Abstract
Background: Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic intestinal inflammation with fluctuating clinical severity. Although fecal calprotectin (FC) and fecal occult blood (FOBT) are established noninvasive biomarkers of intestinal inflammation, their interplay with nutritional status and disease activity has not been fully elucidated. This study aimed to explore the relationship between FC, FOBT, and disease activity in IBD, and to assess the potential mediating role of nutritional status as measured by the prognostic nutritional index (PNI). Methods: This retrospective study includes 128 adult patients with confirmed IBD (50 UC and 78 CD) examined at a tertiary care center between December 2023 and August 2025. Disease activity was assessed using the Mayo score for UC and the Harvey–Bradshaw Index for CD. FC levels were quantitatively measured using an enzyme-linked immunosorbent assay (ELISA), and fecal occult blood testing was performed with an automated latex agglutination-based system. Multivariable linear regression models were conducted to identify independent predictors of disease activity. Results: UC patients had significantly higher FC levels (278.0 vs. 133.5 µg/g, p < 0.001), FOBT positivity rates (76.7% vs. 43.6%, p = 0.002), and lower PNI (49.2 ± 4.2 vs. 51.5 ± 4.6, p = 0.048) compared to CD patients. In both UC and CD, disease activity scores were positively correlated with FC, FOBT positivity, CRP, and duration of illness, and negatively correlated with PNI (p < 0.05). In multivariable regression, PNI lost predictive value when FC and FOBT were included; FC and FOBT remained strong independent predictors of disease activity. Conclusions: FC and fecal occult blood are independently associated with higher disease activity in IBD, and may mediate the observed relationship between poor nutritional status and inflammation severity. The loss of significance of PNI in adjusted models suggests that intestinal inflammation and bleeding may act as intermediaries linking malnutrition to disease activity.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder characterized by periods of remission and relapse. It comprises two primary subtypes: Ulcerative colitis (UC), generally confined to the colon with inflammation typically restricted to the mucosal and submucosal layers, and Crohn’s disease (CD), which is distinguished by transmural inflammation and skip lesions that can affect any part of the gastrointestinal tract, from the mouth to the anus [1,2]. Although the precise etiology of IBD remains unclear, various genetic, microbial, familial, and environmental factors are implicated, alongside immune system dysregulation [3]. IBD severity is assessed using clinical activity indices that incorporate patient symptoms, inflammatory markers, and endoscopic findings [4].
One noninvasive approach to gauge intestinal inflammation in IBD is measuring fecal calprotectin (FC). Calprotectin is a calcium-binding protein released by activated neutrophils, and its concentration in stool rises sharply during active gut inflammation [5]. FC has become an established biomarker for distinguishing inflammatory disease from functional disorders and for monitoring IBD activity [6]. Several studies have demonstrated that FC correlates well with endoscopic disease severity in both UC and CD [6,7,8,9]. In addition to calprotectin, fecal occult blood is another stool marker that reflects intestinal disease activity. Occult blood testing (particularly immunochemical fecal occult blood tests, iFOBT) detects minute bleeding in the GI tract, which frequently occurs with active IBD due to mucosal ulceration. A positive fecal occult blood is common during IBD flares and serves as a quick, inexpensive indicator of mucosal injury [6]. Given its reduced sensitivity relative to FC and its status as a late marker of substantial intestinal hemorrhage in IBD, the fecal occult blood test is not employed in routine practice [10]. On the other hand, studies in UC have shown that serial iFOBT measurements can predict flare-ups: for about 20% of patients, a spike in fecal hemoglobin was observed weeks before clinical symptoms reappeared [11]. This suggests that rising occult blood in stool accompanies subclinical inflammation and heralds impending relapse.
Active inflammation can lead to malabsorption, reduced intake, and metabolic derangements, causing weight loss and nutrient deficiencies; conversely, poor nutritional status may impair immune responses and gut barrier function, potentially exacerbating mucosal inflammation [12,13]. In addition, clinically, fecal biomarkers also tie into nutritional consequences: chronic intestinal blood loss can contribute to iron-deficiency anemia, and severe inflammation can diminish appetite and nutrient absorption, worsening the patient’s nutritional status. Conversely, patients with poor nutrition (low protein stores or sarcopenia) may have less robust mucosal healing, thereby sustaining inflammation and bleeding [14]. Recent evidence indicates that IBD patients with sarcopenia or hypoalbuminemia tend to have higher inflammatory markers and more aggressive disease courses [15,16].
Although malnutrition is common in IBD and strongly associated with poorer outcomes, traditional disease severity scores often overlook nutritional status [14]. Given the interrelationships above, we hypothesize that IBD patients with poor nutritional status tend to exhibit higher FC levels and more frequent fecal occult blood, which correspond to increased disease activity. To explore this, the aim of our study is to investigate the relationship between disease activity and fecal markers (calprotectin and occult blood) in IBD patients, and to evaluate the role of nutritional status as measured by prognostic nutritional index (PNI) in this relationship.
2. Materials and Methods
This retrospective study was conducted on adult patients diagnosed with IBD and followed at the IBD Outpatient Clinic of the Anka Hospital between December 2023 and August 2025. The study adhered to the ethical regulations and principles specified in the Declaration of Helsinki and received approval from the Ethical Committee of Gaziantep City Hospital Clinical Research Ethics Committee (Date: 17 September 2025, Decision No: 286/2025). The requirement for obtaining informed consent was exempted by the Ethics Committee due to the retrospective design of the study.
