Prevalence and Impact of Zinc Deficiency on Clinical Outcomes in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Outcomes
2.3. Statistical Analysis
3. Results
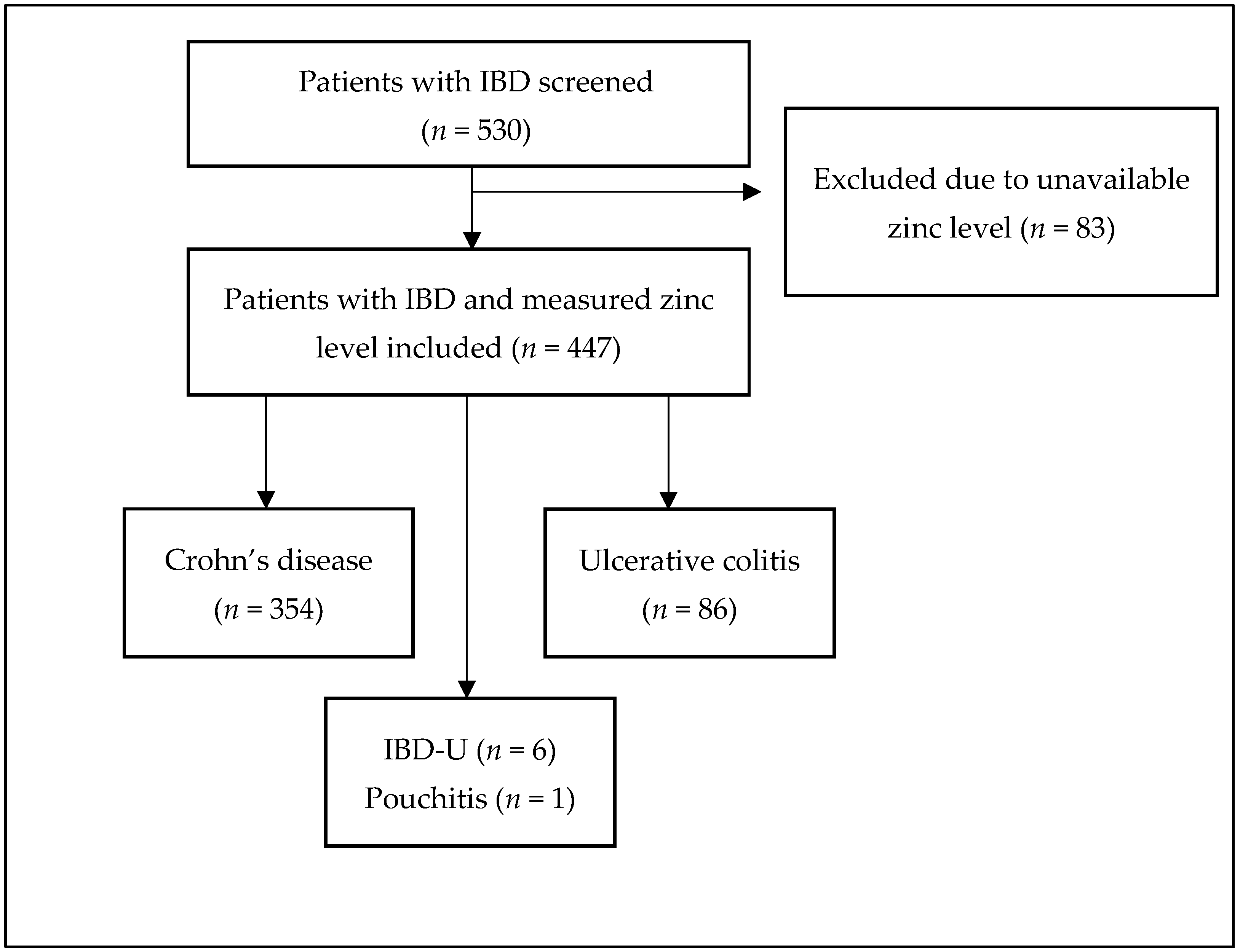
3.1. Study Population
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. Reply. N. Engl. J. Med. 2021, 384, 1378. [Google Scholar] [CrossRef]
- Krugliak Cleveland, N.; Torres, J.; Rubin, D.T. What Does Disease Progression Look Like in Ulcerative Colitis, and How Might It Be Prevented? Gastroenterology 2022, 162, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Vigano, C.; Palermo, A.; Mulinacci, G.; Pirola, L.; Losco, A.; Meucci, G.; Saibeni, S.; Pastorelli, L.; Amato, A.; Gatti, M.; et al. Prevalence of Disease-Related Malnutrition and Micronutrient Deficit in Patients with Inflammatory Bowel Disease: A Multicentric Cross-Sectional Study by the GSMII (Inflammatory Bowel Disease Study Group). Inflamm. Bowel Dis. 2024, 30, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, X.; Ouyang, C.; Wang, D.; Zhang, X.; Liang, J.; Zhu, W.; Cao, Q. Prevalence of Malnutrition, Its Risk Factors, and the Use of Nutrition Support in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, S59–S66. [Google Scholar] [CrossRef]
- Viser, A.C.; Cooke, A.R.; Herfarth, H.H.; Anderson, C.; Proch, C.; Peery, A.F. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Adv. 2024, 3, 181–183. [Google Scholar] [CrossRef]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef]
