Neuroprotective and Neurotrophic Potential of Flammulina velutipes Extracts in Primary Hippocampal Neuronal Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals/Reagents
2.2. Preparation of Extract
2.3. Standardization of the Extracts
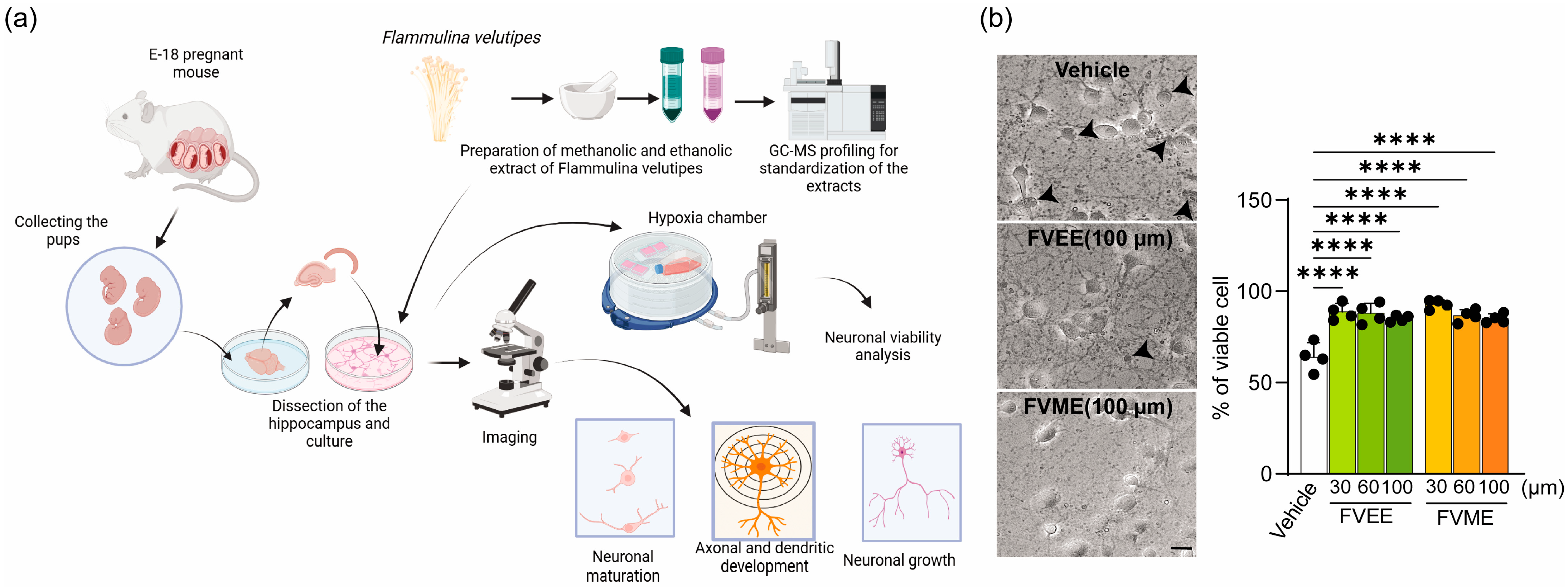
2.4. Primary Hippocampal Neuronal Culture
2.5. Hypoxia/Reoxygenation (H/R)-Induced Oxidative Stress
2.6. Trypan Blue Exclusion Assay for Neuronal Viability
2.7. Immunocytochemistry
2.8. Image Analysis and Quantification
2.9. In Silico Identification of Potential Bioactive Compounds
2.9.1. Glide-Based Molecular Docking Analysis
2.9.2. Binding Energy (MM-GBSA) Analysis
2.10. Statistical Analysis
3. Results
3.1. Chemical Characterization of FVME and FVEE
3.2. Effects of FVME and FVEE on Cell Viability
3.3. Dose-Dependent Effects of FVME and FVEE on Neuronal Development
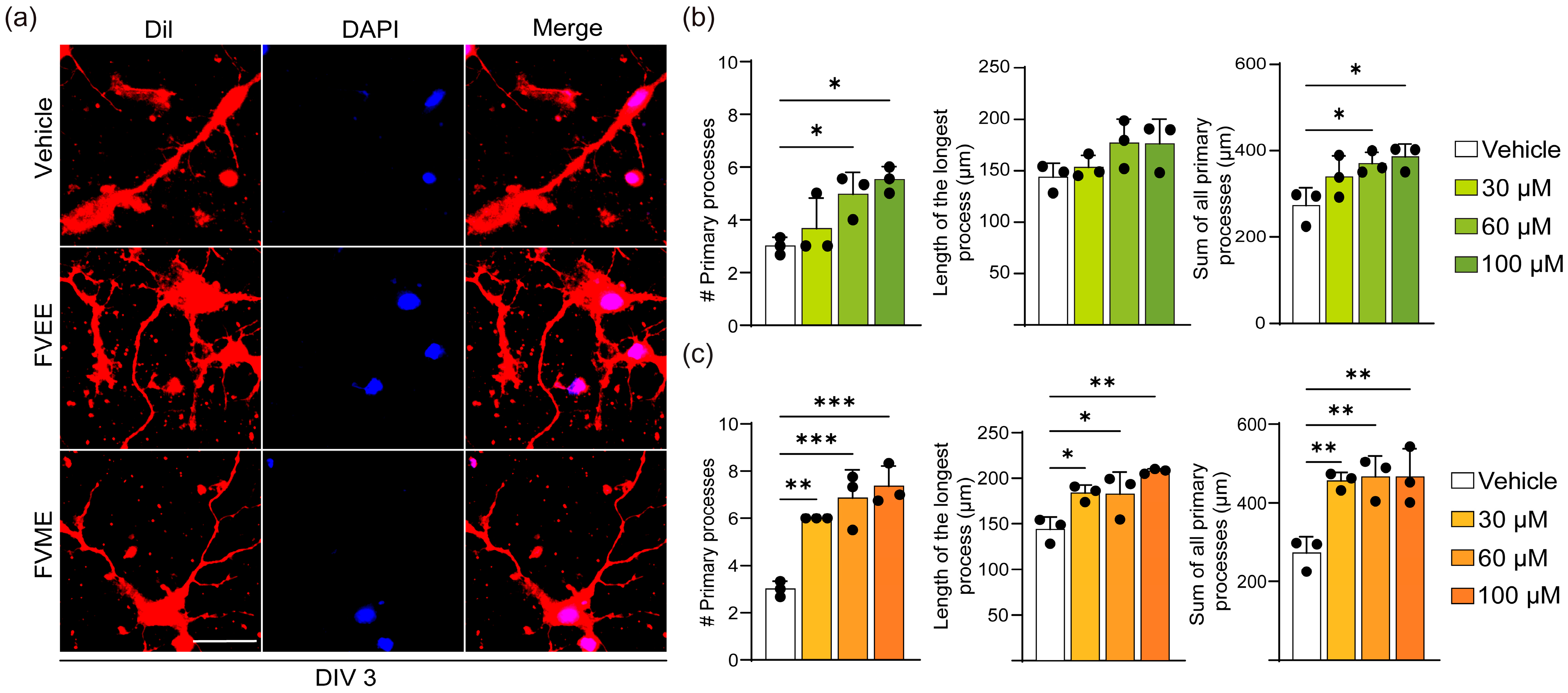
3.3.1. FVME and FVEE Promote Neurite Outgrowth
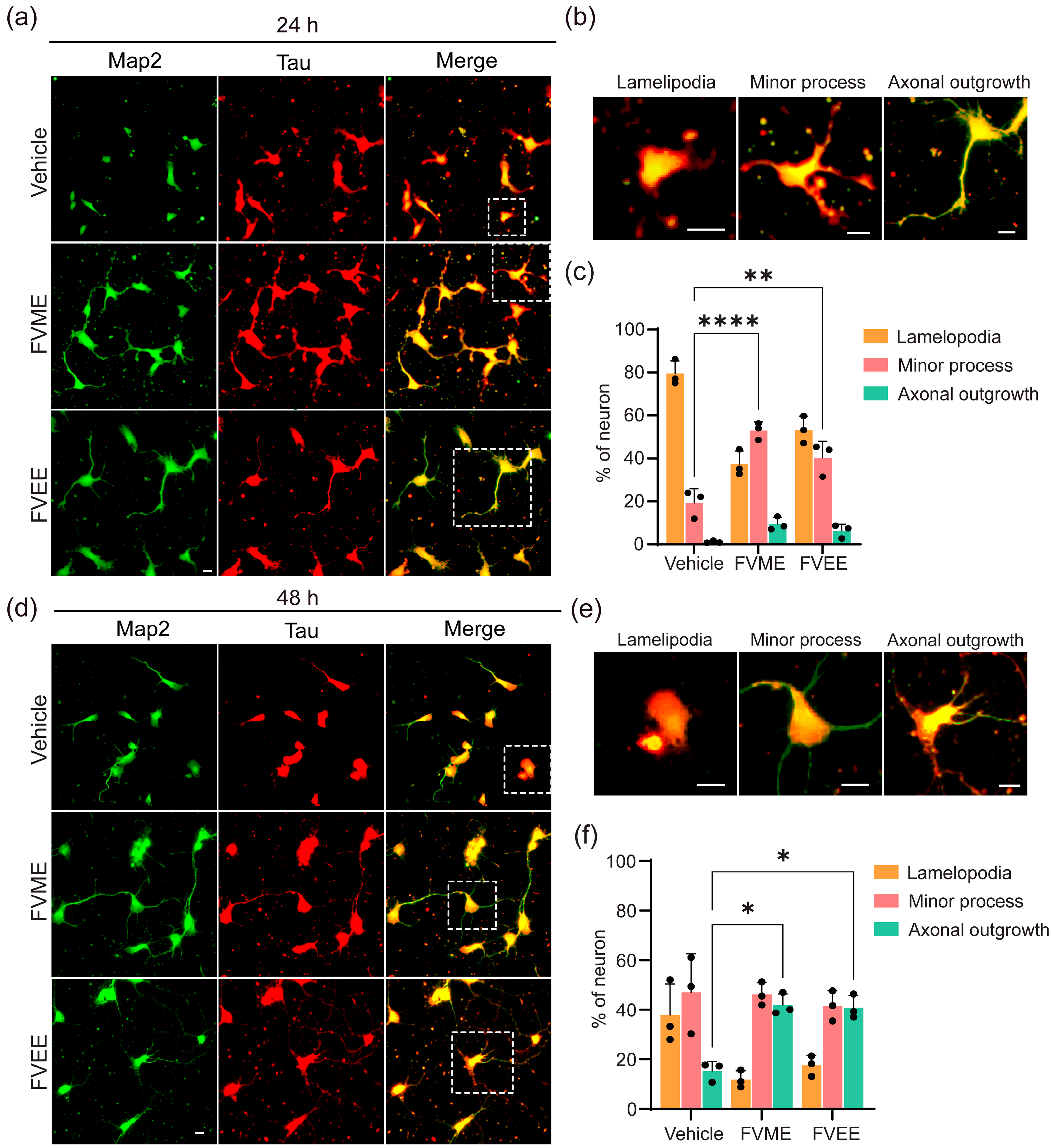
3.3.2. FVME and FVEE Promote Early Neuronal Development
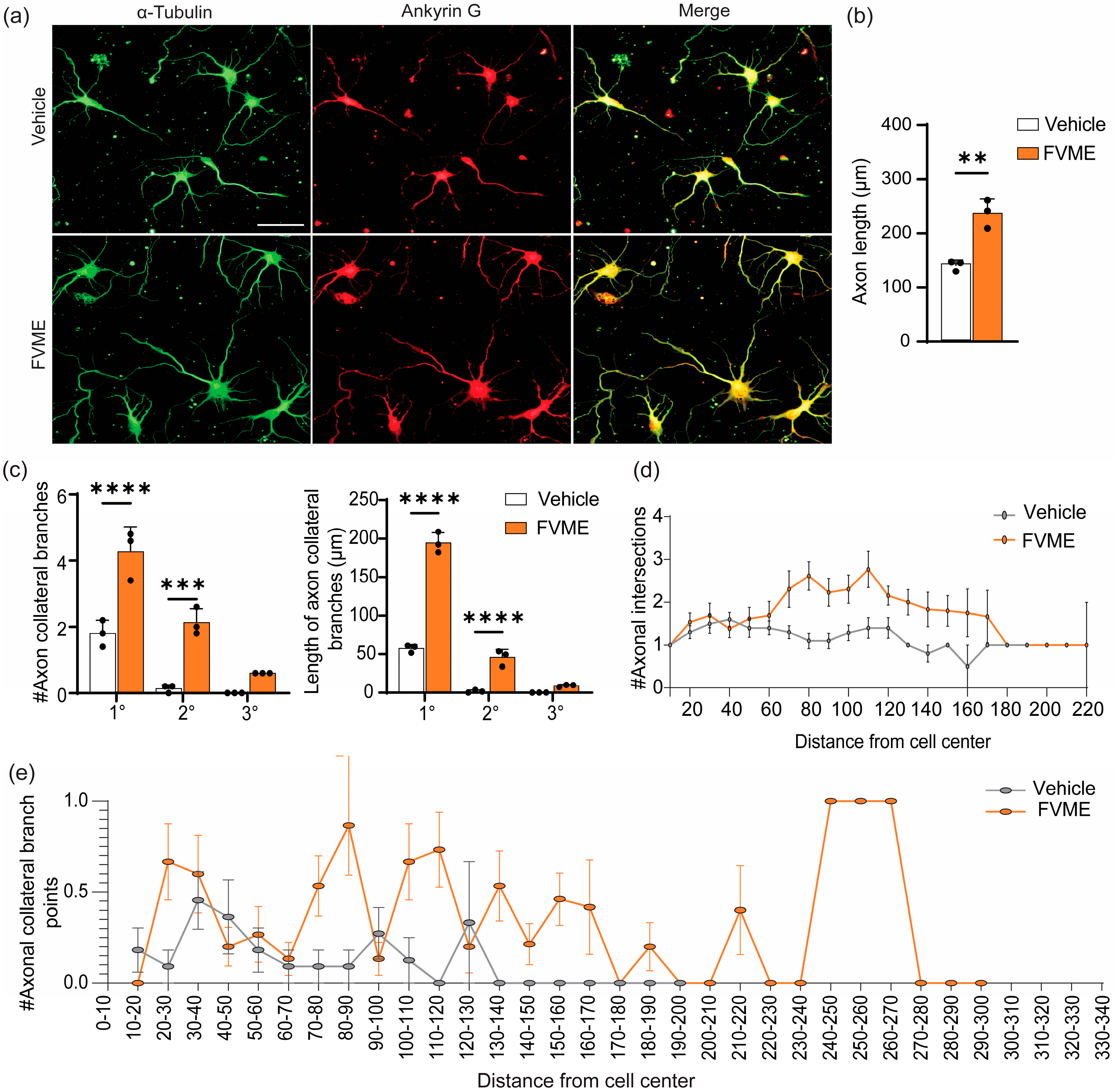
3.3.3. Axonal Development Due to FVME
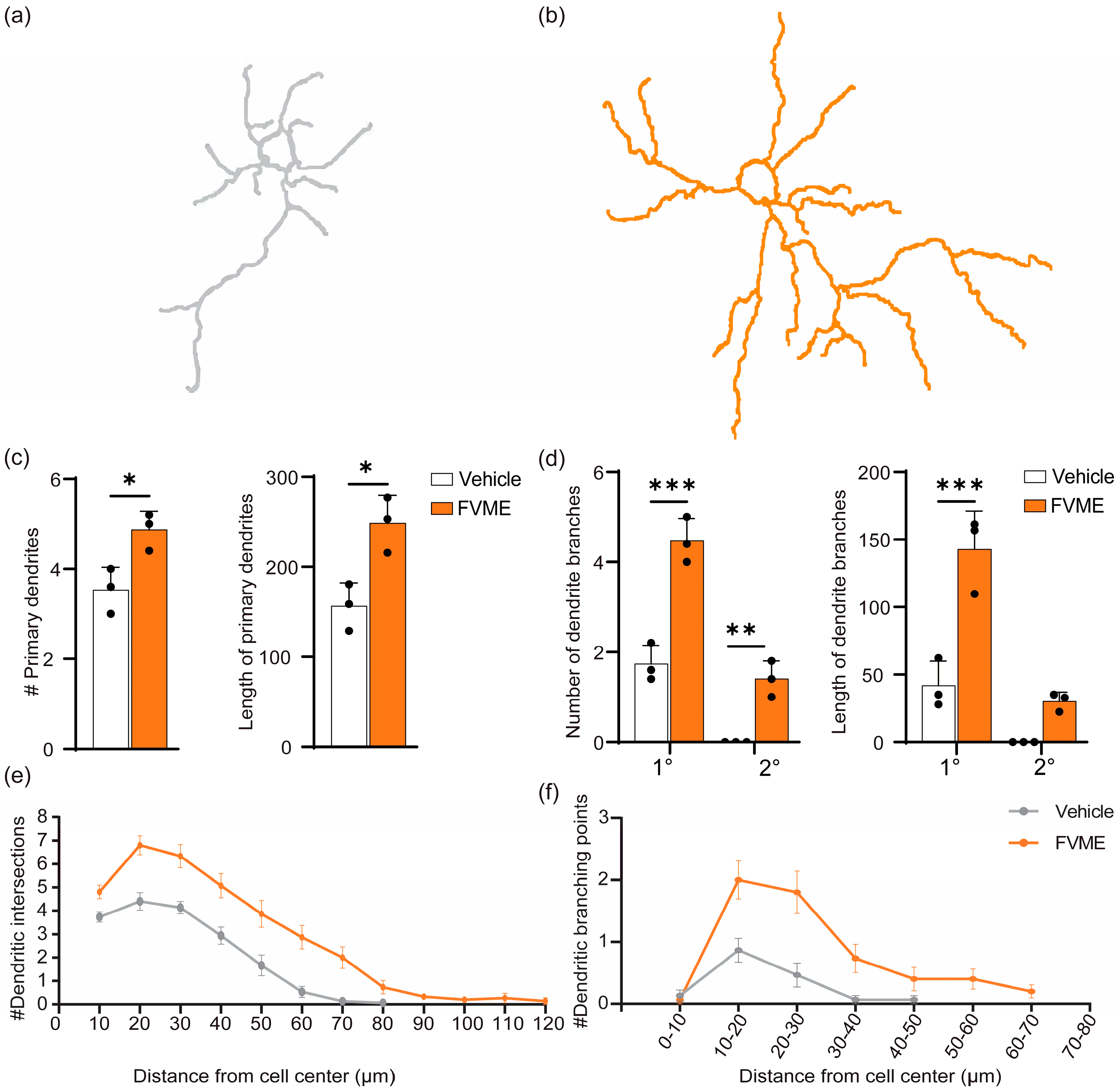
3.3.4. Dendritogenic Arborization Due to FVME
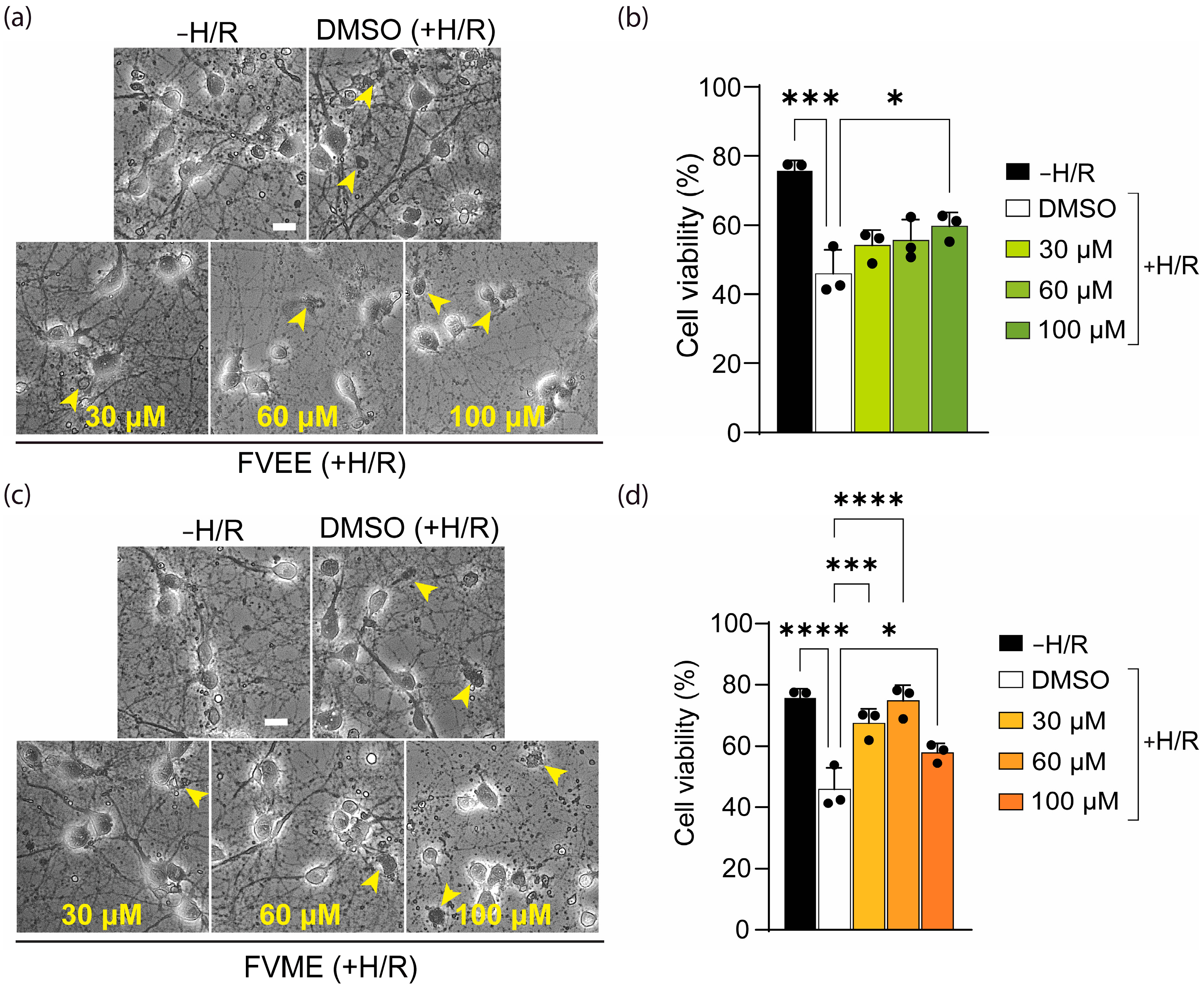
3.4. FVME and FVEE Protect Neurons Against H/R Injury
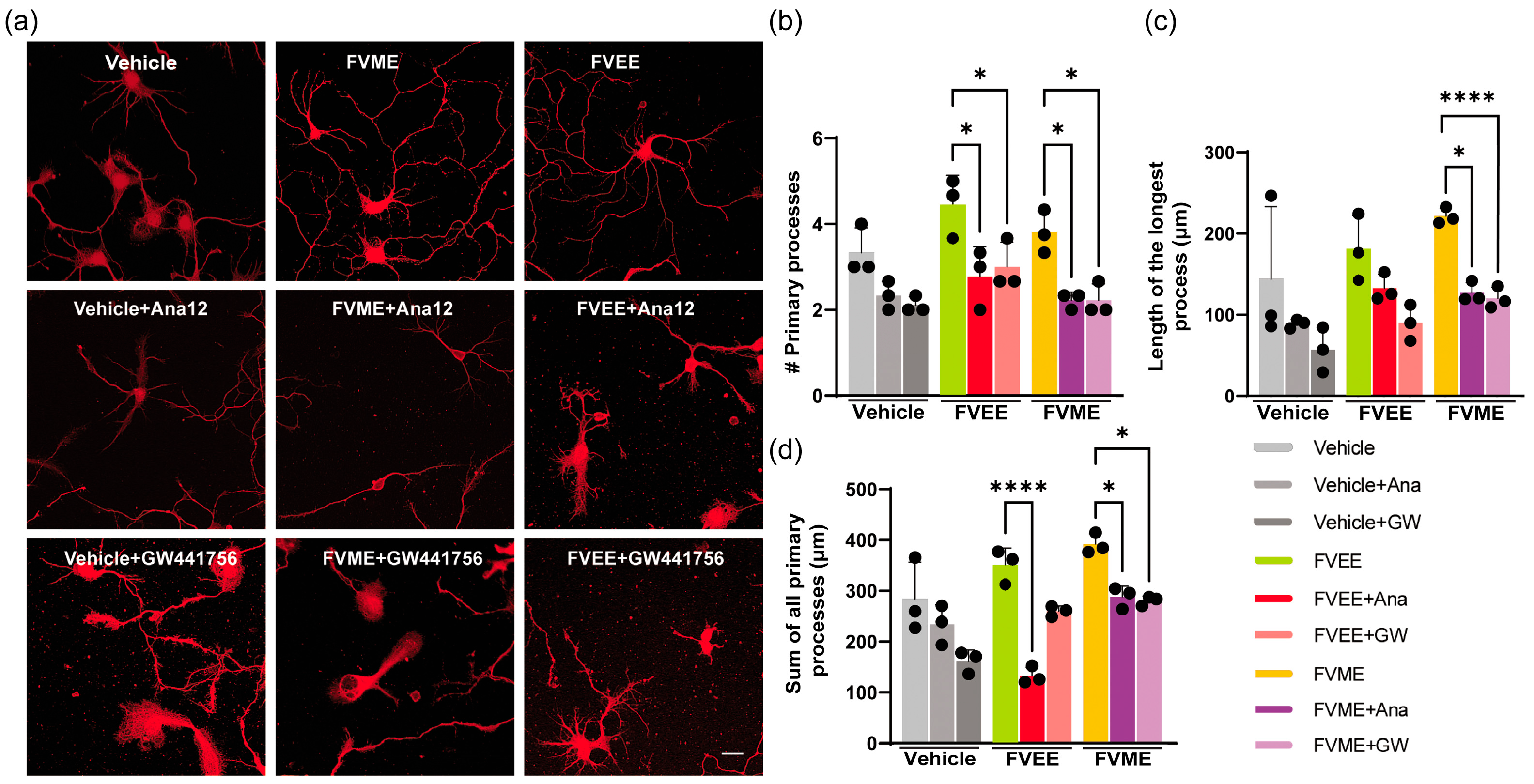
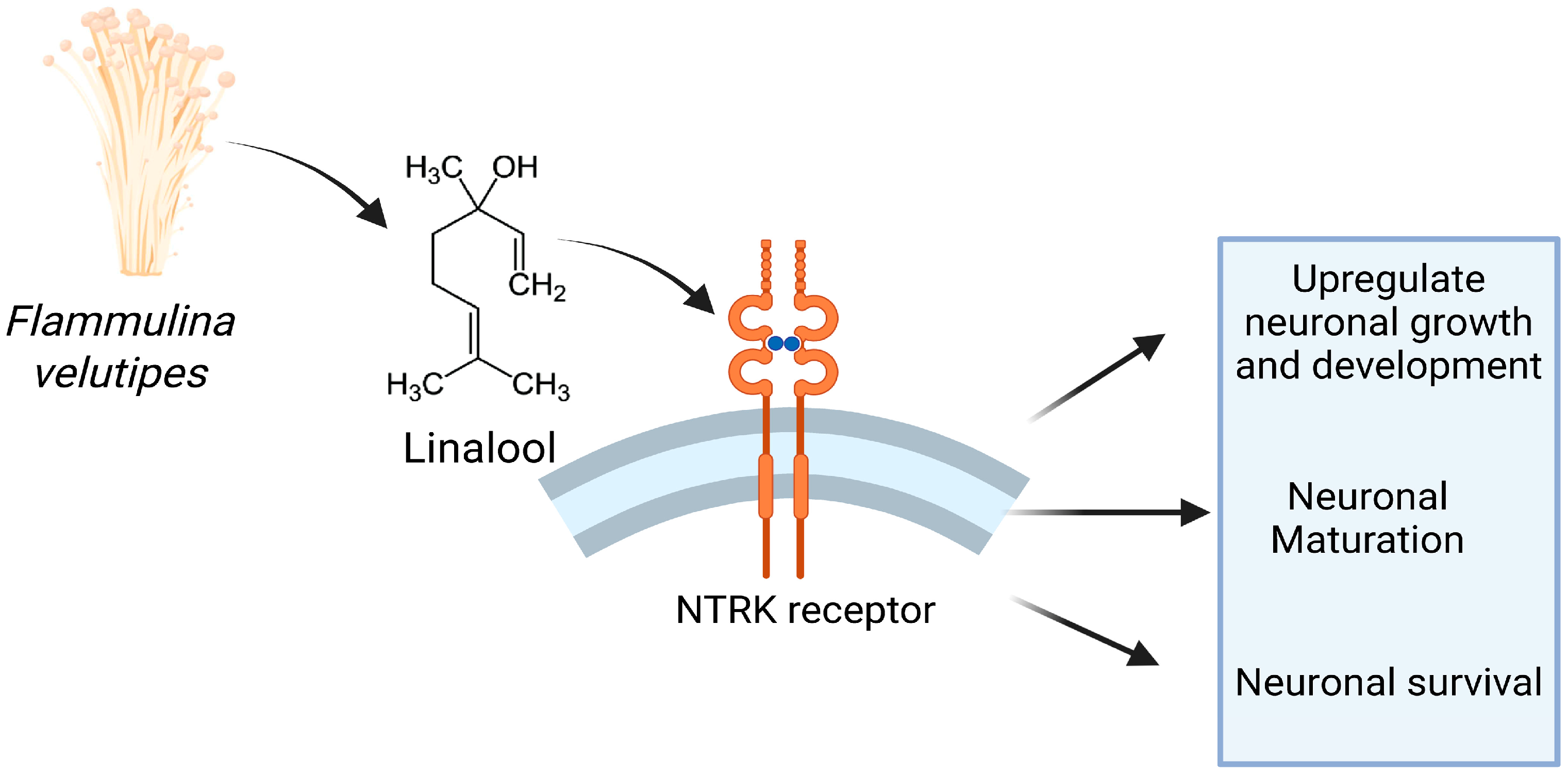
3.5. Mechanistic Analysis of the Neurogenic Effect of FVME and FVEE
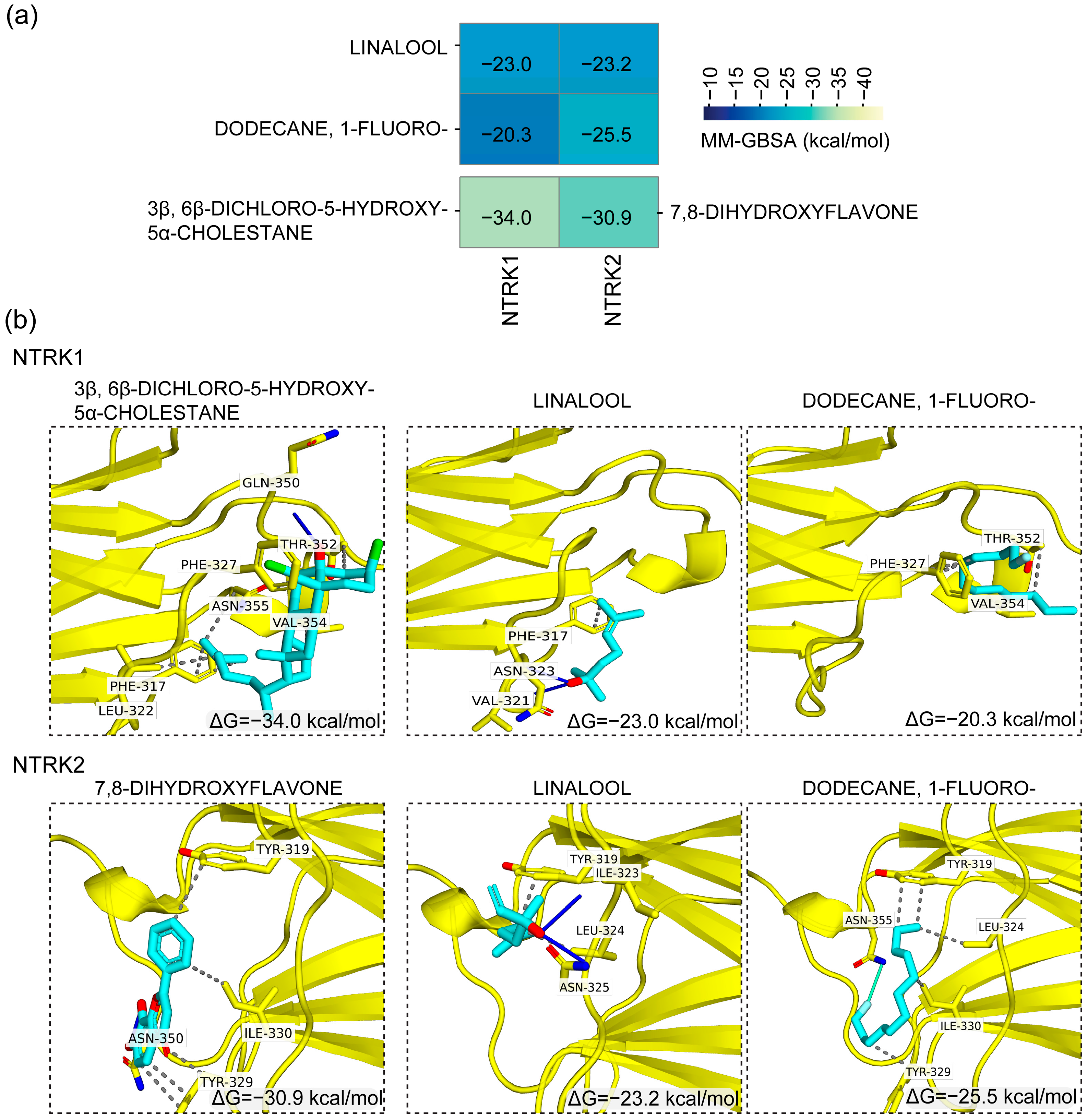
3.6. In Silico-Based Identification of Potential NTRK Receptor Modulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Ganesan, K.; Xu, B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. J. Fungi 2024, 10, 215. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Fulgoni Iii, V.L. Nutritional impact of adding a serving of mushrooms to USDA Food Patterns—A dietary modeling analysis. Food Nutr. Res. 2021, 65, 5618. [Google Scholar] [CrossRef] [PubMed]