2.1. Study Population
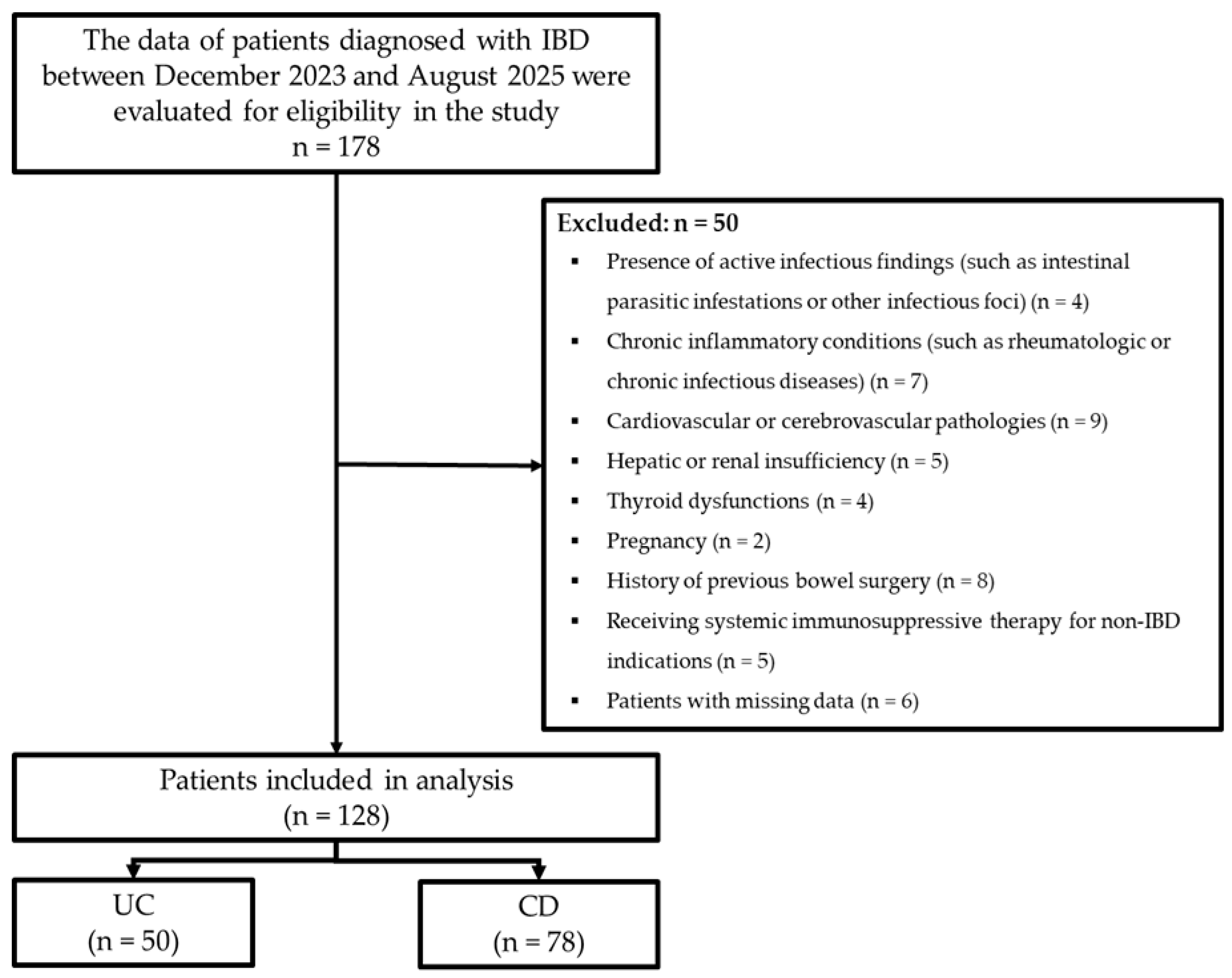
During the study period, a total of 178 patients diagnosed with IBD were retrospectively evaluated. Inclusion criteria comprised patients aged 18–95 years with a diagnosis of IBD, irrespective of gender. Exclusion criteria encompassed patients exhibiting active infectious findings (including intestinal parasitic infestations and/or other infective foci), chronic inflammatory conditions (such as rheumatologic or chronic infectious diseases), cardiovascular or cerebrovascular pathologies, hepatic or renal insufficiency, thyroid abnormalities, pregnancy, previous bowel surgery, and receipt of systemic immunosuppressive therapy for non-IBD indications (such as rheumatologic/oncologic conditions), and any individuals with missing data. After applying the exclusion criteria, the study comprised 128 IBD patients, including 50 with UC and 78 with CD (Figure 1).
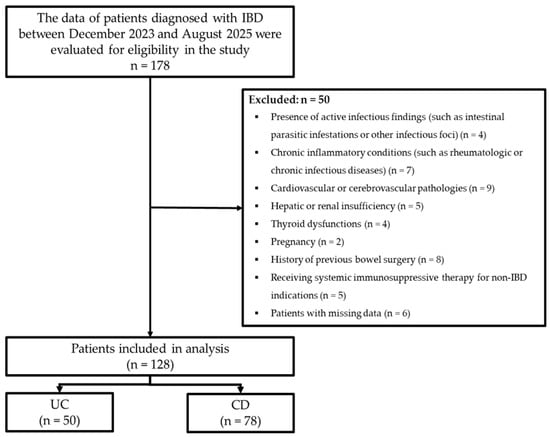
Figure 1.
Flowchart of the study.
2.2. Data Collection and Definitions
Demographic and clinical data were extracted from the hospital’s electronic information system and patient records. The dataset comprised patient age, gender, body mass index, smoking history, duration of illness, disease activity indices, pharmacotherapy regimens, laboratory values, fecal occult blood test results, and FC measurements.