- Kiouri, D.P.; Tsoupra, E.; Peana, M.; Perlepes, S.P.; Stefanidou, M.E.; Chasapis, C.T. Multifunctional role of zinc in human health: An update. EXCLI J. 2023, 22, 809–827. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447s–463s. [Google Scholar] [CrossRef]
- Zupo, R.; Sila, A.; Castellana, F.; Bringiotti, R.; Curlo, M.; De Pergola, G.; De Nucci, S.; Giannelli, G.; Mastronardi, M.; Sardone, R. Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4052. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Molokhia, M.M.; Shields, R.; Turnberg, L.A. Zinc absorption in Crohn’s disease. Gut 1980, 21, 387–391. [Google Scholar] [CrossRef]
- Griffin, I.J.; Kim, S.C.; Hicks, P.D.; Liang, L.K.; Abrams, S.A. Zinc metabolism in adolescents with Crohn’s disease. Pediatr. Res. 2004, 56, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Higashimura, Y.; Takagi, T.; Naito, Y.; Uchiyama, K.; Mizushima, K.; Tanaka, M.; Hamaguchi, M.; Itoh, Y. Zinc Deficiency Activates the IL-23/Th17 Axis to Aggravate Experimental Colitis in Mice. J. Crohns Colitis 2020, 14, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Mechie, N.C.; Mavropoulou, E.; Ellenrieder, V.; Petzold, G.; Kunsch, S.; Neesse, A.; Amanzada, A. Serum vitamin D but not zinc levels are associated with different disease activity status in patients with inflammatory bowel disease. Medicine 2019, 98, e15172. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Adler, J.; Chachu, K.A.; Cohen, B.L.; Singh, S.; on behalf of the AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023, 165, 1367–1399. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Adler, J.; Chachu, K.A.; on behalf of the AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- Cole, C.R.; Grant, F.K.; Swaby-Ellis, E.D.; Smith, J.L.; Jacques, A.; Northrop-Clewes, C.A.; Caldwell, K.L.; Pfeiffer, C.M.; Ziegler, T.R. Zinc and iron deficiency and their interrelations in low-income African American and Hispanic children in Atlanta. Am. J. Clin. Nutr. 2010, 91, 1027–1034. [Google Scholar] [CrossRef]
- Brownson, E.; Saunders, J.; Jatkowska, A.; White, B.; Gerasimidis, K.; Seenan, J.P.; Macdonald, J. Micronutrient Status and Prediction of Disease Outcome in Adults With Inflammatory Bowel Disease Receiving Biologic Therapy. Inflamm. Bowel Dis. 2024, 30, 1233–1240. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.L.; Manning, L.; Kohler, D.; Ungaro, R.; Sands, B.; Raman, M. Micronutrients and Their Role in Inflammatory Bowel Disease: Function, Assessment, Supplementation, and Impact on Clinical Outcomes Including Muscle Health. Inflamm. Bowel Dis. 2023, 29, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Furukawa, S.; Katsurada, T.; Otagiri, S.; Yamanashi, K.; Nagashima, K.; Onishi, R.; Yagisawa, K.; Nishimura, H.; Ito, T.; et al. Effectiveness of administering zinc acetate hydrate to patients with inflammatory bowel disease and zinc deficiency: A retrospective observational two-center study. Intest. Res. 2022, 20, 78–89. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Chan, A.T. Zinc intake and risk of Crohn’s disease and ulcerative colitis: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1995–2005. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Y.; Zhong, R.; Yang, Y.; Yan, F.; Liang, N.; Yuan, S. Zinc and Inflammatory Bowel Disease: From Clinical Study to Animal Experiment. Biol. Trace Elem. Res. 2025, 203, 624–634. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Wang, B.; Cai, C.; Yang, X.; Chai, Z.; Feng, W. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci. Rep. 2017, 7, 43126. [Google Scholar] [CrossRef]
- McMillan, E.M.; Rowe, D.J. Clinical significance of diurnal variation in the estimation of plasma zinc. Clin. Exp. Dermatol. 1982, 7, 629–632. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Age (years), median (IQR) | 29 (22–38) |
| Male, n (%) | 243 (54.4) |
| Disease duration (years), median (IQR) | 5.5 (2–12) |
| Follow-up time (months), median (IQR) | |
| IBD subtype | |
| Crohn’s disease, n (%) * | 354 (79.2) |
| L1 | 56 (15.8) |
| L2 | 34 (9.6) |
| L3 | 261 (73.7) |
| L4 | 8 (2.3) |
| B1 | 105 (29.7) |
| B2 | 64 (18.2) |
| B3 | 154 (43.5) |
| B2 + B3 | 30 (8.5) |
| Perianal disease, n (%) | 136 (38.3) |
| Ulcerative colitis, n (%) | 86 (19.3) |
| Proctitis | 9 (10.5) |
| Left-sided colitis | 22 (25.6) |
| Pancolitis | 55 (63.9) |
| Pouchitis | 1 (0.2) |
| IBD-U | 6 (1.3) |
| Prior bowel resection, n (%) | 128 (28.6) |
| Current smoker, n (%) | 48/297 (16.2) |
| Extraintestinal manifestation, n (%) | 52 (11.6) |
| Characteristics | Normal Zinc Level (n = 243) | Zinc Deficiency (n = 204) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Male, n (%) | 143 (58.9) | 100 (49.0) | 1.48 (1.02–2.16) | 0.04 |
