Developmental Regulation of the Murine Selenoproteome Across Embryonic and Postnatal Stages: Implications for Human Nutrition and Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Tissue Collection and Processing
2.3. RNA Extraction and Quality Assessment
2.4. cDNA Synthesis
2.5. Quantitative Real-Time PCR (qPCR) Analysis
2.6. Data Analysis and Normalization
2.7. Statistical Analysis
3. Results
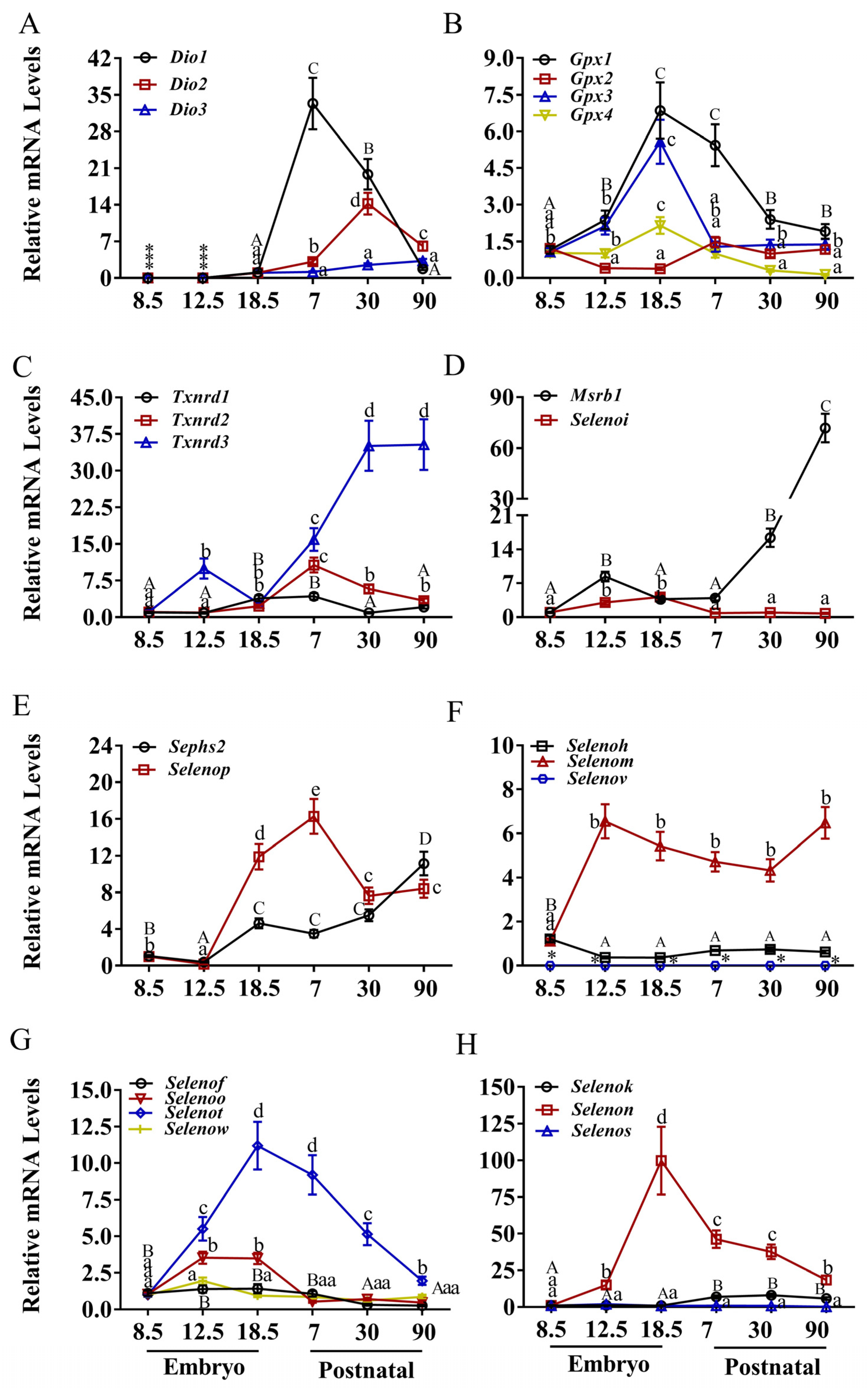
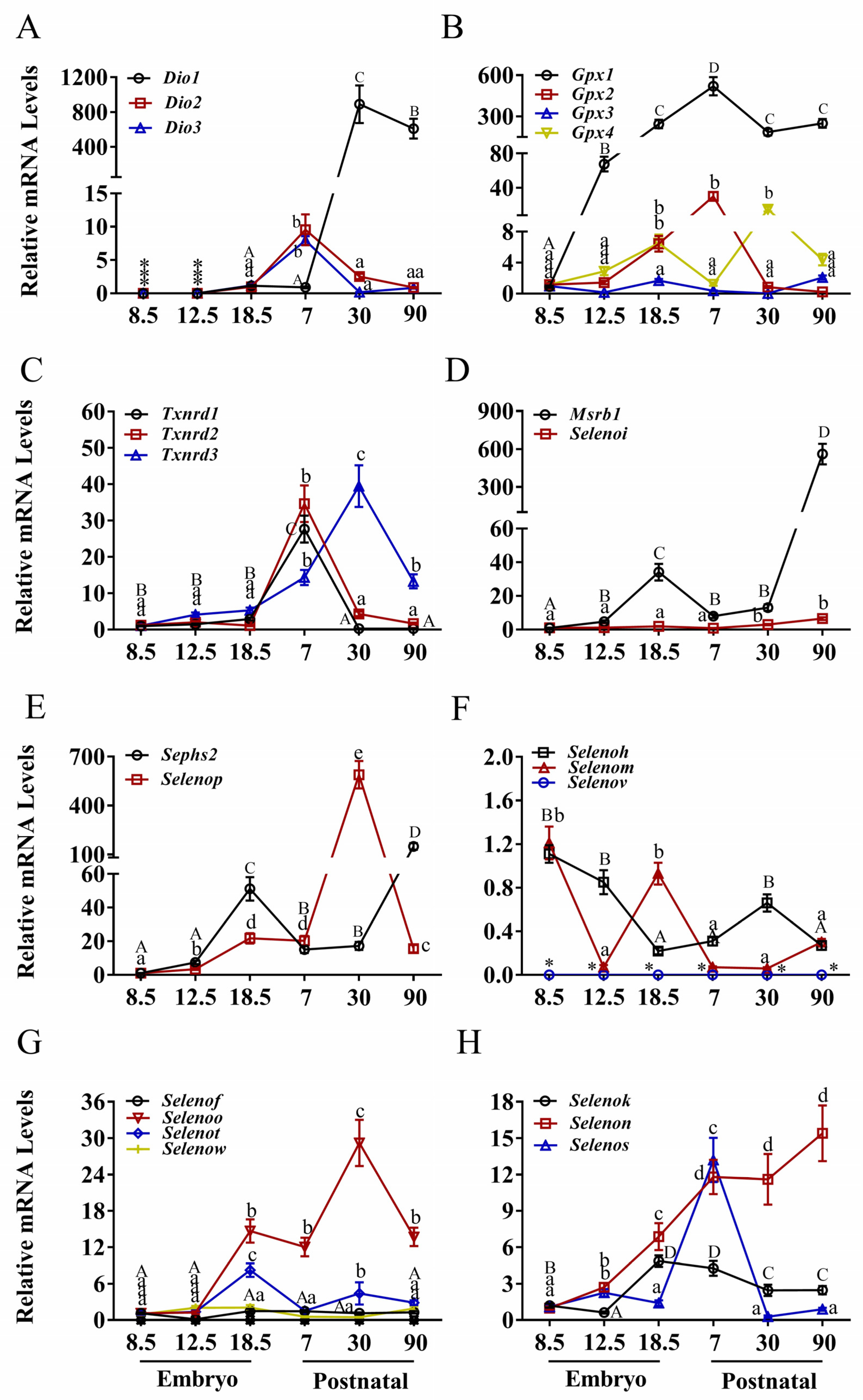
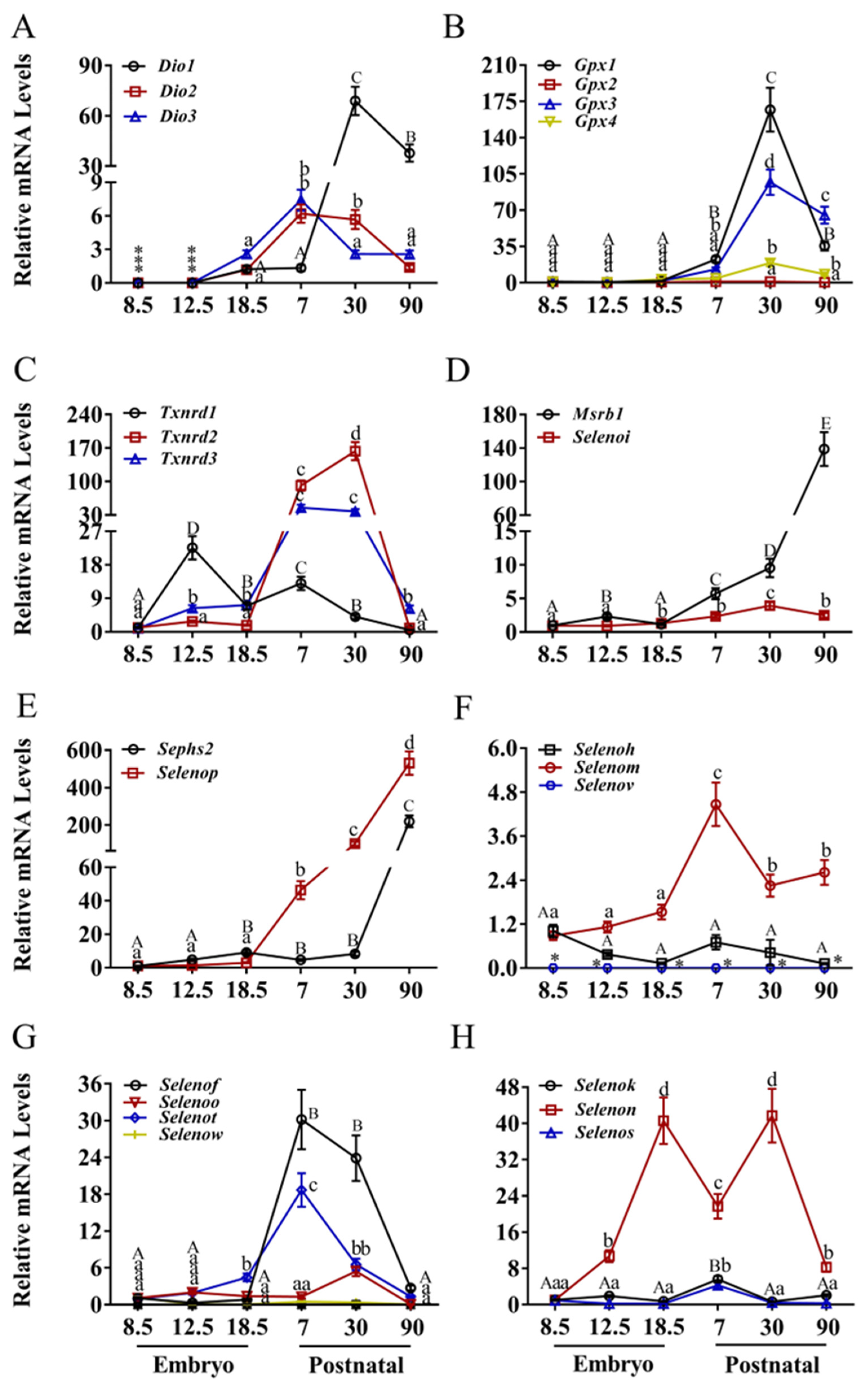
3.1. Expression Patterns of Selenoprotein Genes in the Heart
3.2. Expression Patterns of Selenoprotein Genes in the Brain
3.3. Expression Patterns of Selenoprotein Genes in the Liver
3.4. Expression Patterns of Selenoprotein Genes in the Kidney
4. Discussion
4.1. Expression Dynamics Across Gene Families and Tissues
4.2. Expression Characteristics of Selenoproteins Essential for Embryonic Survival
4.3. Developmental Regulation of Selenoprotein Biosynthesis Genes
4.4. Conservation and Divergence of Expression Across Tissues
4.5. Implications for Tissue-Specific Vulnerability to Selenium Deficiency
4.6. Metabolic Transitions and Corresponding Expression Changes
4.7. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mu, J.; Wang, Y.; Wang, M.; Zhang, D.; Liu, M. Identification of Reliable Reference Genes for Gene Expression Studies in Mouse Models under Microplastics Stress. Ecotoxicol. Environ. Saf. 2023, 252, 114569. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.K. Trace Metals and Animal Health: Interplay of the Gut Microbiota with Iron, Manganese, Zinc, and Copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Li, D.; Tang, H.; Wei, P.; Zheng, J.; Daniel, C.R.; Hassan, M.M. Vitamin C and Vitamin E Mitigate the Risk of Pancreatic Ductal Adenocarcinoma from