To assess disease activity, a comprehensive history was taken at admission and all blood tests—including inflammatory markers—were performed. To ensure consistency in endoscopic assessment, all colonoscopic procedures were carried out by the same endoscopist with a FUJINON XL-4450 colonoscope (Fujifilm Medical Co., Tokyo, Japan). The severity of disease was determined by the Harvey–Bradshaw Index (HBI) in patients with CD and by the Mayo scoring system (Disease Activity Index, DAI) in those with UC [17,18]. Those with endoscopic activity index score of 5 or above were accepted to be an active disease.
Combination therapy was defined as the concurrent use of ≥2 systemic IBD agents at the index assessment—namely biologic plus immunomodulator (e.g., infliximab/adalimumab with azathioprine), biologic plus systemic corticosteroid (e.g., infliximab/adalimumab with prednisone), or immunomodulator plus systemic corticosteroid (e.g., azathioprine with prednisone).
2.3. Laboratory Measurements
Venous blood samples were obtained from all patients at their most recent hospital presentation after an 8 h fast, and analyzed using the Mindray MC6800 (Mindray, Shenzhen, China) and Architect Plus (Abbott Diagnostics, Abbott Park, IL, USA) systems. Leukocyte counts were obtained via impedance; C-reactive protein levels by immunoturbidimetry; and albumin by the bromocresol green method. The prognostic nutritional index (PNI) was calculated as: PNI = 10 × (albumin level in g/dL) + 0.005 × (total lymphocyte count) [19]. PNI values ≥ 50 were considered normal, while values < 50 were classified as low, indicating poor nutritional status [20,21,22].
FC was measured quantitatively using an enzyme-linked immunosorbent assay (ELISA) by IDS calprotectin extraction device (Immunodiagnostic Systems Ltd., Boldon, UK) [23]. Patients’ stool samples underwent fecal occult blood testing (FOBT) (within 20 h post-collection) using the HM-Jack autoanalyzer (Extel Hemo-Auto, Tokyo, Japan). The methodology relies on the agglutination of latex microparticles coated with anti-human hemoglobin antibodies upon interaction with hemoglobin, with resultant turbidity shifts quantitatively reflecting fecal blood levels. Test outcomes were classified as negative (0–12 ng/mL) or positive (>12 ng/mL).
2.4. Statistical Analysis
Statistical analyses were conducted in IBM SPSS Statistics for Windows (v. 20.0; IBM Corp., Armonk, NY, USA). Distributional normality was evaluated using the Kolmogorov–Smirnov test. Continuous variables conforming to a normal distribution are reported as mean ± SD; those deviating from normality are reported as median (IQR). Categorical data are presented as frequencies and percentages. Two-group comparisons of normally distributed continuous variables employed Student’s t-test, while one-way ANOVA was used for comparisons across three or more groups. For non-normally distributed continuous variables, the Mann–Whitney U test and Kruskal–Wallis H test were used for two-group and multi-group comparisons, respectively. Categorical comparisons utilized Pearson’s Chi-square or Fisher’s exact test based on expected cell counts. To determine potential predictors of disease activity scores, univariate linear regression analyses were performed. Parameters failing to meet normality assumptions underwent logarithmic transformation before inclusion in the multivariate linear regression model. Significance was accepted at p < 0.05 for all statistical analyses.
3. Results
A total of 128 IBD patients were analyzed, with 50 (39.1%) diagnosed with UC and 78 (60.9%) with CD. The mean age was 44.4 ± 14.1 years, and no significant difference was observed between UC and CD (42.7 ± 15.1 vs. 45.1 ± 13.7 years, p = 0.432). Females comprised 57% of the total population, with similar distributions in UC and CD (50.0% vs. 61.5%, p = 0.207). BMI, smoking status, and duration of illness were not significantly different between groups. Median disease activity score was higher in UC patients compared to CD patients (66.7% vs. 33.3%, p < 0.001). Regarding treatment, the use of ASA (52.0% vs. 20.5%, p < 0.001) was higher in UC patients, while biological agent use was higher in CD patients (14.1% vs. 0%, p = 0.030). No significant difference was found in the use of other therapies. Mean PNI level was lower in UC patients compared to CD patients (49.2 ± 4.2 vs. 51.5 ± 4.6, p = 0.048). Also, rate of poor nutrition status was higher in UC patients compared to CD patients (62.0% vs. 35.9%, p = 0.004). Positivity for FOBT was significantly greater in UC patients than in CD (76.7% vs. 43.6%, p = 0.002), and FC levels were markedly higher in UC patients compared to CD (278.0 vs. 133.5 µg/g, p < 0.001) (Table 1).

Table 1.
Distribution of demographic characteristics and clinical findings.
In patients with UC, disease activity scores showed significant positive correlations with duration of illness (r = 0.380, p < 0.001), leukocyte count (r = 0.326, p = 0.029), neutrophils count (r = 0.349, p < 0.001), CRP levels (r = 0.376, p < 0.001), and FC levels (r = 0.675, p < 0.001). In contrast, significant negative correlations were observed with albumin levels (r = −0.371, p < 0.001) and PNI values (r = −0.420, p < 0.001). Additionally, poor nutritional status and FOBT positivity were associated with higher disease activity scores (p < 0.05). Similar associations were observed in patients with CD patient (Table 2).

Table 2.
Findings associated with disease activity score.
