Polimeni, A.; Possenti, R.; Mercuri, N.B.; Severini, C. What Substance P Might Tell Us about the Prognosis and Mechanism of Parkinson’s Disease? Neurosci. Biobehav. Rev. 2021, 131, 899–911. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal Neuroendocrine Peptides/Amines in Inflammatory Bowel Disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef] [PubMed]
- Budnik, A.F.; Masliukov, P.M. Postnatal Development of the Enteric Neurons Expressing Neuronal Nitric Oxide Synthase. Anat. Rec. 2023, 306, 2276–2291. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric Glial Biology, Intercellular Signalling and Roles in Gastrointestinal Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Gabella, G. Enteric Glia and Enteric Neurons, Associated. Adv. Exp. Med. Biol. 2022, 1383, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Capuano, R.; Sarnelli, G.; Esposito, G. Play in Advance against Neurodegeneration: Exploring Enteric Glial Cells in Gut-Brain Axis during Neurodegenerative Diseases. Expert Rev. Clin. Pharmacol. 2019, 12, 555–564. [Google Scholar] [CrossRef]
- Lefèvre, M.A.; Godefroid, Z.; Soret, R.; Pilon, N. Enteric Glial Cell Diversification Is Influenced by Spatiotemporal Factors and Source of Neural Progenitors in Mice. Front. Neurosci. 2024, 18, 1392703. [Google Scholar] [CrossRef]
- Seguella, L.; McClain, J.L.; Esposito, G.; Gulbransen, B.D. Functional Intraregional and Interregional Heterogeneity between Myenteric Glial Cells of the Colon and Duodenum in Mice. J. Neurosci. 2022, 42, 8694–8708. [Google Scholar] [CrossRef] [PubMed]
- Guyer, R.A.; Stavely, R.; Robertson, K.; Bhave, S.; Mueller, J.L.; Picard, N.M.; Hotta, R.; Kaltschmidt, J.A.; Goldstein, A.M. Single-Cell Multiome Sequencing Clarifies Enteric Glial Diversity and Identifies an Intraganglionic Population Poised for Neurogenesis. Cell Rep. 2023, 42, 112194. [Google Scholar] [CrossRef]
- Baghdadi, M.B.; Kim, T.-H. The Multiple Roles of Enteric Glial Cells in Intestinal Homeostasis and Regeneration. Semin. Cell Dev. Biol. 2023, 150–151, 43–49. [Google Scholar] [CrossRef]
- Kim, M.; Heo, G.; Kim, S.-Y. Neural Signalling of Gut Mechanosensation in Ingestive and Digestive Processes. Nat. Rev. Neurosci. 2022, 23, 135–156. [Google Scholar] [CrossRef]
- Ameku, T.; Beckwith, H.; Blackie, L.; Miguel-Aliaga, I. Food, Microbes, Sex and Old Age: On the Plasticity of Gastrointestinal Innervation. Curr. Opin. Neurobiol. 2020, 62, 83–91. [Google Scholar] [CrossRef]
- Travagli, R.A.; Anselmi, L. Vagal Neurocircuitry and Its Influence on Gastric Motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef]
- Kumral, D.; Zfass, A.M. Gut Movements: A Review of the Physiology of Gastrointestinal Transit. Dig. Dis. Sci. 2018, 63, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Roosen, L.; Boesmans, W.; Dondeyne, M.; Depoortere, I.; Tack, J.; Vanden Berghe, P. Specific Hunger- and Satiety-Induced Tuning of Guinea Pig Enteric Nerve Activity. J. Physiol. 2012, 590, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Cortellino, S.; Quagliariello, V.; Delfanti, G.; Blaževitš, O.; Chiodoni, C.; Maurea, N.; Di Mauro, A.; Tatangelo, F.; Pisati, F.; Shmahala, A.; et al. Fasting Mimicking Diet in Mice Delays Cancer Growth and Reduces Immunotherapy-Associated Cardiovascular and Systemic Side Effects. Nat. Commun. 2023, 14, 5529. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Handb. Exp. Pharmacol. 2017, 239, 1–16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Su, N.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y.; Zhao, F. Microbiota-Gut-Brain Axis in Health and Neurological Disease: Interactions between Gut Microbiota and the Nervous System. J. Cell Mol. Med. 2024, 28, e70099. [Google Scholar] [CrossRef]