- You, S.W.; Hoskin, R.T.; Komarnytsky, S.; Moncada, M. Mushrooms as Functional and Nutritious Food Ingredients for Multiple Applications. ACS Food Sci. Technol. 2022, 2, 1184–1195. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, S.; Wang, H.; Li, S.; Ma, C.; Wang, J.; Cui, L. Identification of Secondary Metabolites in Flammulina velutipes by UPLC-Q-Exactive-Orbitrap MS. J. Food Qual. 2021, 2021, 4103952. [Google Scholar] [CrossRef]
- Su, A.; Yang, W.; Zhao, L.; Pei, F.; Yuan, B.; Zhong, L.; Ma, G.; Hu, Q. Flammulina velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. 2018, 9, 1424–1432. [Google Scholar] [CrossRef]
- Yang, W.; Fang, Y.; Liang, J.; Hu, Q. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res. Int. 2011, 44, 1269–1275. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Zhao, L.; Ma, N.; Fang, Y.; Pei, F.; Mariga, A.M.; Hu, Q. Polysaccharides from Flammulina velutipes improve scopolamine-induced impairment of learning and memory of rats. J. Funct. Foods 2015, 18, 411–422. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, Q.; Zhao, L.; Ma, G.; Ma, N.; Zhang, J.; Ji, Y.; Liu, L. A novel neuroprotective peptide YVYAETY identified and screened from Flammulina velutipes protein hydrolysates attenuates scopolamine-induced cognitive impairment in mice. Food Funct. 2024, 15, 6082–6094. [Google Scholar] [CrossRef]