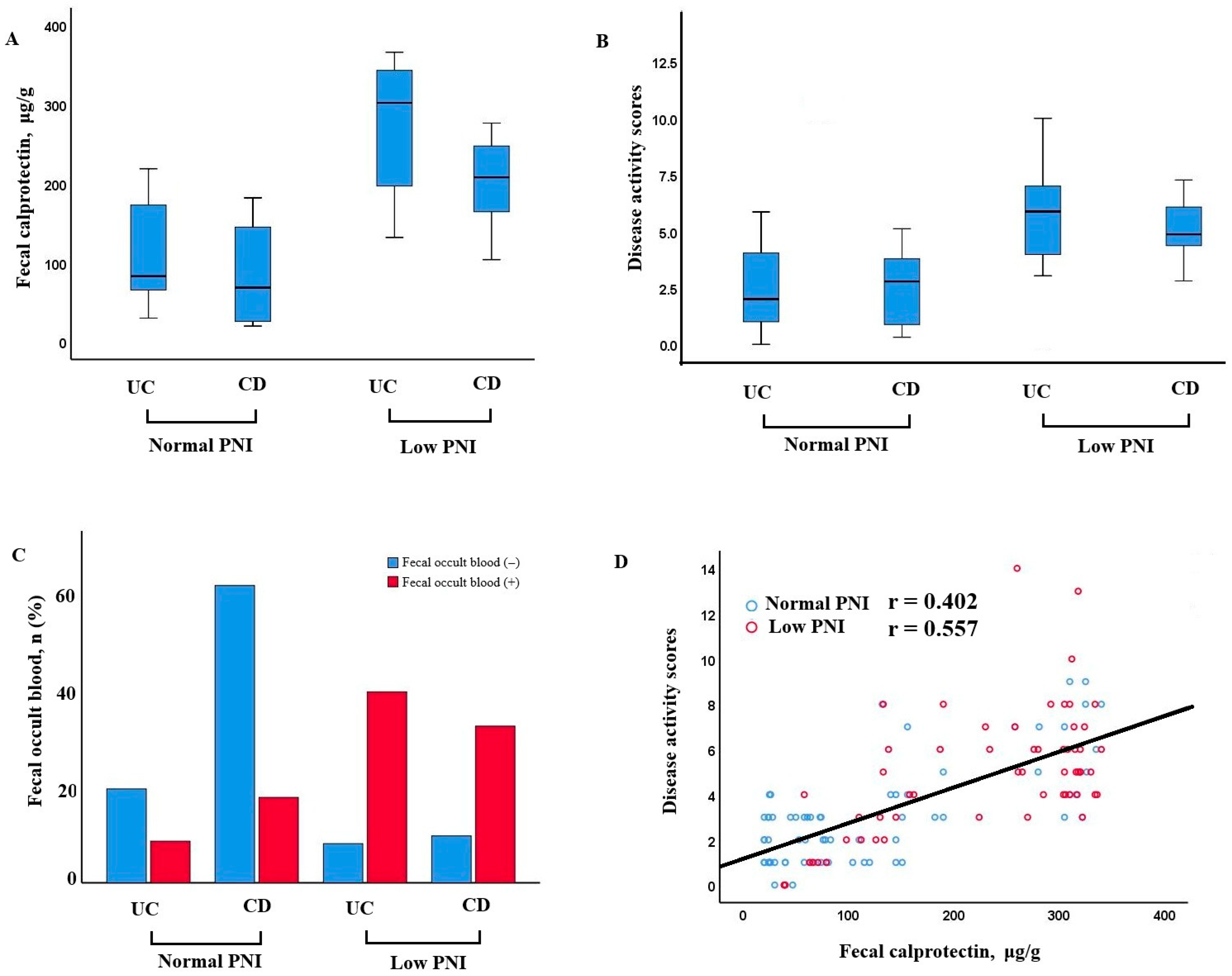
Patients with low PNI demonstrated significantly higher fecal calprotectin levels and disease activity scores in both UC and CD groups. Additionally, fecal occult blood positivity was more frequent in patients with low PNI. A positive correlation was observed between fecal calprotectin and disease activity, especially in those with poor nutritional status (r = 0.402, p < 0.001 for normal nutrition; r = 0.557, p < 0.001 for poor nutrition) (Figure 2).
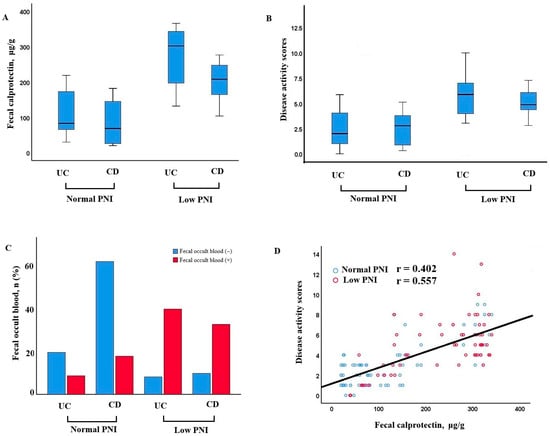
Figure 2.
Relationship between nutritional status, fecal calprotectin, disease activity, and occult blood in UC and CD patients. (A) Fecal calprotectin levels in patients with UC and CD, stratified by normal and low PNI. Patients with low PNI exhibited higher calprotectin levels, particularly in the CD group. (B) Disease activity scores in UC and CD patients according to PNI status. Higher scores were observed in the low PNI groups. (C) Distribution of fecal occult blood positivity in UC and CD patients with normal and low PNI. Fecal occult blood was more frequently positive in patients with low PNI, especially in CD. (D) Correlation between fecal calprotectin and disease activity scores. A positive association was identified, more prominent among patients with low PNI.