Meat-Derived Mutagen Exposure in Adults in a Case-Control Study. J. Nutr. 2019, 149, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and Impact of Selenium on Human Health, A Review. Molecules 2025, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The Role of Selenium in Type-2 Diabetes Mellitus and Its Metabolic Comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Staneviciene, I.; Sulinskiene, J.; Sadauskiene, I.; Liekis, A.; Ruzgaite, A.; Naginiene, R.; Baranauskiene, D.; Simakauskiene, V.; Krusnauskas, R.; Viezeliene, D. Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice. Antioxidants 2022, 11, 1216. [Google Scholar] [CrossRef]
- Reeves, M.A.; Hoffmann, P.R. The Human Selenoproteome: Recent Insights into Functions and Regulation. Cell. Mol. Life Sci. 2009, 66, 2457–2478. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Santesmasses, D.; Lee, B.J.; Gladyshev, V.N.; Hatfield, D.L. Historical Roles of Selenium and Selenoproteins in Health and Development: The Good, the Bad and the Ugly. Int. J. Mol. Sci. 2022, 23, 5. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary Selenium Across Species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Chen, S.; Yin, H.; Tammela, T.; Papagiannakopoulos, T.; Joshi, N.S.; Cai, W.; Yang, G.; Bronson, R.; Crowley, D.G.; et al. CRISPR-Mediated Direct Mutation of Cancer Genes in the Mouse Liver. Nature 2014, 514, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A. From selenium to selenoproteins: Synthesis, identity, and their role in hu-man health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Avery, J.C.; Yamazaki, Y.; Hoffmann, F.K.W.; Folgelgren, B.; Hoffmann, P.R. Selenoprotein I Is Essential for Murine Embryogenesis. Arch. Biochem. Biophys. 