- Burns, A.; Zaudig, M. Mild cognitive impairment in older people. Lancet 2002, 360, 1963–1965. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W. What we need to know about age related memory loss. BMJ 2002, 324, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Watson, R. Research into ageing and older people. J. Nurs. Manag. 2008, 16, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Willis, R.; Feng, Z. Factors influencing quality of life of elderly people with dementia and care implications: A systematic review. Arch. Gerontol. Geriatr. 2016, 66, 23–41. [Google Scholar] [CrossRef]
- Holopainen, A.; Siltanen, H.; Pohjanvuori, A.; Mäkisalo-Ropponen, M.; Okkonen, E. Factors Associated with the Quality of Life of People with Dementia and with Quality of Life-Improving Interventions: Scoping Review. Dementia 2019, 18, 1507–1537. [Google Scholar] [CrossRef]
- Orme, T.; Hernandez, D.; Ross, O.A.; Kun-Rodrigues, C.; Darwent, L.; Shepherd, C.E.; Parkkinen, L.; Ansorge, O.; Clark, L.; Honig, L.S.; et al. Analysis of neurodegenerative disease-causing genes in dementia with Lewy bodies. Acta Neuropathol. Commun. 2020, 8, 5. [Google Scholar] [CrossRef]
- Birajdar, S.V.; Mulchandani, M.; Mazahir, F.; Yadav, A.K. Chapter 1—Dementia and neurodegenerative disorder: An introduction. In Nanomedicine-Based Approaches for the Treatment of Dementia; Gupta, U., Kesharwani, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–36. [Google Scholar]
- Nie, R.-Z.; Luo, H.-M.; Liu, Y.-P.; Wang, S.-S.; Hou, Y.-J.; Chen, C.; Wang, H.; Lv, H.-L.; Tao, X.-Y.; Jing, Z.-H.; et al. Food Functional Factors in Alzheimer’s Disease Intervention: Current Research Progress. Nutrients 2024, 16, 3998. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yang, X.; Jin, G.; Zhang, Y. Cognitive-enhancing effect of polysaccharides from Flammulina velutipes on Alzheimer’s disease by compatibilizing with ginsenosides. Int. J. Biol. Macromol. 2018, 112, 788–795. [Google Scholar] [CrossRef]
