Exploring the Role of Plant-Based Nutrition in Polycystic Kidney Disease
Abstract
1. Introduction
1.1. Definition and Prevalence
1.2. Pathophysiology
1.3. Importance of Dietary Management in Chronic Kidney Disease
2. Overview of Plant-Based Diets
- Vegan: excludes all animal products.
- Lacto-Ovo Vegetarian: includes dairy and eggs.
- Pescatarian: includes fish, seafood, dairy, and eggs.
2.1. Benefits Within the Nutritional Context of Plant-Based Diets
2.2. Risks Within Nutritional Context of Plant-Based Diets
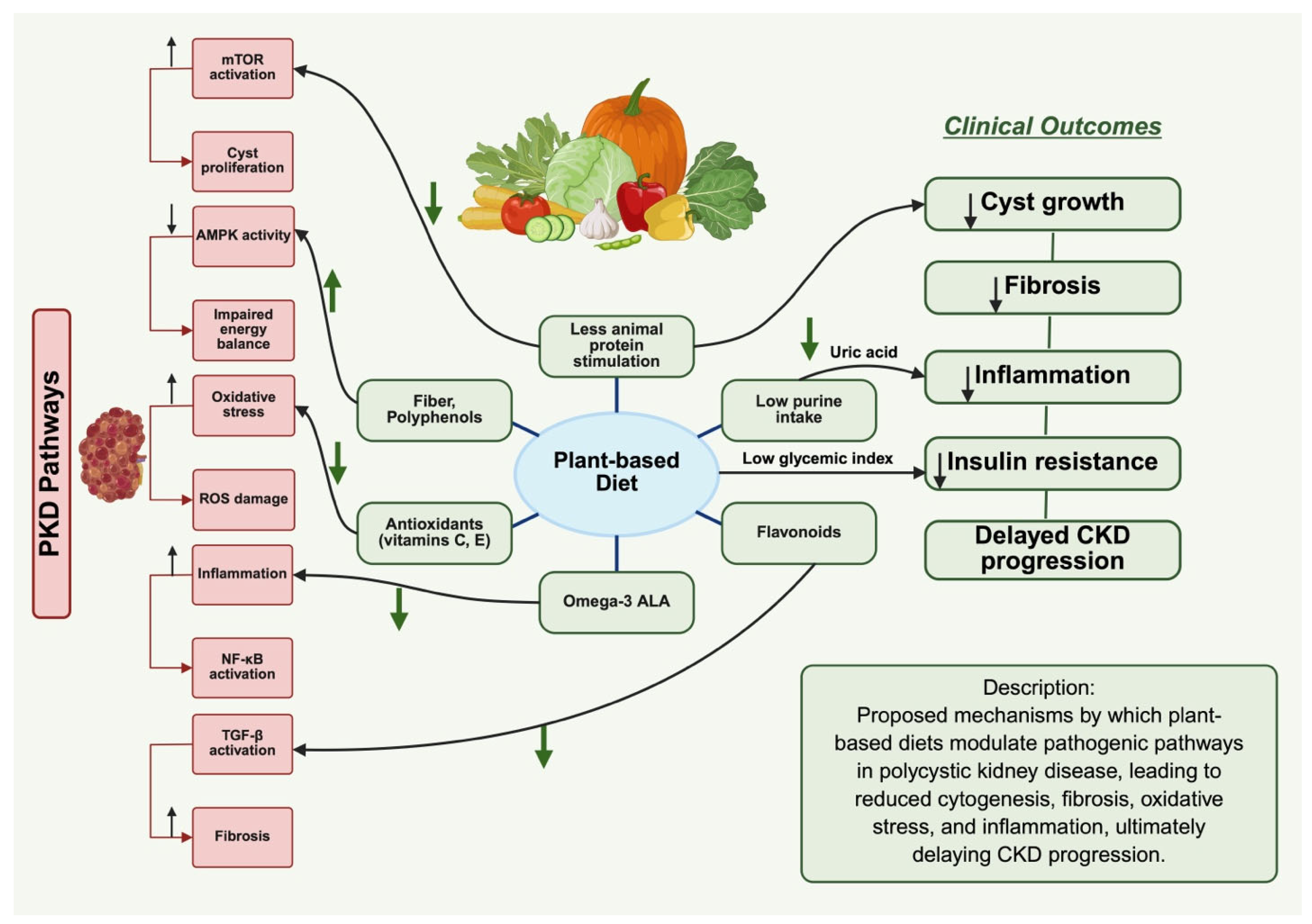
3. Impact of Plant-Based Diets on PKD
3.1. Impact on Primary Molecular Mechanisms in PKD
3.2. Impact on Secondary Pathways and Complications
3.3. Clinical Evidence and Observational Studies
4. Conclusions
Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahboob, M.; Rout, P.; Leslie, S.W.; Bokhari, S.R.A. Autosomal Dominant Polycystic Kidney Disease; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chebib, F.T.; Hanna, C.; Harris, P.C.; Torres, V.E.; Dahl, N.K. Autosomal Dominant Polycystic Kidney Disease: A Review. JAMA 2025, 333, 1708–1719. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Dahl, N.K.; Garimella, P.S.; Chebib, F.T. Clinical Practice Guidelines for Genetic Testing in ADPKD. Kidney News 2025, 17, 12–13. [Google Scholar] [CrossRef]
- Harris, P.C.; Torres, V.E. Polycystic kidney disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Nauli, S.M.; Li, X.; Starremans, P.G.; Luo, Y.; Roberts, K.A.; Zhou, J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell Biol. 2007, 27, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D. Polycystin: New aspects of structure, function, and regulation. J. Am. Soc. Nephrol. 2001, 12, 834–845. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 18–32. [Google Scholar] [CrossRef]
- Parnell, S.C.; Magenheimer, B.S.; Maser, R.L.; Rankin, C.A.; Smine, A.; Okamoto, T.; Calvet, J.P. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem. Biophys. Res. Commun. 1998, 251, 625–631. [Google Scholar] [CrossRef]
- Choi, Y.H.; Suzuki, A.; Hajarnis, S.; Ma, Z.; Chapin, H.C.; Caplan, M.J.; Pontoglio, M.; Somlo, S.; Igarashi, P. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc. Natl. Acad. Sci. USA 2011, 108, 10679–10684. [Google Scholar] [CrossRef]
- Bruen, D.M.; Kingaard, J.J.; Munits, M.; Paimanta, C.S.; Torres, J.A.; Saville, J.; Weimbs, T. Ren. Nu, a Dietary Program for Individuals with Autosomal-Dominant Polycystic Kidney Disease Implementing a Sustainable, Plant-Focused, Kidney-Safe, Ketogenic Approach with Avoidance of Renal Stressors. Kidney Dial. 2022, 2, 183–203. [Google Scholar] [CrossRef]
- Lake, I. Nutritional ketosis is well-tolerated, even in type 1 diabetes: The ZeroFive100 Project; a proof-of-concept study. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Lupsa, B.C.; Kibbey, R.G.; Inzucchi, S.E. Ketones: The double-edged sword of SGLT2 inhibitors? Diabetologia 2023, 66, 23–32. [Google Scholar] [CrossRef] [PubMed]