2020, 689, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, J.H.; Zhang, X.; Cheng, W.H. The Thioredoxin-Like Family of Selenoproteins: Implications in Aging and Age-Related Degeneration. Biol. Trace Elem. Res. 2019, 188, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Boukhzar, L.; Hamieh, A.; Cartier, D.; Tanguy, Y.; Alsharif, I.; Castex, M.; Arabo, A.; Hajji, S.E.; Bonnet, J.J.; Errami, M.; et al. Selenoprotein T Exerts an Essential Oxidoreductase Activity That Protects Dopaminergic Neurons in Mouse Models of Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 557–574. [Google Scholar] [CrossRef]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential Role for Mitochondrial Thioredoxin Reductase in Hematopoiesis, Heart Development, and Heart Function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef]
- Imai, H.; Hirao, F.; Sakamoto, T.; Sekine, K.; Mizukura, Y.; Saito, M.; Kitamoto, T.; Hayasaka, M.; Hanaoka, K.; Nakagawa, Y. Early Embryonic Lethality Caused by Targeted Disruption of the Mouse PHGPx Gene. Biochem. Biophys. Res. Commun. 2003, 305, 278–286. [Google Scholar] [CrossRef]
- Bondareva, A.A.; Capecchi, M.R.; Iverson, S.V.; Li, Y.; Lopez, N.I.; Lucas, O.; Merrill, G.F.; Prigge, J.R.; Siders, A.M.; Wakamiya, M.; et al. Effects of Thioredoxin Reductase-1 Deletion on Embryogenesis and Transcriptome. Free Radic. Biol. Med. 2007, 43, 911–923. [Google Scholar] [CrossRef]
- Chen, L.L.; Huang, J.Q.; Xiao, Y.; Wu, Y.Y.; Ren, F.Z.; Lei, X.G. Knockout of Selenoprotein v Affects Regulation of Selenoprotein Expression by Dietary Selenium and Fat Intakes in Mice. J. Nutr. 2020, 150, 483–491. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, W.; Chen, L.L.; Huang, J.Q.; Lei, X.G. Selenoprotein V Protects against Endoplasmic Reticulum Stress and Oxidative Injury Induced by Pro-Oxidants. Free Radic. Biol. Med. 2020, 160, 670–679. [Google Scholar] [CrossRef]