Variables significantly associated with disease activity scores in univariable analysis are presented in Table 3. Multivariable regression analysis was performed to determine independent predictors of disease activity, and results are shown as Model I and Model II. In Model I, all potential risk factors except FOBT positivity and FC were included. Model II incorporated the FOBT positivity and FC in addition to those in Model I. All models were adjusted for age, sex, BMI, smoking status, and medication effects. For UC, Model I included log(Duration of illness), log(CRP), and PNI. All variables emerged as independent predictors: longer duration of illness (β = 0.43, 95% CI: 0.20–0.68, p = 0.001), higher CRP levels (β = 0.20, 95% CI: 0.04–0.36, p = 0.014), and lower PNI values (β = −0.03, 95% CI: −0.04 to −0.01, p = 0.008). Model II included log(Duration of illness), log(CRP), fecal occult blood, and log(Fecal calprotectin), revealing that longer illness duration (β = 0.48, 95% CI: 0.18–0.78, p = 0.003), higher CRP (β = 0.13, 95% CI: 0.04–0.22, p = 0.009), FOBT positivity (β = 0.34, 95% CI: 0.13–0.55, p = 0.002), and elevated FC (β = 0.91, 95% CI: 0.68–1.14, p < 0.001) remained independent predictors (Table 3). Similar analyses for CD in Model I identified log(Duration of illness) (β = 0.30, 95% CI: 0.14–0.47, p < 0.001), log(CRP) (β = 0.18, 95% CI: 0.03–0.32, p = 0.018), and lower PNI (β = −0.02, 95% CI: −0.03 to −0.01, p = 0.013) as significant independent predictors. Model II confirmed log(Duration of illness) (β = 0.27, 95% CI: 0.13–0.42, p < 0.001), log(CRP) (β = 0.12, 95% CI: 0.02–0.25, p = 0.042), FOBT positivity (β = 0.19, 95% CI: 0.07–0.32, p = 0.002), and elevated FC (β = 0.33, 95% CI: 0.20–0.46, p < 0.001) as independent predictors of disease activity (Table 3).

Table 3.
Independent predictors for disease activity score in IBD patients.
4. Discussion
In this study of patients with IBD, several key biomarkers were found to correlate with disease activity. Fecal calprotectin (FC) levels were significantly elevated in patients with active IBD compared to those in remission, reflecting a higher inflammatory burden in the gut. Similarly, a greater proportion of patients in active disease had a positive FOBT, indicating occult gastrointestinal bleeding associated with mucosal ulceration. Markers of systemic inflammation were also higher during active disease—patients in flare showed elevated CRP levels and often had increased leukocytes. In contrast, nutritional status indicators were poorer in active IBD: serum albumin was significantly lower and the PNI was depressed, consistent with malnutrition and systemic inflammation. Notably, PNI independently predicted disease activity in the initial model but lost significance upon inclusion of FC and FOBT, suggesting that nutritional status may indirectly influence disease activity via these fecal biomarkers.
Numerous studies confirm that FC correlates strongly with IBD activity [24,25,26,27]. A study involving patients with UC and CD reported that fecal calprotectin levels exhibited sensitivity greater than 90% and specificity greater than 80% in differentiating active disease from remission [24]. Consistent with these findings, a strong correlation was observed between IBD activity and FC. Nevertheless, compared with Crohn’s disease, ulcerative colitis involved a higher proportion of patients in an active state [28], who also demonstrated increased FC levels. There may be several potential mechanisms underlying this condition. In active UC, neutrophils infiltrate the colonic mucosa and release calprotectin into the lumen, so stool FC levels rise in proportion to inflammatory burden [29]. However, CD can involve patchy, transmural inflammation and segments of small intestine where fecal markers may be less uniformly elevated [30]. Another important mechanism may be the higher proportion of FOBT-positive patients in UC than in those with UC [31]. Rectal bleeding (hematochezia) is a hallmark symptom of active UC, so it is unsurprising that FOBT frequently turn positive during UC flares. Comparative studies highlight this difference: in UC, fecal immunochemical test (FIT) and FC perform equally well in detecting mucosal disease, but in CD, FIT is markedly less sensitive for small-bowel lesions compared to FC [32]. This is because CD inflammation that is confined to the ileum or jejunum may not lead to substantial bleeding into feces, or blood is degraded during transit. The fecal immunochemical test “misses” purely inflammatory lesions if they do not bleed, whereas FC will still be elevated from neutrophil influx [32]. These findings suggest that both FOBT positivity and FC may be important predictors in UC patients, whereas in CD patients, FC may serve as a more important marker than FOBT.
An additional noteworthy factor may involve the nutritional status of IBD patients. It is estimated that 20–85% of IBD patients suffer from malnutrition at some point, with the prevalence on the higher end (up to ~75%) during active disease flares [33]. Although clinical remission and the absence of symptoms do not necessitate dietary restrictions, studies have reported that macronutrient and fiber intake in IBD patients differs from that of healthy individuals, with no significant differences observed between CD and UC patients in terms of total caloric, protein, lipid, carbohydrate, or fiber intake [34]. Despite this, malnutrition tends to be more pronounced in patients with CD. Because CD can affect extensive portions of the GI tract, it more commonly leads to protein–energy malnutrition and specific nutrient deficiencies than UC [35,36]. In UC, malabsorption is less pronounced, but chronic blood loss and inflammation can still lead to deficiencies (e.g., iron, vitamin D) and protein-losing enteropathy [33]. The inflammatory state itself exacerbates malnutrition by increasing catabolism and muscle breakdown, as well as by inducing anorexia and altering gut hormone signals that regulate appetite and metabolism [14]. This may explain the lower PNI values and the stronger correlation with FC observed in UC patients, who had a higher rate of active disease.