| Age, median (IQR) | 30 (24–41) | 27 (21–35.8) | 0.97 (0.95–0.98) | <0.001 |
| Disease duration, median (IQR) | 7 (3–13) | 4 (1–9) | 0.94 (0.91–0.97) | <0.001 |
| Smoking, n (%) * | 35 (22.3) | 13 (9.3) | 0.36 (0.18–0.71) | 0.002 |
| Previous surgery, n (%) | 72 (29.6) | 56 (27.5) | 0.89 (0.59–1.36) | 0.61 |
| Disease subtype, n (%) | 0.14 | |||
| Crohn’s disease | 183 (75.3) | 171 (83.8) | ||
| Ulcerative colitis | 55 (22.6) | 31 (15.2) | 0.60 (0.37–0.98) | |
| IBD-U | 4 (1.7) | 2 (1.0) | 0.53 (0.097–2.96) | |
| Pouchitis | 1 (0.4) | 0 | ||
| Perianal Crohn’s disease, n (%) | 69 (28.4) | 67 (32.8) | 1.23 (0.82–1.85) | 0.31 |
| Extraintestinal Manifestations, n (%) | 25 (10.3) | 27 (13.2) | 1.33 (0.74–2.37) | 0.33 |
| Labs | ||||
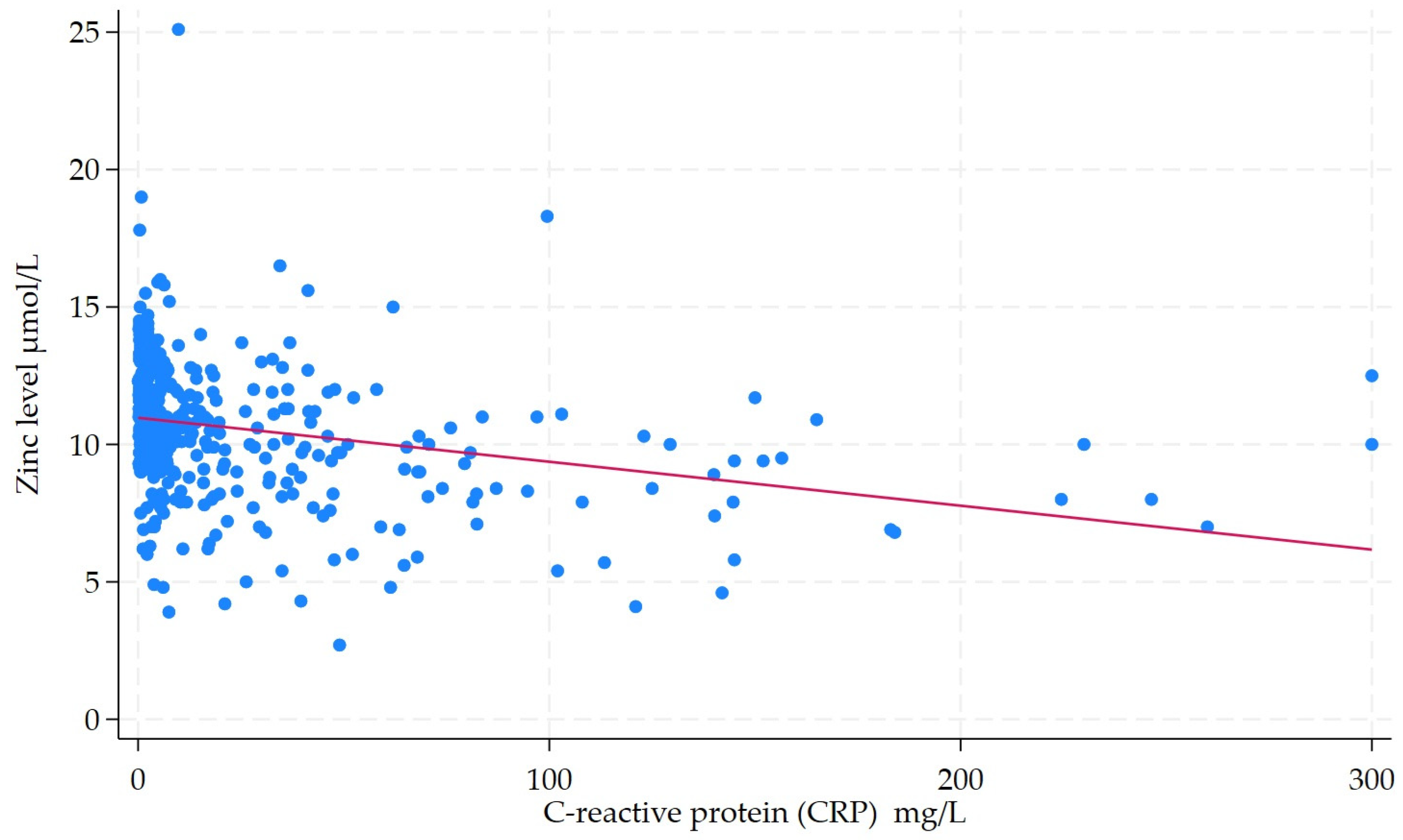
| Median CRP (IQR) | 3.4 (0.9–10.6) | 12.1 (3.8–46.8) | 3.34 (2.24–4.99) | <0.001 |
| Median FCP (IQR) | 177 (49–750) | 660 (146–1000) | 2.79 (1.57–4.96) | <0.001 |
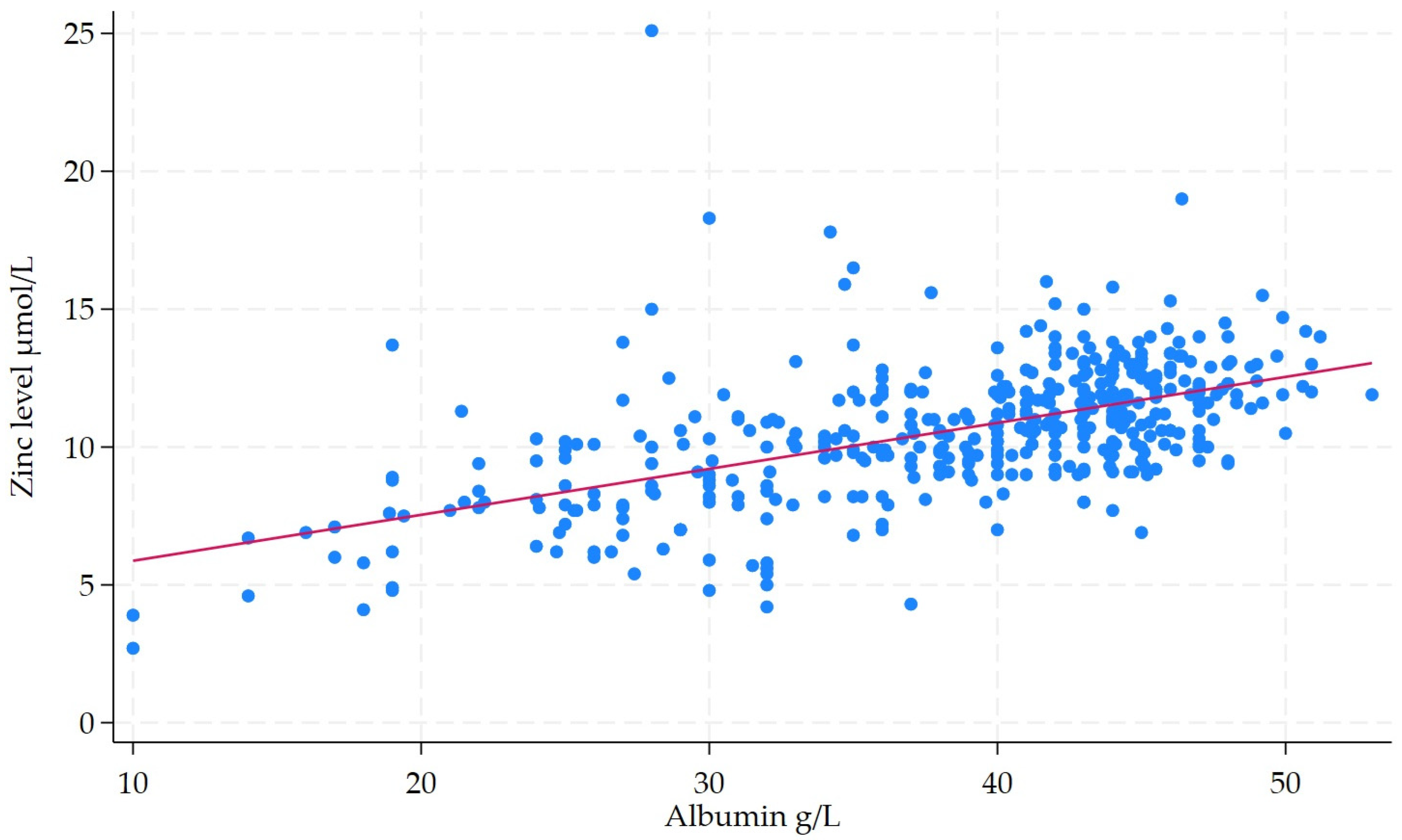
| Median Albumin (IQR) | 43.3 (40.4–45.7) | 35.2 (28–40.5) | 7.84 (4.73–12.99) | <0.001 |
| Medications | ||||
| Immunomodulators, n (%) | 113 (46.5) | 98 (48.0) | 1.06 (0.73–1.54) | 0.75 |
| Advanced Therapies, n (%) | 128 (52.7) | 109 (53.4) | 1.03 (0.71–1.49) | 0.87 |
| Corticosteroids, n (%) | 31 (12.8) | 41 (20.1) | 1.72 (1.03–2.86) | 0.04 |
| Outcome | Normal Zinc Level (n = 243) | Zinc Deficiency (n = 204) | p-Value |
|---|---|---|---|
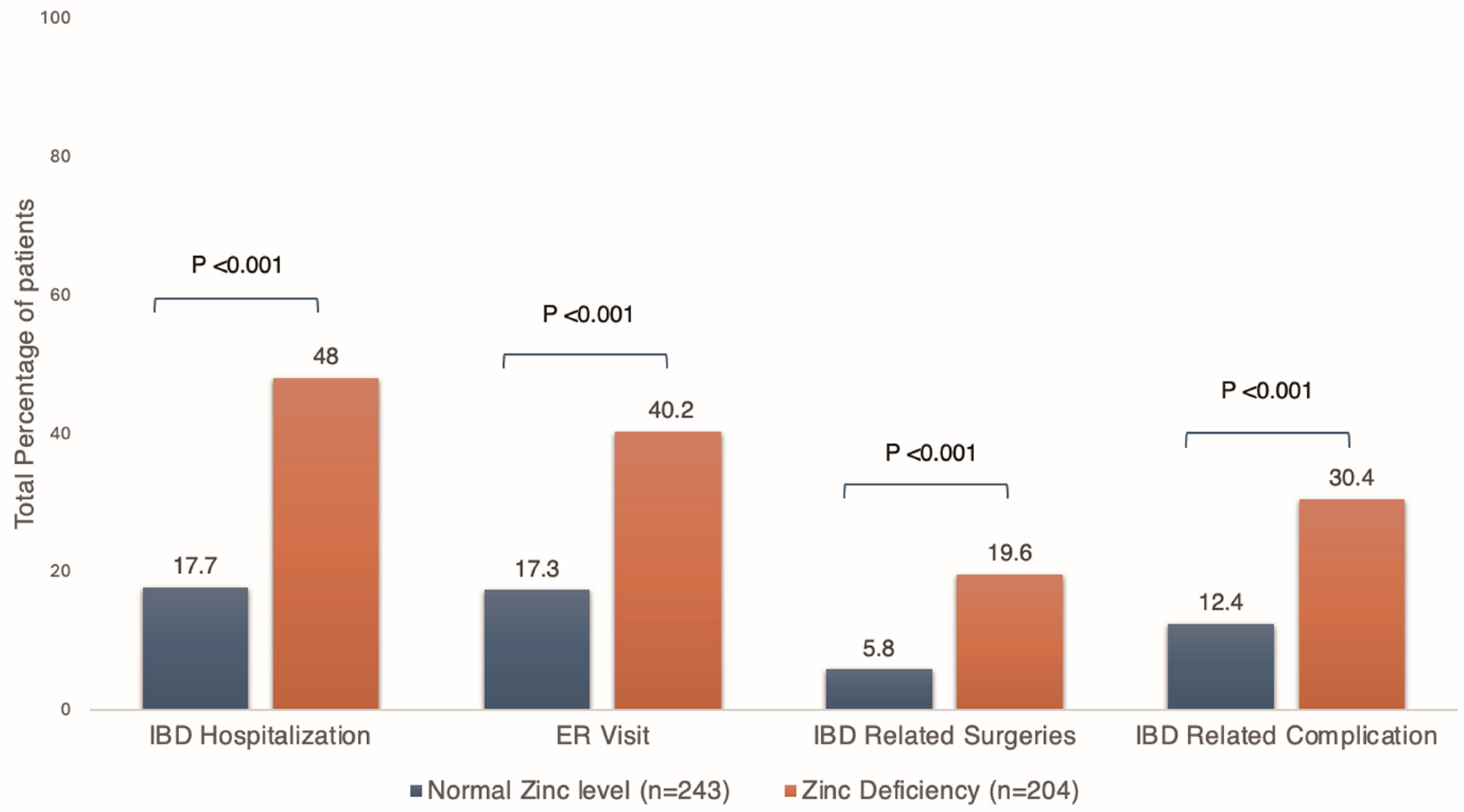
