Bioactive Phenolics from Vinegar–Egg Accelerates Acute Wound Healing by Activation of Focal Adhesion and Mitogen-Activated Protein Kinase Signaling
Highlights
- Four phenolics were isolated from vinegar-eggs by LC–MS and HPLC.
- 4-Hydroxybenzoic and vanillic acids enhance fibroblast motility and invasion.
- Both compounds activate p-FAK, MMP-2, and p38 MAPK wound-healing signaling.
- Potential wound-healing agents were identified in a traditional beverage.
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Preparation of Vinegar–Egg
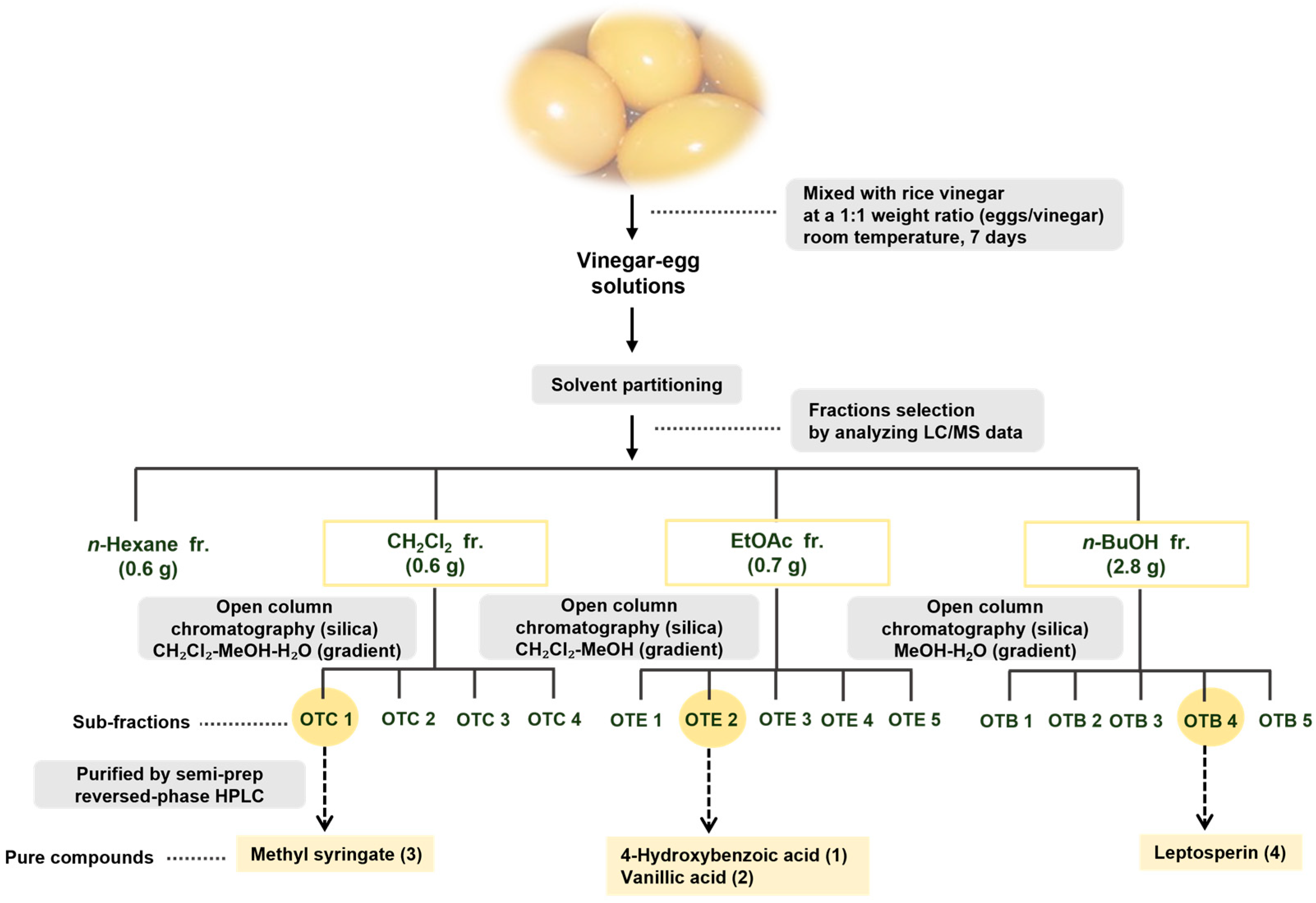
2.3. Extraction and Isolation
2.4. Reagents and Antibodies
2.5. Cell Culture
2.6. Cell Viability and Cell Proliferation Assay
2.7. Scratch Wound Healing Assay
2.8. Transwell Chamber Invasion Assay
2.9. Time-Lapse Cell Tracking Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results and Discussion
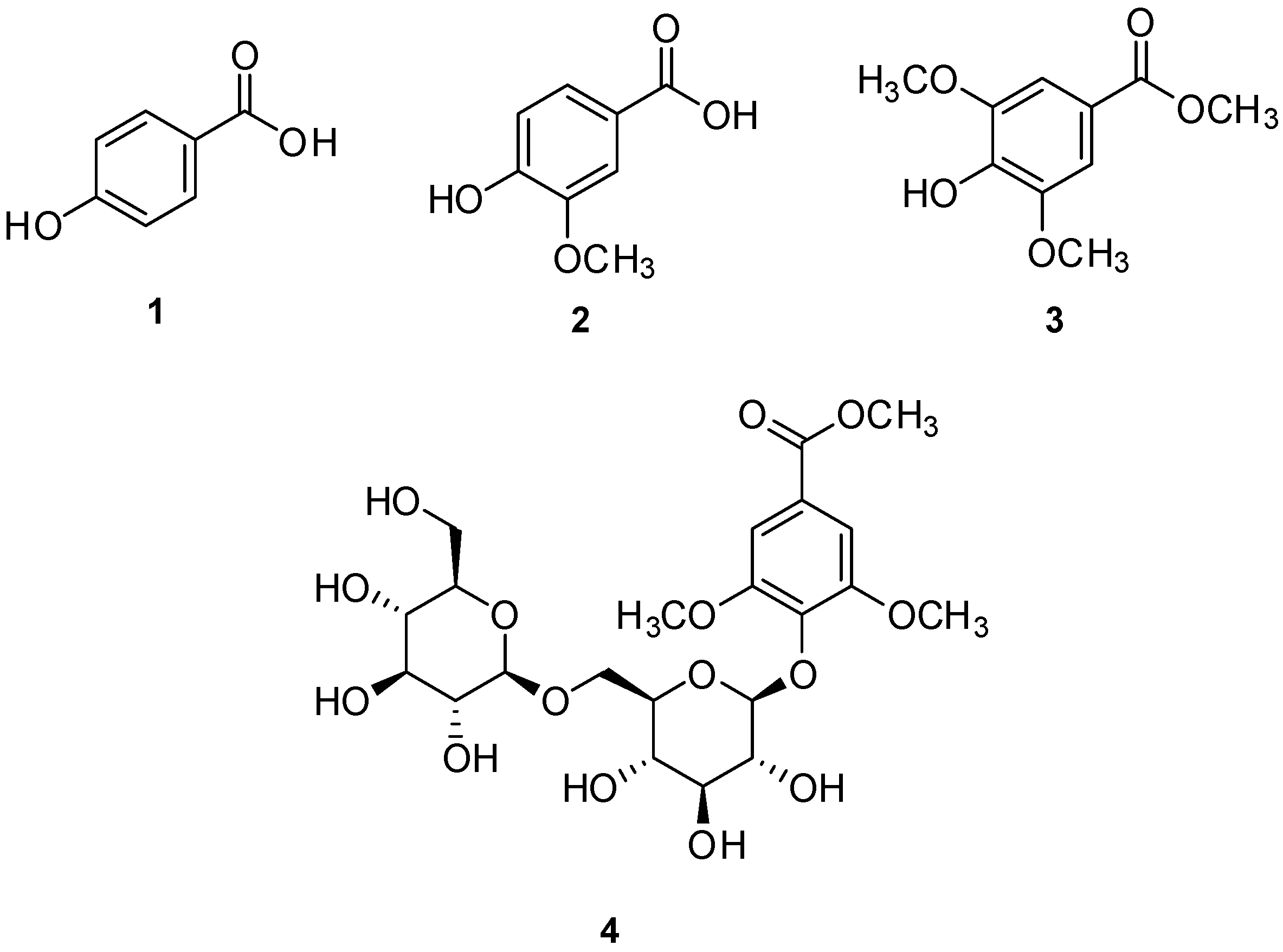
3.1. Isolation and Structure Elucidation of Compounds
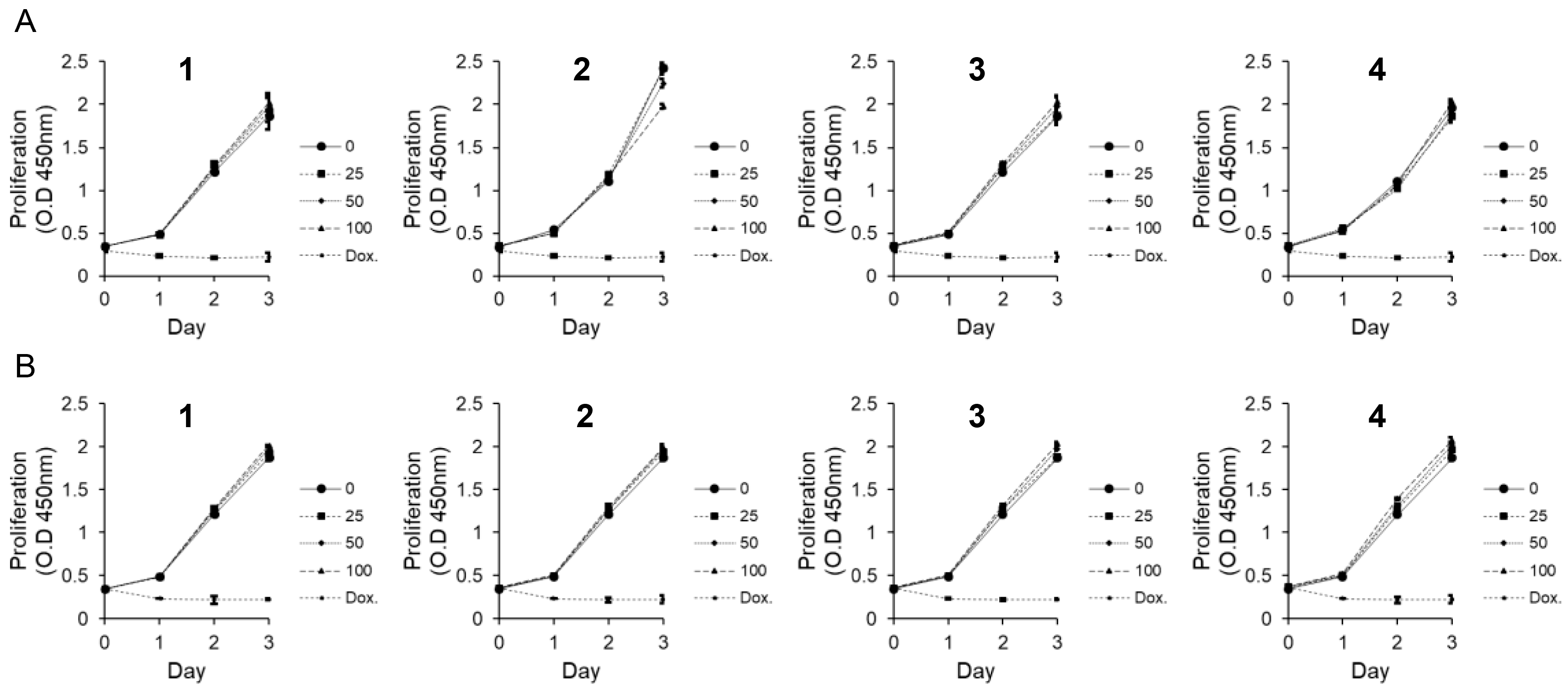
3.2. Effects of Compounds 1–4 on Cytotoxicity
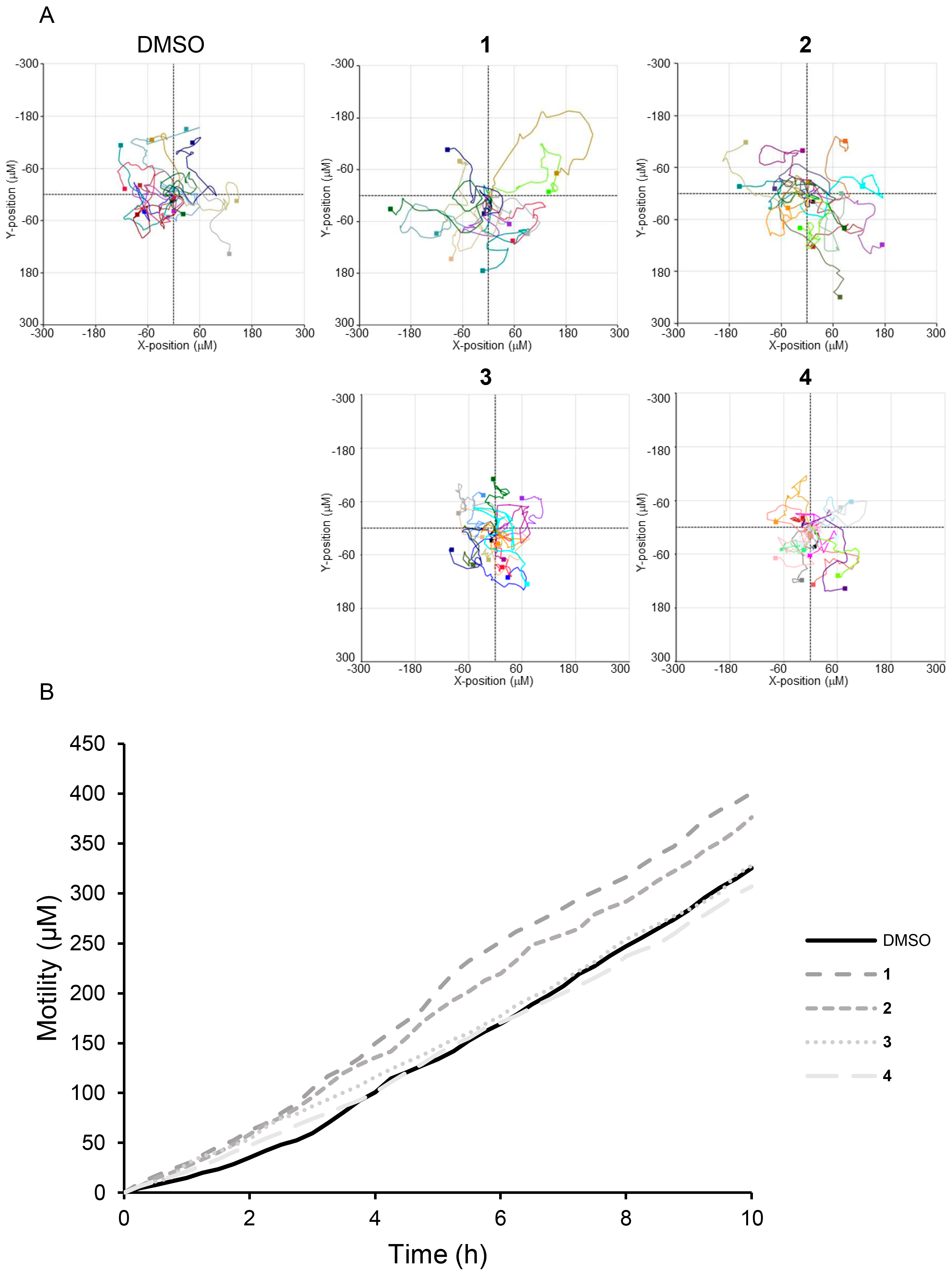
3.3. Effect of Compounds 1–4 on MEF Cells Motility
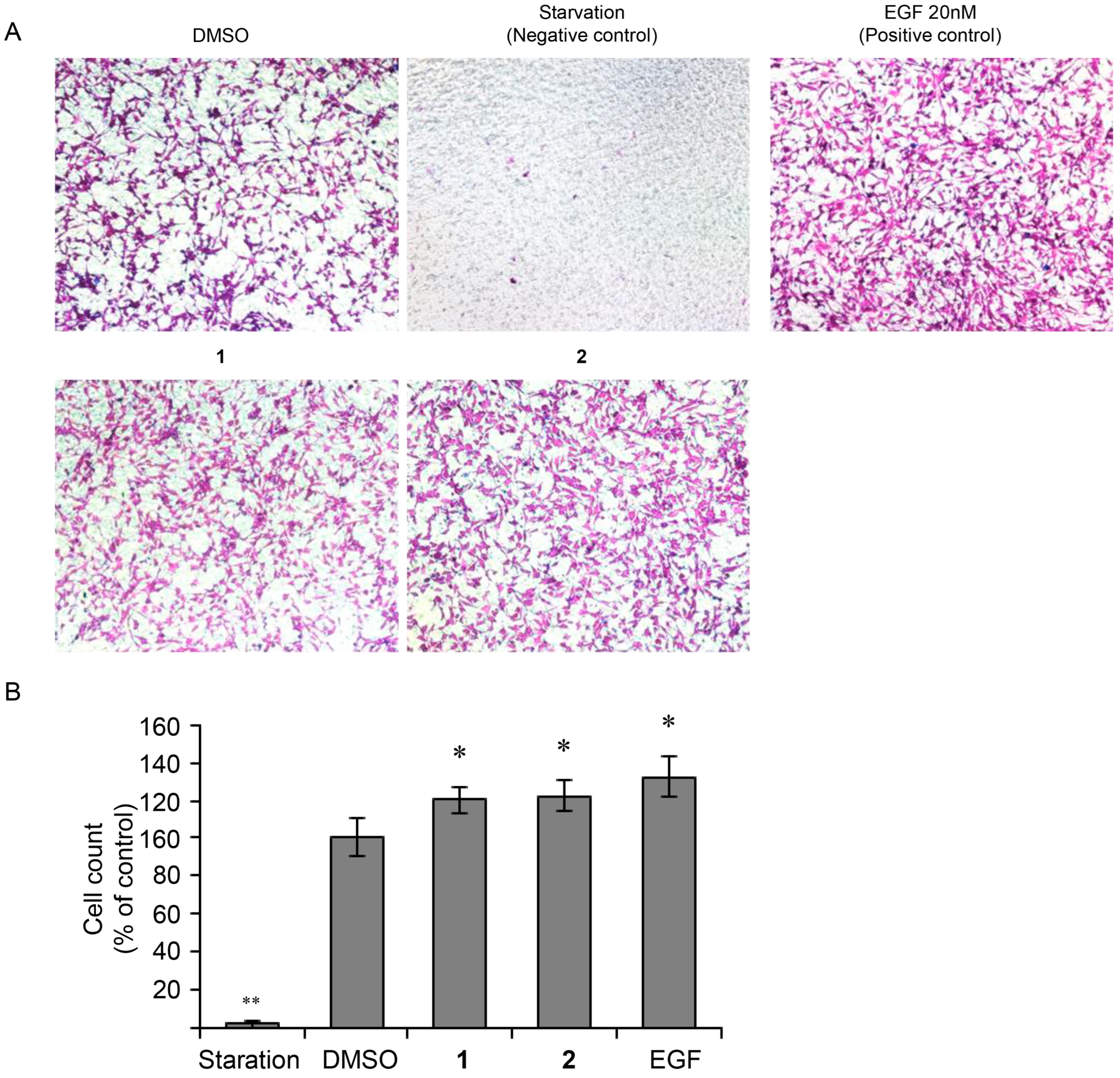
3.4. Effect of 4-Hydroxy-Benzoic Acid (1) and Vanillic Acid (2) on MEF Cells Invasion