- James, J.D.; James, O.K. Low-grade metabolic acidosis as a driver of chronic disease: A 21st century public health crisis. Open Heart 2021, 8, e001730. [Google Scholar] [CrossRef]
- Blijdorp, C.J.; Severs, D.; Musterd-Bhaggoe, U.M.; Gansevoort, R.T.; Zietse, R.; Hoorn, E.J. Serum bicarbonate is associated with kidney outcomes in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 2248–2255. [Google Scholar] [CrossRef]
- Torres, J.A.; Rezaei, M.; Broderick, C.; Lin, L.; Wang, X.; Hoppe, B.; Cowley, B.D., Jr.; Savica, V.; Torres, V.E.; Khan, S.; et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J. Clin. Investig. 2019, 129, 4506–4522. [Google Scholar] [CrossRef]
- Yokus, B.; Maccioni, L.; Fu, L.; Haskó, G.; Nagy, L.E.; Gao, B.; Pacher, P. The Link between Alcohol Consumption and Kidney Injury. Am. J. Pathol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Narasaki, Y.; Kalantar-Zadeh, K.; Rhee, C.M.; Brunori, G.; Zarantonello, D. Vegetarian Nutrition in Chronic Kidney Disease. Nutrients 2023, 16, 66. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. Vegetarian Diet in Chronic Kidney Disease-A Friend or Foe. Nutrients 2017, 9, 374. [Google Scholar] [CrossRef]
- Torres, V.E.; Ahn, C.; Barten, T.R.M.; Brosnahan, G.; Cadnapaphornchai, M.A.; Chapman, A.B.; Cornec-Le Gall, E.; Drenth, J.P.H.; Gansevoort, R.T.; Harris, P.C.; et al. KDIGO 2025 clinical practice guideline for the evaluation, management, and treatment of autosomal dominant polycystic kidney disease (ADPKD): Executive summary. Kidney Int. 2025, 107, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Wesson, D.E. Plant-Based Diets across the Spectrum of Kidney Disease. Clin. J. Am. Soc. Nephrol. 2025, 20, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, A.; Cavati, G.; Valenti, R.; Mingiano, C.; Cosso, R.; Gennari, L.; Chiodini, I.; Merlotti, D. The effects of vegetarian diets on bone health: A literature review. Front. Endocrinol. 2022, 13, 899375. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, L.A. Dietary fiber influence on overall health, with an emphasis on CVD, diabetes, obesity, colon cancer, and inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Jones, D.W.; Ferdinand, K.C.; Taler, S.J.; Johnson, H.M.; Shimbo, D.; Abdalla, M.; Altieri, M.M.; Bansal, N.; Bello, N.A.; Bress, A.P.; et al. 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 152, e114–e218. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Shinde, S.; Sinha, V.; Dixit, V.; Dwivedi, M.; Vishwakarma, N.K.; Tiwari, A.K.; Pandey, S.K.; Shukla, D. Dietary Habits and Global Incidence of Colon Cancer. In Colon Cancer Diagnosis and Therapy: Volume 2; Vishvakarma, N.K., Nagaraju, G.P., Shukla, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 15–52. [Google Scholar]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wilcox, S. Does the type of weight loss diet affect who participates in a behavioral weight loss intervention? A comparison of participants for a plant-based diet versus a standard diet trial. Appetite 2014, 73, 156–162. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef]