- Mitra, S.; Bhartiya, P.; Kaushik, N.; Nhat Nguyen, L.; Wahab, R.; Bekeschus, S.; Choi, E.H.; Kaushik, N.K. Plasma-Treated Flammulina velutipes-Derived Extract Showed Anticancer Potential in Human Breast Cancer Cells. Appl. Sci. 2020, 10, 8395. [Google Scholar] [CrossRef]
- Mitra, S.; Veerana, M.; Choi, E.H.; Park, G. Effects of Pre-Treatment Using Plasma on the Antibacterial Activity of Mushroom Surfaces. Foods 2021, 10, 1888. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Hannan, M.A.; Choi, J.-Y.; Bhuiyan, M.M.H.; Hong, Y.-K.; Choi, J.-S.; Choi, I.S.; Moon, I.S. The Edible Marine Alga Gracilariopsis chorda Alleviates Hypoxia/Reoxygenation-Induced Oxidative Stress in Cultured Hippocampal Neurons. J. Med. Food 2015, 18, 960–971. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, A.-S.; Wu, L.-Y.; Ma, Z.-M.; Fan, M. Establishment of the model of oxygen-glucose deprivation in vitro rat hippocampal neurons. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2003, 19, 197–200. [Google Scholar] [PubMed]
- Chiu, Y.J.; Lin, T.H.; Chang, K.H.; Lin, W.; Hsieh-Li, H.M.; Su, M.T.; Chen, C.M.; Sun, Y.C.; Lee-Chen, G.J. Novel TRKB agonists activate TRKB and downstream ERK and AKT signaling to protect Aβ-GFP SH-SY5Y cells against Aβ toxicity. Aging 2022, 14, 7568–7586. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Haque, M.N.; Dash, R.; Alam, M.; Moon, I.S. 3β, 6β-dichloro-5-hydroxy-5α-cholestane facilitates neuronal development through modulating TrkA signaling regulated proteins in primary hippocampal neuron. Sci. Rep. 2019, 9, 18919. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Gupta, U.; Gupta, S.; Wadhwa, G.; Gabrani, R.; Sharma, S.K.; Jain, C.K. Molecular docking of glucosamine-6-phosphate synthase in Rhizopus oryzae. Bioinformation 2011, 7, 285–290. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Vijayakumar, B.; Umamaheswari, A.; Puratchikody, A.; Velmurugan, D. Selection of an improved HDAC8 inhibitor through structure-based drug design. Bioinformation 2011, 7, 134–141. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Zhang, F.; Liu, L.; Lei, B.-H.; Dong, X.; Yang, Z.; Li, H.; Pan, S. Li3AlSiO5: The first aluminosilicate as a potential deep-ultraviolet nonlinear optical crystal with the quaternary diamond-like structure. Phys. Chem. Chem. Phys. 2016, 18, 4362–4369. [Google Scholar] [CrossRef]
- Hou, T.; Li, N.; Li, Y.; Wang, W. Characterization of domain-peptide interaction interface: Prediction of SH3 domain-mediated protein-protein interaction network in yeast by generic structure-based models. J. Proteome Res. 2012, 11, 2982–2995. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys. Chem. Chem. Phys. 2014, 16, 16719–16729. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Shen, M.; Tian, S.; Xu, L.; Pan, P.; Guan, Y.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 5. Improved docking performance using high solute dielectric constant MM/GBSA and MM/PBSA rescoring. Phys. Chem. Chem. Phys. 2014, 16, 22035–22045. [Google Scholar] [CrossRef]
- Li, J.; Abel, R.; Zhu, K.; Cao, Y.; Zhao, S.; Friesner, R.A. The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling. Proteins 2011, 79, 2794–2812. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Munni, Y.A.; Dash, R.; Sultana, A.; Moon, I.S. Unveiling the effect of Withania somnifera on neuronal cytoarchitecture and synaptogenesis: A combined in vitro and network pharmacology approach. Phytother. Res. 2022, 36, 2524–2541. [Google Scholar] [CrossRef] [PubMed]