- Theiler, K. The House Mouse: Atlas of Embryonic Development; Springer: New York, NY, USA, 1989. [Google Scholar]
- Virágh, S.; Challice, C.E. The Development of the Conduction System in the Mouse Embryo Heart. II. Histogenesis of the Atrioventricular Node and Bundle. Dev. Biol. 1977, 56, 397–411. [Google Scholar] [CrossRef]
- Seale, L.A.; Ha, H.Y.; Hashimoto, A.C.; Berry, M.J. Relationship between Selenoprotein P and Selenocysteine Lyase: Insights into Selenium Metabolism. Free Radic. Biol. Med. 2018, 127, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Yamamoto, M.; Lin, Y.; Hogg, N.; Akiyama, H.; Behringer, R.R.; Yamazaki, Y. Male Differentiation of Germ Cells Induced by Embryonic Age-Specific Sertoli Cells in Mice. Biol. Reprod. 2012, 86, 112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Yokoyama, T.; Ng, D.; Ulu, F.; Yamazaki, Y. Retinoic Acid-Stimulated ERK1/2 Pathway Regulates Meiotic Initiation in Cultured Fetal Germ Cells. PLoS ONE 2019, 14, e0224628. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, X.; Liu, Y.; Luan, Y.; Wang, L.; Zhao, L.; Zhang, J.; Tian, Y.; Lu, H.; Chen, X.; et al. Activation of Metabotropic Glutamate Receptor 4 Regulates Proliferation and Neural Differentiation in Neural Stem/Progenitor Cells of the Rat Subventricular Zone and Increases Phosphatide and Tensin Homolog Protein Expression. J. Neurochem. 2021, 156, 465–480. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Hoffmann, P.R.; Schomburg, L. Editorial: Selenium and Selenoproteins in Brain Development, Function, and Disease. Front. Neurosci. 2022, 15, 2021–2022. [Google Scholar] [CrossRef]