- Haider, L.M.; Schwingshackl, L.; Hoffmann, G.; Ekmekcioglu, C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1359–1374. [Google Scholar] [CrossRef]
- Sanders, T.A. Plant compared with marine n-3 fatty acid effects on cardiovascular risk factors and outcomes: What is the verdict? Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 453s–458s. [Google Scholar] [CrossRef]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets-A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Nowak, K.L.; Chonchol, M.B.; Bing, K.; Ghanem, A.; Rahbari-Oskoui, F.F.; Dahl, N.K.; Mrug, M. Polycystic Kidney Disease Diet: What is Known and What is Safe. Clin. J. Am. Soc. Nephrol. 2024, 19, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, V.Y.; Sultanova, R.F.; Sudarikova, A.V.; Ilatovskaya, D.V. Insights Into the Molecular Mechanisms of Polycystic Kidney Diseases. Front. Physiol. 2021, 12, 693130. [Google Scholar] [CrossRef] [PubMed]
- Cordido, A.; Vizoso-Gonzalez, M.; Garcia-Gonzalez, M.A. Molecular Pathophysiology of Autosomal Recessive Polycystic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 6523. [Google Scholar] [CrossRef]
- Valim, A.; Carpes, L.S.; Nicoletto, B.B. Effect of vegetarian diets on renal function in patients with chronic kidney disease under non-dialysis treatment: A scoping review. J. Bras. Nefrol. 2022, 44, 395–402. [Google Scholar] [CrossRef]
- Capelli, I.; Lerario, S.; Aiello, V.; Provenzano, M.; Di Costanzo, R.; Squadrani, A.; Vella, A.; Vicennati, V.; Poli, C.; La Manna, G.; et al. Diet and Physical Activity in Adult Dominant Polycystic Kidney Disease: A Review of the Literature. Nutrients 2023, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Kim, H.; Hu, E.A.; Ricardo, A.C.; Mills, K.T.; He, J.; Fischer, M.J.; Pradhan, N.; Tan, T.C.; Navaneethan, S.D.; et al. Adherence to Plant-Based Diets and Risk of CKD Progression and All-Cause Mortality: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2024, 83, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gattone, V., 2nd; Harris, P.C.; Torres, V.E. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J. Am. Soc. Nephrol. 2005, 16, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Zuo, C.D.; Schilling, W.P. Role of cAMP/PKA signaling cascade in vasopressin-induced trafficking of TRPC3 channels in principal cells of the collecting duct. Am. J. Physiol. Renal Physiol. 2010, 298, F988–F996. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C. Mechanisms of Disease: Autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2006, 2, 40–55. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Cupisti, A.; D’Alessandro, C.; Bellasi, A.; Barbera, V.; Di Lullo, L. Nutritional therapy in autosomal dominant polycystic kidney disease. J. Nephrol. 2018, 31, 635–643. [Google Scholar] [CrossRef]
- Pickel, L.; Iliuta, I.A.; Scholey, J.; Pei, Y.; Sung, H.K. Dietary Interventions in Autosomal Dominant Polycystic Kidney Disease. Adv. Nutr. 2022, 13, 652–666. [Google Scholar] [CrossRef]