- Nieber, K. Hypoxia and Neuronal Function Under In Vitro Conditions. Pharmacol. Ther. 1999, 82, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Kim, C.K.; Cho, J.H.; Lee, K.H.; Ahn, J.Y. Neuroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19-7/IGF-IR [corrected]. Exp. Mol. Med. 2010, 42, 583–595. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef]
- Saito, A.; Tominaga, T.; Chan, P.H. Neuroprotective role of neurotrophins: Relationship between nerve growth factor and apoptotic cell survival pathway after cerebral ischemia. Curr. Atheroscler. Rep. 2005, 7, 268–273. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Chang, W.-H.; Hsu, H.-T.; Lin, C.-C.; An, L.-M.; Lee, C.-H.; Ko, H.-H.; Lin, C.-L.; Lo, Y.-C. Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 2514. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.-S.; Won, S.-Y.; Cho, K.S. Linalool Alleviates Aβ42-Induced Neurodegeneration via Suppressing ROS Production and Inflammation in Fly and Rat Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Major, B.; Rák, T.; Sipos, K.; Csutak, A.; Horváth, G. Linalool and Geraniol Defend Neurons from Oxidative Stress, Inflammation, and Iron Accumulation in In Vitro Parkinson’s Models. Antioxidants 2024, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Jung, A.N.; Jung, Y.S. Linalool Ameliorates Memory Loss and Behavioral Impairment Induced by REM-Sleep Deprivation through the Serotonergic Pathway. Biomol. Ther. 2018, 26, 368–373. [Google Scholar] [CrossRef]
- Lanoue, V.; Cooper, H.M. Branching mechanisms shaping dendrite architecture. Dev. Biol. 2019, 451, 16–24. [Google Scholar] [CrossRef]
- Parekh, R.; Ascoli, G.A. Quantitative Investigations of Axonal and Dendritic Arbors:Development, Structure, Function, and Pathology. Neuroscientist 2015, 21, 241–254. [Google Scholar] [CrossRef]