- Pothion, H.; Jehan, C.; Tostivint, H.; Cartier, D.; Bucharles, C.; Falluel-Morel, A.; Boukhzar, L.; Anouar, Y.; Lihrmann, I. Selenoprotein T: An Essential Oxidoreductase Serving as a Guardian of Endoplasmic Reticulum Homeostasis. Antioxid. Redox Signal. 2020, 33, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Kuria, A.; Tian, H.; Li, M.; Wang, Y.; Aaseth, J.O.; Zang, J.; Cao, Y. Selenium Status in the Body and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 3616–3625. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- Jehan, C.; Cartier, D.; Bucharles, C.; Anouar, Y.; Lihrmann, I. Emerging Roles of ER-Resident Selenoproteins in Brain Physiology and Physiopathology. Redox Biol. 2022, 55, 102412. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in Thyroid Disorders—Essential Knowledge for Clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and Selenium Co-Supplementation, and the Effects on Clinical, Metabolic and Genetic Status in Alzheimer’s Disease: A Randomized, Double-Blind, Controlled Trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]

| Genes | Oligonucleotide (5′–3′) | Oligonucleotide (5′–3′) |
|---|---|---|
| β-actin | CTATTGGCAACGAGCGGT | GGTCTTTACGGATGTCAACG |
| GAPDH | GGTGCTAAGCGTGTTATCATCTCA | CATGGTTGACACCCATCACAA |
| Dio1 | GGAACCATAGGCATTGGAAA | AGTGCCAGAGAGCCAGATTC |
| Dio2 | GTGCTGATGTGTTGTTCCTGCCAA | TACACCCTTCACTCAGCACCCAAA |
| Dio3 | AGCGCAGCGAGAGTACTACAACAA | ACATGATGGTGCCACTCTGGATGA |
| Gpx1 | GGTTCGAGCCCAATTTTACA | CCCACCAGGAACTTCTCAAA |
| Gpx2 | GCATGGCTTACATTGCCAAGTCGT | AGCCCTGCCTCTGAACGTATTGAA |
| Gpx3 | GATGTGAACGGGGAGAAAGA | CCCACCAGGAACTTCTCAAA |
| Gpx4 | CTCCATGCACGAATTCTCAG | ACGTCAGTTTTGCCTCATTG |
| Msrb1 | ACAGTTGTTGCCCCATTAGC | GGAGTGGGTCTCAGCTTCAG |
| Selenof | CTCACCAGTGAAACGCTTTG | TCAAAGAGCACACAGCAAGG |
| Selenoh | GCGAGATTTGAACTTTGCATC | TTGTCCACCGTCTCCATAGG |
| Selenoi | ATAGTGACTGCGGTTGTGGGAGTT | TGGCTTCATAGACGGACTTGTGCT |
| Selenok | GCTGGTGGATGAGGAAGGTA | CTCATTCATCTGTGGGGACA |