- Ogborn, M.R.; Bankovic-Calic, N.; Shoesmith, C.; Buist, R.; Peeling, J. Soy protein modification of rat polycystic kidney disease. Am. J. Physiol. 1998, 274, F541–F549. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Raman, A.; Daniel, E.; Metcalf, J.; Reif, G.; Pierucci-Alves, F.; Wallace, D.P. Overexpression of TGF-β1 induces renal fibrosis and accelerates the decline in kidney function in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 319, F1135–F1148. [Google Scholar] [CrossRef]
- Wang, M.C. Natural plant resource flavonoids as potential therapeutic drugs for pulmonary fibrosis. Heliyon 2023, 9, e19308. [Google Scholar] [CrossRef]
- Brosnahan, G.M.; You, Z.; Wang, W.; Gitomer, B.Y.; Chonchol, M. Serum Uric Acid and Progression of Autosomal Dominant Polycystic Kidney Disease: Results from the HALT PKD Trials. Curr. Hypertens. Rev. 2021, 17, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Kassianos, A.J.; Hoy, W.E.; Alam, A.K.; Healy, H.G.; Gobe, G.C. Promoting Plant-Based Therapies for Chronic Kidney Disease. J. Evid. Based Integr. Med. 2022, 27, 2515690x221079688. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Han, M.; Ryu, H.; Kang, E.; Kim, M.; Ahn, C.; Yang, S.J.; Oh, K.H. Compliance with a Healthful Plant-Based Diet Is Associated with Kidney Function in Patients with Autosomal Dominant Polycystic Kidney Disease. Nutrients 2024, 16, 2749. [Google Scholar] [CrossRef]
- Nowak, K.L.; Hopp, K. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin. J. Am. Soc. Nephrol. 2020, 15, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.G.; Selvaraj, A.; Thomas, G. mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007, 13, 252–259. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Jagannathan, R.; Gutekunst, L.; Kalantar-Zadeh, K.; Sevick, M.A. Examining the Proportion of Dietary Phosphorus From Plants, Animals, and Food Additives Excreted in Urine. J. Ren. Nutr. 2017, 27, 78–83. [Google Scholar] [CrossRef]
- Torreggiani, M.; Avesani, C.M.; Contzen, B.; Cupisti, A.; Czaja-Stolc, S.; D’Alessandro, C.; Garneata, L.; Gutierrez, A.; Lippi, F.; Mocanu, C.A.; et al. Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease. Nutrients 2025, 17, 2002. [Google Scholar] [CrossRef]
- Zixin, Y.; Lulu, C.; Xiangchang, Z.; Qing, F.; Binjie, Z.; Chunyang, L.; Tai, R.; Dongsheng, O. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef]
- Wang, K.; Zelnick, L.R.; Chen, Y.; Hoofnagle, A.N.; Watnick, T.; Seliger, S.; Kestenbaum, B. Alterations of Proximal Tubular Secretion in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 80–88. [Google Scholar] [CrossRef]
- Gao, J.; Yu, X. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Role in Cystogenesis and Novel Therapeutic Approaches. Biomedicines 2025, 13, 1596. [Google Scholar] [CrossRef]
- Oehm, S.; Steinke, K.; Schmidt, J.; Arjune, S.; Todorova, P.; Heinrich Lindemann, C.; Wostmann, F.; Meyer, F.; Siedek, F.; Weimbs, T.; et al. RESET-PKD: A pilot trial on short-term ketogenic interventions in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 1623–1635. [Google Scholar] [CrossRef]