Locally, inflammation also disrupts the epithelial barrier and absorptive function of the gut. The inflamed mucosa has blunted villi and increased permeability (“leaky gut”), reducing the surface area and integrity needed for nutrient absorption [36,37]. Tight junctions between cells are weakened, allowing microbial products and proteins to leak across, which in turn sustains immune activation and can worsen systemic inflammation [33]. In the intestinal mucosa, neutrophils flood into the tissue and lumen, drawn by chemokines. These activated neutrophils release calprotectin (among other proteins), which explains why fecal calprotectin rises in proportion to neutrophilic inflammation. Essentially, FC is a direct readout of neutrophil activity at the mucosal surface [38]. Neutrophils also release myeloperoxidase, elastase, and other enzymes that contribute to mucosal injury, potentially leading to erosions and ulcers [39,40]. When ulcers breach the submucosa, capillaries are exposed and bleed—resulting in blood entering the stool. Accordingly, FC—a proximal index of mucosal neutrophilic inflammation—exhibits a more immediate and specific correspondence with disease activity, whereas PNI, which is depressed via inflammation-induced malabsorption and negative acute-phase responses, represents a downstream systemic consequence. Hence, PNI may correlate with disease activity when considered alone (Model 1 regression analysis), but typically loses independent significance after adjustment for FC (Model 2 regression analysis), consistent with FC capturing variance more causally proximate to the inflammatory disease process.
4.1. Limitations
This study has several limitations. First, the retrospective nature of the study may have introduced selection bias and restricted the ability to draw causal inferences. Additionally, this was a single-center study. Between-hospital variability in stool-biomarker analytics (e.g., brand- and lot-specific FIT/FOBT and fecal calprotectin assays) and differences in local therapeutic algorithms may influence absolute values and operating characteristics [41,42]. Moreover, regional or geographic variability may contribute to differences in the sociodemographic profiles of populations, which play an important role in shaping IBD phenotypes and malnutrition risk [43,44,45]. These factors may limit the generalizability of the study findings to community-based or multiethnic cohorts. Second, disease activity was assessed by symptom-based indices (Partial Mayo for UC and HBI for CD) rather than uniformly paired endoscopic or histologic measures, which may lead to misclassification. Third, phenotypic characterization based on the Montreal classification (e.g., UC extent, CD location and behavior) was not systematically included due to incomplete documentation in the retrospective records. As such, the potential influence of disease phenotype on biomarker levels and nutritional status could not be fully analyzed. Research indicates that fecal calprotectin’s accuracy in CD can be influenced by disease location: colonic CD behaves similarly to UC with high FC levels, whereas isolated small-bowel CD might produce somewhat lower FC despite active inflammation [46]. Finally, it lacked longitudinal follow-up, precluding assessment of changes in fecal marker levels or nutritional status over time.
4.2. Future Directions
Future prospective, multi-center studies with larger, phenotype-stratified cohorts that directly address these limitations are warranted to validate our findings and to determine whether nutrition-focused interventions, coupled with biomarker-guided treat-to-target care, can disrupt the inflammation–malnutrition cycle and improve outcomes. Emerging evidence suggests that integrating stool biomarkers with nutritional indices could enable a more personalized approach to IBD management moving forward. Biomarker-guided nutritional interventions may represent a promising strategy. An elevated FC or positive FOBT in a stable patient could prompt earlier dietary support (such as exclusive enteral nutrition or targeted supplementation) to preempt an impending flare [47,48]. Likewise, trends such as a declining PNI or persistent hypoalbuminemia might signal deteriorating nutritional status and justify intensifying nutritional interventions in anticipation of clinical deterioration [14]. In parallel, these biomarkers offer value for clinical risk stratification. Patients with persistently high FC or recurrent FOBT positivity are at heightened risk of relapse and complications, and combining such stool markers with indices of nutritional status can identify high-risk individuals who warrant closer monitoring and aggressive, multidisciplinary management. Notably, malnourished IBD patients (e.g., those with sarcopenia or low PNI) tend to experience more active disease and worse outcomes, underscoring the importance of early nutritional optimization as part of the care plan [14]. Furthermore, current guidelines issued by organizations such as the European Crohn’s and Colitis Organization (ECCO) and ESPEN emphasize the importance of routine nutritional risk screening and support in the management of IBD [49]. In addition, treat-to-target strategies—such as STRIDE—incorporate objective biomarkers like FC to guide proactive treatment adjustments before irreversible tissue damage occurs [50,51]. Therefore, future research and clinical guidelines may provide direction on how stool biomarkers and nutritional indices can be used to guide the timing and intensity of both medical and nutritional interventions. This approach may ultimately help disrupt the inflammation–malnutrition cycle, prevent impending flares, and improve long-term clinical outcomes.
5. Conclusions
In these IBD patients, disease activity scores were associated with higher fecal biomarker burden and more pronounced nutritional impairment. In both the UC and CD groups, patients with malnutrition exhibited higher fecal biomarker burden. In both groups, fecal biomarkers remained an independent indicator of disease activity in the regression model, whereas PNI lost its significance. These findings suggest that malnutrition is a consequence of severe mucosal inflammation rather than an independent trigger for disease flare. Integrating fecal biomarkers into routine IBD monitoring, while utilizing PNI as a supplementary risk indicator, may offer a simple and noninvasive strategy for stratifying patients by flare risk. In practice, regular FC and FOBT assessments can help identify those at higher risk of an impending flare, prompting clinicians to initiate timely therapeutic escalation and provide targeted nutritional support. Adopting this pragmatic, biomarker-first approach with PNI as an adjunct offers a path to earlier escalation and tailored nutrition care, mitigating flare risk and enhancing long-term clinical trajectories.