- Fung, C.; Venneman, T.; Holland, A.M.; Martens, T.; Alata, M.I.; Hao, M.M.; Alar, C.; Obata, Y.; Tack, J.; Sifrim, A.; et al. Nutrients Activate Distinct Patterns of Small-Intestinal Enteric Neurons. Nature 2025, 644, 1069–1077. [Google Scholar] [CrossRef]
- Petsakou, A.; Liu, Y.; Liu, Y.; Comjean, A.; Hu, Y.; Perrimon, N. Cholinergic Neurons Trigger Epithelial Ca2+ Currents to Heal the Gut. Nature 2023, 623, 122–131. [Google Scholar] [CrossRef] [PubMed]
- van Baarle, L.; Stakenborg, M.; Matteoli, G. Enteric Neuro-Immune Interactions in Intestinal Health and Disease. Semin. Immunol. 2023, 70, 101819. [Google Scholar] [CrossRef]
- Progatzky, F.; Shapiro, M.; Chng, S.H.; Garcia-Cassani, B.; Classon, C.H.; Sevgi, S.; Laddach, A.; Bon-Frauches, A.C.; Lasrado, R.; Rahim, M.; et al. Regulation of Intestinal Immunity and Tissue Repair by Enteric Glia. Nature 2021, 599, 125–130. [Google Scholar] [CrossRef]
- Rahman, A.A.; Stavely, R.; Pan, W.; Ott, L.; Ohishi, K.; Ohkura, T.; Han, C.; Hotta, R.; Goldstein, A.M. Optogenetic Activation of Cholinergic Enteric Neurons Reduces Inflammation in Experimental Colitis. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 907–921. [Google Scholar] [CrossRef]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The Intestinal Neuro-Immune Axis: Crosstalk between Neurons, Immune Cells, and Microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef]
- Becker, L.; Nguyen, L.; Gill, J.; Kulkarni, S.; Pasricha, P.J.; Habtezion, A. Age-Dependent Shift in Macrophage Polarisation Causes Inflammation-Mediated Degeneration of Enteric Nervous System. Gut 2018, 67, 827–836. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Wall, E.S.; Robinson, C.D.; Guillemin, K.; Eisen, J.S. Enteric Nervous System Modulation of Luminal pH Modifies the Microbial Environment to Promote Intestinal Health. PLoS Pathog. 2022, 18, e1009989. [Google Scholar] [CrossRef]
- Rolig, A.S.; Mittge, E.K.; Ganz, J.; Troll, J.V.; Melancon, E.; Wiles, T.J.; Alligood, K.; Stephens, W.Z.; Eisen, J.S.; Guillemin, K. The Enteric Nervous System Promotes Intestinal Health by Constraining Microbiota Composition. PLoS Biol. 2017, 15, e2000689. [Google Scholar] [CrossRef]
- Heymans, C.; de Lange, I.H.; Hütten, M.C.; Lenaerts, K.; de Ruijter, N.J.E.; Kessels, L.C.G.A.; Rademakers, G.; Melotte, V.; Boesmans, W.; Saito, M.; et al. Chronic Intra-Uterine Ureaplasma Parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses. Front. Immunol. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Klotz, M.; Hirschberg, D.L.; Waldura, B.; Schofer, O.; Ehnert, S.; Schwarz, L.K.; Ginneken, C.V.; Schäfer, K.-H. Breast Milk Contains Relevant Neurotrophic Factors and Cytokines for Enteric Nervous System Development. Mol. Nutr. Food Res. 2011, 55, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation-An Overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Benea, S.