- Kirchner, J.H.; Euler, L.; Fritz, I.; Ferreira Castro, A.; Gjorgjieva, J. Dendritic growth and synaptic organization from activity-independent cues and local activity-dependent plasticity. eLife 2025, 12, RP87527. [Google Scholar] [CrossRef]
- Ledda, F.; Paratcha, G. Mechanisms regulating dendritic arbor patterning. Cell. Mol. Life Sci. 2017, 74, 4511–4537. [Google Scholar] [CrossRef]
- Peng, Y.R.; He, S.; Marie, H.; Zeng, S.Y.; Ma, J.; Tan, Z.J.; Lee, S.Y.; Malenka, R.C.; Yu, X. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron 2009, 61, 71–84. [Google Scholar] [CrossRef]
- Jamann, N.; Jordan, M.; Engelhardt, M. Activity-Dependent Axonal Plasticity in Sensory Systems. Neuroscience 2018, 368, 268–282. [Google Scholar] [CrossRef]
- Ginglinger, J.F.; Boachon, B.; Höfer, R.; Paetz, C.; Köllner, T.G.; Miesch, L.; Lugan, R.; Baltenweck, R.; Mutterer, J.; Ullmann, P.; et al. Gene coexpression analysis reveals complex metabolism of the monoterpene alcohol linalool in Arabidopsis flowers. Plant Cell 2013, 25, 4640–4657. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.-N.; Zhong, X.-X.; He, L.; Gao, Y.; Zhang, X.; Wang, C.-M.; Du, X. Comparison of different aroma-active compounds of Sichuan Dark brick tea (Camellia sinensis) and Sichuan Fuzhuan brick tea using gas chromatography–mass spectrometry (GC–MS) and aroma descriptive profile tests. Eur. Food Res. Technol. 2019, 245, 1963–1979. [Google Scholar] [CrossRef]
- Xiao, M.; Zheng, F.; Xiao, M.; Qi, A.; Wang, H.; Dai, Q. Contribution of aroma-active compounds to the aroma of Lu’an Guapian tea. Flavour Fragr. J. 2022, 37, 83–95. [Google Scholar] [CrossRef]