3.5. Acceleration of FAK and MAPK Phosphorylation in MEF Cells Following Treatment with 4-Hydroxy-Benzoic Acid (1) and Vanillic Acid (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound Healing Concepts in Clinical Practice of OMFS. J. Maxillofac. Oral. Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Schmitt, S.; Safferling, K.; Westphal, K.; Hrabowski, M.; Müller, U.; Angel, P.; Wiechert, L.; Ehemann, V.; Müller, B.; Holland-Cunz, S.; et al. Stathmin regulates keratinocyte proliferation and migration during cutaneous regeneration. PLoS ONE 2013, 8, e75075. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Manuel, F.; Nascimento, D.S. The bright side of fibroblasts: Molecular signature and regenerative cues in major organs. NPJ Regen. Med. 2021, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Moravvej, H.; Hosseinzadeh, S.; Mostafavi, E.; Bayat, H.; Kazemi, B.; Bandehpour, M.; Rostami, E.; Rahimpour, A.; Moosavian, H. Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: In vitro and in vivo studies. Sci. Rep. 2022, 12, 18529. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- El Ghalbzouri, A.; Hensbergen, P.; Gibbs, S.; Kempenaar, J.; van der Schors, R.; Ponec, M. Fibroblasts facilitate re-epithelialization in wounded human skin equivalents. Lab. Investig. 2004, 84, 102–112. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Wang, J.; Zhang, C.; Sun, Y. Promoted Skin Wound Healing by Tail-Amputated Eisenia foetida Proteins via the Ras/Raf/MEK/ERK Signaling Pathway. ACS Omega 2023, 8, 13935–13943. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Katz, M.; Amit, I.; Yarden, Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 2007, 1773, 1161–1176. [Google Scholar] [CrossRef]
- Kong, T.; Liu, M.; Ji, B.; Bai, B.; Cheng, B.; Wang, C. Role of the Extracellular Signal-Regulated Kinase 1/2 Signaling Pathway in Ischemia-Reperfusion Injury. Front. Physiol. 2019, 10, 1038. [Google Scholar] [CrossRef]
- Kurtzeborn, K.; Kwon, H.N.; Kuure, S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int. J. Mol. Sci. 2019, 20, 1779. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Sang, Q.X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Siméon, A.; Monier, F.; Emonard, H.; Gillery, P.; Birembaut, P.; Hornebeck, W.; Maquart, F.X. Expression and activation of matrix metalloproteinases in wounds: Modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J. Investig. Dermatol. 1999, 112, 957–964. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Chen, P.C.; Tang, C.H.; Lin, L.W.; Tsai, C.H.; Chu, C.Y.; Lin, T.H.; Huang, Y.L. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J. Hematol. Oncol. 2017, 10, 33. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, Y.M.; Jeoung, D.I.; Kim, K.C.; Lee, H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp. Mol. Med. 2005, 37, 230–239. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Lee, W.; Chen, T. Functional characteristics of egg white solids obtained from papain treated albumen. J. Food Eng. 2002, 51, 263–266. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Zhao, W.; Lin, S.; Wang, E.; Zhang, Y.; Hao, H.; Wang, Z.; Chen, F. Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates. Food Chem. 2010, 122, 1159–1163. [Google Scholar] [CrossRef]
- Mine, Y.; Ma, F.; Lauriau, S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef]
- Qing, M.; Zang, J.; Ma, Y.; Chi, Y.; Chi, Y. Effects of rice vinegar treatment on the antioxidant activities and protein structures of whole egg liquid before and after gastrointestinal digestion. Food Chem. 2023, 404, 134574. [Google Scholar] [CrossRef]
- You, S.-J.; Udenigwe, C.C.; Aluko, R.E.; Wu, J. Multifunctional peptides from egg white lysozyme. Food Res. Int. 2010, 43, 848–855. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–16. [Google Scholar]
- Chen, Y.J.; Ma, M.H.; Fu, X. Changes in physicochemical properties of protein in vinegar-egg emulsion processing. Mod. Food Sci. Technol. 2019, 35, 80–86. [Google Scholar]