| IBD-related complications, n (%) | 30 (12.4) | 62 (30.4) | <0.001 |
| New-onset perianal fistula, n (%) | 2 (0.8) | 18 (8.8) | <0.001 |
| Small bowel obstruction, n (%) | 3 (1.2) | 17 (8.3) | <0.001 |
| New-onset anemia, n (%) | 18 (7.4) | 26 (12.8) | 0.06 |
| Intra-abdominal abscess, n (%) | 7 (2.88) | 18 (8.8) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuhaya, H.; Alsalamah, R.; Sallam, A.; Alonazy, A.; AlAwwad, A.; Mohamed, G.; Almutairdi, A.; Attamimi, M.; Al-Bawardy, B. Prevalence and Impact of Zinc Deficiency on Clinical Outcomes in Inflammatory Bowel Disease. Nutrients 2025, 17, 3378. https://doi.org/10.3390/nu17213378
Almuhaya H, Alsalamah R, Sallam A, Alonazy A, AlAwwad A, Mohamed G, Almutairdi A, Attamimi M, Al-Bawardy B. Prevalence and Impact of Zinc Deficiency on Clinical Outcomes in Inflammatory Bowel Disease. Nutrients. 2025; 17(21):3378. https://doi.org/10.3390/nu17213378
Chicago/Turabian StyleAlmuhaya, Hend, Raghad Alsalamah, Asma Sallam, Amgad Alonazy, Atheer AlAwwad, Gamal Mohamed, Abdulelah Almutairdi, Mashary Attamimi, and Badr Al-Bawardy. 2025. "Prevalence and Impact of Zinc Deficiency on Clinical Outcomes in Inflammatory Bowel Disease" Nutrients 17, no. 21: 3378. https://doi.org/10.3390/nu17213378
APA StyleAlmuhaya, H., Alsalamah, R., Sallam, A., Alonazy, A., AlAwwad, A., Mohamed, G., Almutairdi, A., Attamimi, M., & Al-Bawardy, B. (2025). Prevalence and Impact of Zinc Deficiency on Clinical Outcomes in Inflammatory Bowel Disease. Nutrients, 17(21), 3378. https://doi.org/10.3390/nu17213378