-N.; Căruntu, C.; Nancoff, A.-S.; Homentcovschi, C.; Bucurica, S. Rewiring the Brain Through the Gut: Insights into Microbiota-Nervous System Interactions. Curr. Issues Mol. Biol. 2025, 47, 489. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Murray, P.J. Tryptophan and Indole Metabolism in Immune Regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-Aminobutyric Acid (GABA): A Comprehensive Review of Dietary Sources, Enrichment Technologies, Processing Effects, Health Benefits, and Its Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Our Mental Health Is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nerves, and Gut Microbiota. Int. J. Mol. Sci. 2023, 25, 38. [Google Scholar] [CrossRef]
- Gao, J.; Azad, M.A.K.; Han, H.; Wan, D.; Li, T. Impact of Prebiotics on Enteric Diseases and Oxidative Stress. Curr. Pharm. Des. 2020, 26, 2630–2641. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Seifi, M.; Brown, J.F.; Mills, J.; Bhandari, P.; Belelli, D.; Lambert, J.J.; Rudolph, U.; Swinny, J.D. Molecular and Functional Diversity of GABA-A Receptors in the Enteric Nervous System of the Mouse Colon. J. Neurosci. 2014, 34, 10361–10378. [Google Scholar] [CrossRef]
- Seifi, M.; Swinny, J.D. Developmental and Age-Dependent Plasticity of GABAA Receptors in the Mouse Colon: Implications in Colonic Motility and Inflammation. Auton. Neurosci. 2019, 221, 102579. [Google Scholar] [CrossRef] [PubMed]
- Suply, E.; de Vries, P.; Soret, R.; Cossais, F.; Neunlist, M. Butyrate Enemas Enhance Both Cholinergic and Nitrergic Phenotype of Myenteric Neurons and Neuromuscular Transmission in Newborn Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1373–G1380. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, B.; Saeedi, B.J.; Alam, A.; Houser, M.; Srinivasan, S.; Tansey, M.; Jones, R.; Nusrat, A.; Neish, A.S. Interactions Between Commensal Bacteria and Enteric Neurons, via FPR1 Induction of ROS, Increase Gastrointestinal Motility in Mice. Gastroenterology 2019, 157, 179–192.e2. [Google Scholar] [CrossRef]
- Legan, T.B.; Lavoie, B.; Norberg, E.; Ley, I.C.; Tack, S.; Tompkins, T.A.; Wargo, M.J.; Mawe, G.M. Tryptophan-Synthesizing Bacteria Enhance Colonic Motility. Neurogastroenterol. Motil. 2023, 35, e14629. [Google Scholar] [CrossRef]
- Wang, L.; Martínez, V.; Kimura, H.; Taché, Y. 5-Hydroxytryptophan Activates Colonic Myenteric Neurons and Propulsive Motor Function through 5-HT4 Receptors in Conscious Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G419–G428. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal Microbiota Shapes Gut Physiology and Regulates Enteric Neurons and Glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Duan, R.; Zhang, Y.; Zheng, H.; Zhang, J.; Zhang, T.; Xu, J.; Li, K.; Pei, F.; et al. Preconception Maternal Gut Dysbiosis Affects Enteric Nervous System Development and Disease Susceptibility in Offspring via the GPR41-GDNF/RET/SOX10 Signaling Pathway. iMeta 2025, 4, e70012. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Karaki, S.-I.; Kubo, Y.; Sugiura, Y.; Kuwahara, A. Fibre-Free Diet Leads to Impairment of Neuronally Mediated Muscle Contractile Response in Rat Distal Colon. Neurogastroenterol. Motil. 2006, 18, 1093–1101. [Google Scholar] [CrossRef]