- Wang, S.-Y.; Chang, C.-Y.; Chen, C.-W. Effects of vinegar–egg on growth inhibition, differentiation human leukemic U937 cells and its immunomodulatory activity. J. Food. Drug. Anal. 2018, 26, 731–740. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, Z.-X.; Liu, Y.-P.; Jin, Y.-G. A comprehensive review on bioactive peptides derived from egg proteins. Food Sci. 2021, 42, 272–287. [Google Scholar]
- Cho, C.H.; Chae, S.H.; Kim, S.H.; Kim, K.H. Phenolic compounds isolated from Juncus decipiens and their effects on osteoblast differentiation in the mouse mesenchymal stem cell line C3H10T1/2. Nat. Prod. Sci. 2024, 30, 135–142. [Google Scholar] [CrossRef]
- Glick, V.J.; Webber, C.A.; Simmons, L.E.; Martin, M.C.; Ahmad, M.; Kim, C.H.; Adams, A.N.; Bang, S.; Chao, M.C.; Howard, N.C. Vaginal lactobacilli produce anti-inflammatory β-carboline compounds. Cell Host Microbe 2024, 32, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Park, K.H.; Hong, J.-H.; Kim, S.H.; Park, K.-M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jeong, S.Y.; Li, Z.; Kim, H.-Y.; Kim, H.-W.; Yoo, M.J.; Jang, H.J.; Kim, D.-K.; Cho, N.; Yoo, H.M. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharm. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Chebaibi, M.; Galvao de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic composition, wound healing, antinociceptive, and anticancer effects of Caralluma europaea extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef]