Author Contributions
Conceptualization, A.B. and H.A.; methodology, A.B. and H.A.; formal analysis, A.B. and H.A.; investigation, A.B. and H.A.; resources, A.B. and H.A.; data curation, A.B. and H.A.; writing—original draft preparation, A.B.; writing—review and editing, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed in accordance with the Declaration of Helsinki, and was approved by the Gaziantep City Hospital Clinical Research Ethics Committee (Date: 17 September 2025, Decision No: 286/2025).
Informed Consent Statement
The need for informed consent was waived under the approval of the Local Ethics Committee due to the retrospective design.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Scholmerich, J.; Brynskov, J.; D’Haens, G.; Hanauer, S.B.; Irvine, E.J.; Jewell, D.P.; Rachmilewitz, D.; Sachar, D.B.; Sandborn, W.J.; et al. A simple classification of Crohn’s disease: Report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm. Bowel Dis. 2000, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bianchi Porro, G. Inflammatory bowel disease: New insights into pathogenesis and treatment. J. Intern. Med. 2002, 252, 475–496. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Abraham, B.P.; Kane, S. Fecal markers: Calprotectin and lactoferrin. Gastroenterol. Clin. 2012, 41, 483–495. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, L.; Xie, C.; Zou, K.; Tu, L.; Yan, W.; Hou, X. Comparison of non-invasive biomarkers faecal BAFF, calprotectin and FOBT in discriminating IBS from IBD and evaluation of intestinal inflammation. Sci. Rep. 2017, 7, 2669. [Google Scholar] [CrossRef]
- Suttichaimongkol, T.; Coelho-Prabhu, N.; Bruining, D.H.; Tariq, R.; Snyder, M.R.; Loftus, E.V., Jr. Diagnostic Performance of a Fecal Calprotectin Assay as a Biomarker for Mayo Endoscopic Subscore in Ulcerative Colitis: Result From a Tertiary Referral Center. Inflamm. Bowel Dis. 2024, 30, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Ishihara, S.; Yuki, T.; Fukuba, N.; Oshima, N.; Kazumori, H.; Sonoyama, H.; Yamashita, N.; Tada, Y.; Kusunoki, R.; et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Qian, W.; Ling, F.; Chen, Y.; Li, S.; Cheng, Y.; Zhu, L. Clinical value of fecal calprotectin for evaluating disease activity in patients with Crohn’s disease. Front. Physiol. 2023, 14, 1186665. [Google Scholar] [CrossRef]
- Durham, K.; Atagozli, T.; Elliott, D.E.; Ince, M.N. Laboratory Tests in Inflammatory Bowel Disease: An Evidence-Based Approach to Daily Practice. Biomedicines 2025, 13, 491. [Google Scholar] [CrossRef]
- Kuriyama, M.; Kato, J.; Takemoto, K.; Hiraoka, S.; Okada, H.; Yamamoto, K. Prediction of flare-ups of ulcerative colitis using quantitative immunochemical fecal occult blood test. World J. Gastroenterol. 2010, 16, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Profir, M.; Enache, R.M.; Rosu, O.A.; Pavelescu, L.A.; Cretoiu, S.M.; Gaspar, B.S. Malnutrition and Its Influence on Gut sIgA–Microbiota Dynamics. Biomedicines 2025, 13, 179. [Google Scholar] [CrossRef]
- Sherif, M.; Fouad, R.; Elbaz, T.; Awadalla, M.; Tantawi, O.; Negm, M.; El-kareem, D.A.; Naguib, I.; Shehab, H.; Badary, H.A. Sarcopenia and low prognostic nutritional index as markers of disease activity in patients with inflammatory bowel disease and predictors of poor outcome: A cohort longitudinal study. Egypt. Rheumatol. Rehabil. 2024, 51, 55. [Google Scholar] [CrossRef]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Shah, Y.; Trivedi, C.; Yang, Y.X. Albumin as a prognostic marker for ulcerative colitis. World J. Gastroenterol. 2017, 23, 8008–8016. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar] [PubMed]
- Jia, G.; Li, K. Prognostic Nutritional Index is A Useful Predictive Marker for Morbidity and Mortality in Surgery for Perforated Peptic Ulcer. J. Inflamm. Res. 2025, 18, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Bakılan, F.; Sarıçimen, G. Prognostic Nutritional Index in Patients with Both Vertebral and Non-Vertebral Osteoporotic Fractures. Turk. J. Osteoporos. 2024, 30, 142–148. [Google Scholar] [CrossRef]
- Wei, J.; Lu, J.; Jia, H.; Yang, X.; Guo, X.; Liu, J.; Li, X. Value of a preoperative prognostic nutritional index for the prognostic evaluation of gastric neuroendocrine carcinoma patients. Front. Nutr. 2023, 10, 1043550. [Google Scholar] [CrossRef]
- Castiglione, V.; Berodes, M.; Lukas, P.; Louis, E.; Cavalier, E.; Lutteri, L. New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method. Diagnostics 2022, 12, 2338. [Google Scholar] [CrossRef]
- Jain, A.V.; Gopal, S.; Shetty, A.J.; Shenoy, S.; Tantry, B.V.; Unnikrishnan, B.; Holla, R.; Anand, R. Predictive accuracy of fecal calprotectin in assessing clinical activity and disease severity in patients with Ulcerative Colitis and Crohn’s disease. BMC Gastroenterol. 2025, 25, 429. [Google Scholar] [CrossRef] [PubMed]