- Liu, N.; He, J.; Yang, Y.; Wang, Y.; Zhang, L.; Xiao, Z.; Xiong, Z.; Zhong, S.; Xu, Y.; Gu, Y.; et al. Enteric GABAergic Neuron-Derived γ-Aminobutyric Acid Initiates Expression of Igfbp7 to Sustain ILC3 Homeostasis. Nat. Immunol. 2025, 26, 404–415. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota Modulate Sympathetic Neurons via a Gut-Brain Circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Capó, J.D.; Ponti, A.; Faggin, S.; Moro, E.; Caputi, V.; Bresesti, I.; Crema, F.; Giron, M.C.; et al. Sex-Dependent Alteration of the Enteric Neuromuscular Function after Antibiotic-Induced Dysbiosis in Juvenile Mice and Effect of Lactocaseibacillus Rhamnosus GG. Biomed. Pharmacother. 2025, 189, 118263. [Google Scholar] [CrossRef]
- Larsson, S.; Voss, U. Neuroprotective Effects of Vitamin D on High Fat Diet- and Palmitic Acid-Induced Enteric Neuronal Loss in Mice. BMC Gastroenterol. 2018, 18, 175. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N.; et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Afridi, M.I.; Zhang, S.; Wan, Z.; Zhang, X.; Stingelin, L.; Wang, Y.; Tu, H. GABAergic Signaling between Enteric Neurons and Intestinal Smooth Muscle Promotes Innate Immunity and Gut Defense in Caenorhabditis Elegans. Immunity 2023, 56, 1515–1532.e9. [Google Scholar] [CrossRef]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.-Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine Cells Sense Bacterial Tryptophan Catabolites to Activate Enteric and Vagal Neuronal Pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, W.-Q.; Yang, J.-T.; Lin, X.; Liu, H.-M.; Tan, H.-J.; Quan, R.-P.; Long, P.-P.; Shen, H.; Shen, J.; et al. Enteric Nervous System Damage Caused by Abnormal Intestinal Butyrate Metabolism May Lead to Functional Constipation. Front. Microbiol. 2023, 14, 1117905. [Google Scholar] [CrossRef]
- Xia, F.; He, Q.; Wang, S.; He, M.; Fang, P.; Yu, Y. Leven PRO-CR Mediates 5-HT and Gut Microbiota via TPH1 to Improve Slow-Transit Constipation. Mol. Immunol. 2025, 185, 81–91. [Google Scholar] [CrossRef]
- Yilmaz, O.; Okullu, S.O.; Catakci, M.; Elmas, M.A.; Pinheiro, Y.; Arbak, S.; Demir, E.; Schaefer, K.H.; Kolgazi, M. Akkermansia Muciniphila Improves Chronic Colitis-Induced Enteric Neuroinflammation in Mice. Neurogastroenterol. Motil. 2024, 36, e14745. [Google Scholar] [CrossRef]
- Caetano, M.A.F.; Magalhães, H.I.R.; Duarte, J.R.L.; Conceição, L.B.; Castelucci, P. Butyrate Protects Myenteric Neurons Loss in Mice Following Experimental Ulcerative Colitis. Cells 2023, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Lin, Y.-H.; Chang, W.-Y.; Lin, L.-Y.; Chang, C.-F.; Kuo, W.-T.; Tu, C.-H.; Wu, M.-S.; Hsin, L.-W.; Yu, L.C.-H. 5-HT7 Antagonists Confer Analgesia via Suppression of Neurotrophin Overproduction in Submucosal Nerves of Mouse Models with Visceral Hypersensitivity. J. Physiol. 2025, 603, 4723–4745. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef]
- White, A.R.; Werner, C.M.; Holmes, G.M. Diminished Enteric Neuromuscular Transmission in the Distal Colon Following Experimental Spinal Cord Injury. Exp. Neurol. 2020, 331, 113377. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Blackmer-Raynolds, L.; Li, Y.; Kelly, S.D.; Kebede, N.; Williams, A.E.; Chang, J.; Garraway, S.M.; Srinivasan, S.; Sampson, T.R. Diet-Microbiome Interactions Promote Enteric Nervous System Resilience Following Spinal Cord Injury. npj Biofilms Microbiomes 2024, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V.; et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521.e4. [Google Scholar] [CrossRef]