- Qu, F.-F.; Li, X.-H.; Wang, P.-Q.; Han, Y.-H.; Wu, Y.; Hu, J.-H.; Zhang, X.-F. Effect of thermal process on the key aroma components of green tea with chestnut-like aroma. J. Sci. Food Agric. 2023, 103, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms-Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Satou, T.; Kamagata, M.; Nakashima, S.; Mori, K.; Yoshinari, M. Effects of Inhaled Linalool on the Autonomic Nervous System in Awake Mice. Nat. Prod. Commun. 2024, 19, 1934578X241232273. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, S.T.; Liao, V.H. Potential anti-Parkinsonian’s effect of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves are associated with mitochondrial regulation via gas-1, nuo-1, and mev-1 in Caenorhabditis elegans. Phytother. Res. 2022, 36, 3325–3334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, S.; Dash, R.; Bashar, M.A.; Mazumder, K.; Moon, I.S. Neuroprotective and Neurotrophic Potential of Flammulina velutipes Extracts in Primary Hippocampal Neuronal Culture. Nutrients 2025, 17, 3107. https://doi.org/10.3390/nu17193107
Mitra S, Dash R, Bashar MA, Mazumder K, Moon IS. Neuroprotective and Neurotrophic Potential of Flammulina velutipes Extracts in Primary Hippocampal Neuronal Culture. Nutrients. 2025; 17(19):3107. https://doi.org/10.3390/nu17193107
Chicago/Turabian StyleMitra, Sarmistha, Raju Dash, Md Abul Bashar, Kishor Mazumder, and Il Soo Moon. 2025. "Neuroprotective and Neurotrophic Potential of Flammulina velutipes Extracts in Primary Hippocampal Neuronal Culture" Nutrients 17, no. 19: 3107. https://doi.org/10.3390/nu17193107
APA StyleMitra, S., Dash, R., Bashar, M. A., Mazumder, K., & Moon, I. S. (2025). Neuroprotective and Neurotrophic Potential of Flammulina velutipes Extracts in Primary Hippocampal Neuronal Culture. Nutrients, 17(19), 3107. https://doi.org/10.3390/nu17193107