- Erbayrak, M.; Turkay, C.; Eraslan, E.; Cetinkaya, H.; Kasapoglu, B.; Bektas, M. The role of fecal calprotectin in investigating inflammatory bowel diseases. Clinics 2009, 64, 421–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walsham, N.E.; Sherwood, R.A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2016, 9, 21–29. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lee, K.M.; Lee, J.M.; Chung, Y.Y.; Kim, D.B.; Kim, Y.J.; Chung, W.C.; Paik, C.N. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J. Intern. Med. 2019, 34, 72–80. [Google Scholar] [CrossRef]
- Desalegn, Z.; Bane, A.; Merdasa, G.; Arota, A. Active Disease and Its Associated Factors Among Patients with Inflammatory Bowel Disease in Addis Ababa Ethiopia: A Hospital-Based Cross-Sectional Study. Front. Gastroenterol. 2025, 4, 1569933. [Google Scholar] [CrossRef]
- Ortega-Zapero, M.; Gomez-Bris, R.; Pascual-Laguna, I.; Saez, A.; Gonzalez-Granado, J.M. Neutrophils and NETs in Pathophysiology and Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 7098. [Google Scholar] [CrossRef]
- Zittan, E.; Kelly, O.B.; Gralnek, I.M.; Silverberg, M.S.; Hillary Steinhart, A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open 2018, 2, 201–206. [Google Scholar] [CrossRef]
- Lee, E.; Lee, G.H.; Park, B.; Ahn, S.S.; Noh, C.K. Positive faecal immunochemical test predicts the onset of inflammatory bowel disease: A nationwide, propensity score-matched study. Front. Immunol. 2023, 14, 1128736. [Google Scholar] [CrossRef]
- Kato, J.; Hiraoka, S.; Nakarai, A.; Takashima, S.; Inokuchi, T.; Ichinose, M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: Can it rival fecal calprotectin? Intest. Res. 2016, 14, 5–14. [Google Scholar] [CrossRef]
- Bueno-Hernandez, N.; Yamamoto-Furusho, J.K.; Mendoza-Martinez, V.M. Nutrition in Inflammatory Bowel Disease: Strategies to Improve Prognosis and New Therapeutic Approaches. Diseases 2025, 13, 139. [Google Scholar] [CrossRef]
- Principi, M.; Losurdo, G.; Iannone, A.; Contaldo, A.; Deflorio, V.; Ranaldo, N.; Pisani, A.; Ierardi, E.; Di Leo, A.; Barone, M. Differences in dietary habits between patients with inflammatory bowel disease in clinical remission and a healthy population. Ann. Gastroenterol. 2018, 31, 469–473. [Google Scholar] [CrossRef]
- Jablonska, B.; Mrowiec, S. Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Kilby, K.; Mathias, H.; Boisvenue, L.; Heisler, C.; Jones, J.L. Micronutrient Absorption and Related Outcomes in People with Inflammatory Bowel Disease: A Review. Nutrients 2019, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Chan-Diehl, F.; Baygani, S.; Fisher, D.A.; Moses, R.E.; Siegmund, B.; Walsh, A.; Kobayashi, T.; Dulai, P.S.; Travis, S. Fecal Calprotectin and C-Reactive Protein Association With Histologic and Endoscopic Endpoints in Mirikizumab-Treated Patients With Ulcerative Colitis. Crohns Colitis 360 2025, 7, otaf043. [Google Scholar] [CrossRef]
- Riaz, B.; Sohn, S. Neutrophils in Inflammatory Diseases: Unraveling the Impact of Their Derived Molecules and Heterogeneity. Cells 2023, 12, 2621. [Google Scholar] [CrossRef]
- Magalhaes, D.; Peyrin-Biroulet, L.; Estevinho, M.M.; Danese, S.; Magro, F. Pursuing neutrophils: Systematic scoping review on blood-based biomarkers as predictors of treatment outcomes in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2023, 16, 17562848231155987. [Google Scholar] [CrossRef]
- Whitehead, S.J.; French, J.; Brookes, M.J.; Ford, C.; Gama, R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann. Clin. Biochem. 2013, 50, 53–61. [Google Scholar] [CrossRef]
- van Wassenaer, E.A.; Diederen, K.; van Leeuwen, E.M.M.; D’Haens, G.R.; Benninga, M.A.; Koot, B.G.P.; Kindermann, A. Can 2 Different Fecal Calprotectin Assays be Used Interchangeably in IBD Treatment? J. Clin. Gastroenterol. 2022, 56, e27–e30. [Google Scholar] [CrossRef]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinovic, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, L.; Gao, X.; Yang, F.; Yang, Y.; Fang, H.; Ju, L.; Xu, X.; Guo, Q.; Li, S.; et al. Geographic Variations in Dietary Patterns and Their Associations with Overweight/Obesity and Hypertension in China: Findings from China Nutrition and Health Surveillance (2015–2017). Nutrients 2022, 14, 3949. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Simon, E.G.; Wardle, R.; Thi, A.A.; Eldridge, J.; Samuel, S.; Moran, G.W. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease?: A systematic review. Intest. Res. 2019, 17, 160–170. [Google Scholar] [CrossRef]
- Barberio, B.; Bertin, L.; Facchin, S.; Bonazzi, E.; Cusano, S.; Romanelli, G.; Pesenti, F.F.; Cazzaniga, E.; Palestini, P.; Zingone, F.; et al. Dietary Interventions and Oral Nutritional Supplementation in Inflammatory Bowel Disease: Current Evidence and Future Directions. Nutrients 2025, 17, 1879. [Google Scholar] [CrossRef]
- Geesala, R.; Gongloor, P.; Recharla, N.; Shi, X.Z. Mechanisms of Action of Exclusive Enteral Nutrition and Other Nutritional Therapies in Crohn’s Disease. Nutrients 2024, 16, 3581. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hebuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Horst, S.; Caldera, F. Applying Biomarkers in Treat-to-target Approach for IBD. Curr. Gastroenterol. Rep. 2025, 27, 41. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).