- Sehaber-Sierakowski, C.C.; Vieira-Frez, F.C.; Hermes-Uliana, C.; Martins, H.A.; Bossolani, G.D.P.; Lima, M.M.; Blegniski, F.P.; Guarnier, F.A.; Baracat, M.M.; Perles, J.V.C.M.; et al. Protective Effects of Quercetin-Loaded Microcapsules on the Enteric Nervous System of Diabetic Rats. Auton. Neurosci. 2021, 230, 102759. [Google Scholar] [CrossRef]
- Vieira-Frez, F.C.; Sehaber-Sierakowski, C.C.; Perles, J.V.C.M.; Bossolani, G.D.P.; Verri, W.A.; Nascimento, R.C.D.; Guarnier, F.A.; Bordini, H.P.; Blegniski, F.P.; Martins, H.A.; et al. Anti- and pro-Oxidant Effects of Quercetin Stabilized by Microencapsulation on Interstitial Cells of Cajal, Nitrergic Neurons and M2-like Macrophages in the Jejunum of Diabetic Rats. NeuroToxicology 2020, 77, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.E.B.; Beraldi, E.J.; Borges, S.C.; Natali, M.R.M.; Buttow, N.C. Resveratrol Promotes Neuroprotection and Attenuates Oxidative and Nitrosative Stress in the Small Intestine in Diabetic Rats. Biomed. Pharmacother. 2018, 105, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Wemelle, E.; Laurens, C.; Paquot, A.; Pomie, N.; Carper, D.; Bessac, A.; Orea, X.M.; Fremez, C.; Fontanie, M.; et al. Identification of New Enterosynes Using Prebiotics: Roles of Bioactive Lipids and Mu-Opioid Receptor Signalling in Humans and Mice. Gut 2021, 70, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.C.; da Silva de Souza, A.C.; Beraldi, E.J.; Schneider, L.C.L.; Buttow, N.C. Resveratrol Promotes Myenteric Neuroprotection in the Ileum of Rats after Ischemia-Reperfusion Injury. Life Sci. 2016, 166, 54–59. [Google Scholar] [CrossRef]
- da Silva de Souza, A.C.; Borges, S.C.; Beraldi, E.J.; de Sá-Nakanishi, A.B.; Comar, J.F.; Bracht, A.; Natali, M.R.M.; Buttow, N.C. Resveratrol Reduces Morphologic Changes in the Myenteric Plexus and Oxidative Stress in the Ileum in Rats with Ischemia/Reperfusion Injury. Dig. Dis. Sci. 2015, 60, 3252–3263. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, W.; Wang, H.; Zhao, Y.; Wang, P.; Wang, R.; Sun, Y.; Ren, F.; Li, Y. Dietary Modulation of the Enteric Nervous System: From Molecular Mechanisms to Therapeutic Applications. Nutrients 2025, 17, 3519. https://doi.org/10.3390/nu17223519
Wang X, Zhang W, Wang H, Zhao Y, Wang P, Wang R, Sun Y, Ren F, Li Y. Dietary Modulation of the Enteric Nervous System: From Molecular Mechanisms to Therapeutic Applications. Nutrients. 2025; 17(22):3519. https://doi.org/10.3390/nu17223519
Chicago/Turabian StyleWang, Xintong, Wen Zhang, Huihui Wang, Yuzhen Zhao, Pengjie Wang, Ran Wang, Yanan Sun, Fazheng Ren, and Yixuan Li. 2025. "Dietary Modulation of the Enteric Nervous System: From Molecular Mechanisms to Therapeutic Applications" Nutrients 17, no. 22: 3519. https://doi.org/10.3390/nu17223519
APA StyleWang, X., Zhang, W., Wang, H., Zhao, Y., Wang, P., Wang, R., Sun, Y., Ren, F., & Li, Y. (2025). Dietary Modulation of the Enteric Nervous System: From Molecular Mechanisms to Therapeutic Applications. Nutrients, 17(22), 3519. https://doi.org/10.3390/nu17223519