- Badaoui, M.I.; Chabani, S.; Magid, A.A.; Voutquenne-Nazabadioko, L.; Sayagh, C.; Harakat, D.; Haba, H. Chemical constituents of Centaurea dissecta Ten. and sesquiterpenes chemotaxonomic significance. Biochem. Syst. Ecol. 2024, 114, 104808. [Google Scholar] [CrossRef]
- Curir, P.; Dolci, M.; Dolci, P.; Lanzotti, V.; Cooman, L.D. Fungitoxic phenols from carnation (Dianthus caryophyllus) effective against Fusarium oxysporum f. sp. dianthi. Int. J. Plant Chem. Biochem. Tech. 2003, 14, 8–12. [Google Scholar] [CrossRef]
- Jermnak, U.; Yoshinari, T.; Sugiyama, Y.; Tsuyuki, R.; Nagasawa, H.; Sakuda, S. Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compounds. Int. J. Food Microbiol. 2012, 153, 339–344. [Google Scholar] [CrossRef]
- Aitken, H.R.; Johannes, M.; Loomes, K.M.; Brimble, M.A. Synthesis of leptosin, a glycoside isolated from mānuka honey. Tetrahedron Lett. 2013, 54, 6916–6919. [Google Scholar] [CrossRef]
- D’Onofrio, P.M.; Shabanzadeh, A.P.; Choi, B.K.; Bähr, M.; Koeberle, P.D. MMP Inhibition Preserves Integrin Ligation and FAK Activation to Induce Survival and Regeneration in RGCs Following Optic Nerve Damage. Investig. Ophthalmol. Vis. Sci. 2019, 60, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Shutova, M.S.; Boehncke, W.H. Mechanotransduction in Skin Inflammation. Cells 2022, 11, 2026. [Google Scholar] [CrossRef]