| Selenom | TTTGTCACCGAGGACATTCA | TGTACCAGCGCATTGATCTC |
| Selenon | TGACTGCCATCAGCGATTCCTACT | GTGATTGGGCACAAACAGCCTGAA |
| Selenoo | TGCACAGAAAGCCATTGAAG | GGAAGATTGCTCCTCAGTGC |
| Selenop | GCAATTGCTTGACAGTGTGC | TTCATGGGCTGATTTTGTCA |
| Selenos | GCCTTACGCACACTTTCACA | GTGGCCTAATGGCAATGTCT |
| Selenot | TGTGGCAACAGAAAGGGATT | CAGGTGGCATCAACATCAAG |
| Selenov | ACCCTGGAGCATCAATTCCCAAAC | ACCCACAAGTTTCTTCAGACCGGA |
| Selenow | CCCAAGTACCTCCAGCTCAA | GCCATCACCTCTCTTCTTGG |
| Sephs2 | TGCCAATGTGCTCAGTGACCTCTA | TTCACTCATGCTCTGGCTCACACT |
| Txnrd1 | TTCGACCTGATCATCATTGG | CCACATTCACACACGTTCCT |
| Txnrd2 | CCCTAGAGTGTGCTGGCTTC | AAGCATGATCCTCCCAAGTG |
| Txnrd3 | CCTTTCCCAGTTGCTAGTGC | GTGCTACACTCTGGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-S.; Li, T.; Wei, C.-J.; Cui, L.-Y. Developmental Regulation of the Murine Selenoproteome Across Embryonic and Postnatal Stages: Implications for Human Nutrition and Health. Nutrients 2025, 17, 3200. https://doi.org/10.3390/nu17203200
Wang S-S, Li T, Wei C-J, Cui L-Y. Developmental Regulation of the Murine Selenoproteome Across Embryonic and Postnatal Stages: Implications for Human Nutrition and Health. Nutrients. 2025; 17(20):3200. https://doi.org/10.3390/nu17203200
Chicago/Turabian StyleWang, Shan-Shan, Tong Li, Cheng-Jia Wei, and Lan-Yu Cui. 2025. "Developmental Regulation of the Murine Selenoproteome Across Embryonic and Postnatal Stages: Implications for Human Nutrition and Health" Nutrients 17, no. 20: 3200. https://doi.org/10.3390/nu17203200
APA StyleWang, S.-S., Li, T., Wei, C.-J., & Cui, L.-Y. (2025). Developmental Regulation of the Murine Selenoproteome Across Embryonic and Postnatal Stages: Implications for Human Nutrition and Health. Nutrients, 17(20), 3200. https://doi.org/10.3390/nu17203200