- Cukoski, S.; Lindemann, C.H.; Arjune, S.; Todorova, P.; Brecht, T.; Kühn, A.; Oehm, S.; Strubl, S.; Becker, I.; Kämmerer, U.; et al. Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD—A randomized controlled trial. Cell Rep. Med. 2023, 4, 101283. [Google Scholar] [CrossRef]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Del Carmen Fernández-Fígares Jiménez, M.; López-Moreno, M. Ultra-processed Plant Foods: Are They Worse than their Unprocessed Animal-Based Counterparts? Curr. Nutr. Rep. 2025, 14, 115. [Google Scholar] [CrossRef]
| Model/Study | Intervention | Findings | Mechanism/Pathway | Reference |
|---|---|---|---|---|
| ARPKD animal models | Genetic PKHD1 mutations | Elevated cAMP promotes cystogenesis | V2R hyperactivity → ↑cAMP → activation of MAPK and mTOR pathways | Cordido et al. [41] 2021; Wang et al. [45] 2005 |
| PCK rat model | V2 receptor antagonists (OPC-31260, OPC-41061) | Reduced cyst development and preserved kidney architecture | Blockade of vasopressin-V2R signaling → ↓cAMP | Wang et al. [45] 2005 |
| Mouse PKD model | Induced microcrystal deposition | Tubule dilation accelerated cyst growth | Crystal deposition promotes tubular injury and cystogenesis | Torres et al. [18] 2019 |
| Rat PKD model | Soy protein diet vs. animal protein | Attenuated cyst growth | Modulation of growth signaling; anti-inflammatory/antioxidant effects | Ogborn et al. [50] 1998 |
| Transgenic mouse model | TGF-β1 overexpression | Increased kidney fibrosis and accelerated functional decline | TGF-β–mediated extracellular matrix deposition and scarring | Zhang et al. [51] 2020 |
| Fibrosis models | Plant flavonoids | Reduced fibrosis | Inhibition of TGF-β signaling; anti-inflammatory properties | Wang [52] 2023 |
| Animal CKD models (gut–kidney axis) | Meat-based vs. plant-based diets | Meat diet: ↑TMAO, uremic toxins, inflammation; Plant diet: ↑SCFAs, improved gut barrier | Modulation of microbiota-derived metabolites; ↓TNF-α, ↓fibrosis | Zixin et al. [61] 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmad, A.; Ebrahimi, N.; Chebib, F.T.; Garimella, P.S.; Bruen, D.; Abdipour, A.; Norouzi, S. Exploring the Role of Plant-Based Nutrition in Polycystic Kidney Disease. Nutrients 2025, 17, 3518. https://doi.org/10.3390/nu17223518
Sarmad A, Ebrahimi N, Chebib FT, Garimella PS, Bruen D, Abdipour A, Norouzi S. Exploring the Role of Plant-Based Nutrition in Polycystic Kidney Disease. Nutrients. 2025; 17(22):3518. https://doi.org/10.3390/nu17223518
Chicago/Turabian StyleSarmad, Ahmad, Niloufar Ebrahimi, Fouad T. Chebib, Pranav S. Garimella, Diana Bruen, Amir Abdipour, and Sayna Norouzi. 2025. "Exploring the Role of Plant-Based Nutrition in Polycystic Kidney Disease" Nutrients 17, no. 22: 3518. https://doi.org/10.3390/nu17223518
APA StyleSarmad, A., Ebrahimi, N., Chebib, F. T., Garimella, P. S., Bruen, D., Abdipour, A., & Norouzi, S. (2025). Exploring the Role of Plant-Based Nutrition in Polycystic Kidney Disease. Nutrients, 17(22), 3518. https://doi.org/10.3390/nu17223518