- Frey, M.R.; Golovin, A.; Polk, D.B. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J. Biol. Chem. 2004, 279, 44513–44521. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.; Cho, C.H.; Baek, S.C.; Jo, M.S.; Kang, W.B.; Kang, Y.S.; Ko, S.-K.; Kim, K.H. Bioactive Phenolics from Vinegar–Egg Accelerates Acute Wound Healing by Activation of Focal Adhesion and Mitogen-Activated Protein Kinase Signaling. Nutrients 2025, 17, 2584. https://doi.org/10.3390/nu17162584
Oh T, Cho CH, Baek SC, Jo MS, Kang WB, Kang YS, Ko S-K, Kim KH. Bioactive Phenolics from Vinegar–Egg Accelerates Acute Wound Healing by Activation of Focal Adhesion and Mitogen-Activated Protein Kinase Signaling. Nutrients. 2025; 17(16):2584. https://doi.org/10.3390/nu17162584
Chicago/Turabian StyleOh, Taehoon, Chan Hee Cho, Su Cheol Baek, Mun Seok Jo, Woo Bong Kang, Yun Seok Kang, Sung-Kyun Ko, and Ki Hyun Kim. 2025. "Bioactive Phenolics from Vinegar–Egg Accelerates Acute Wound Healing by Activation of Focal Adhesion and Mitogen-Activated Protein Kinase Signaling" Nutrients 17, no. 16: 2584. https://doi.org/10.3390/nu17162584
APA StyleOh, T., Cho, C. H., Baek, S. C., Jo, M. S., Kang, W. B., Kang, Y. S., Ko, S.-K., & Kim, K. H. (2025). Bioactive Phenolics from Vinegar–Egg Accelerates Acute Wound Healing by Activation of Focal Adhesion and Mitogen-Activated Protein Kinase Signaling. Nutrients, 17(16), 2584. https://doi.org/10.3390/nu17162584






