Associations of Birth Size with Physical and Cognitive Function in Men and Women 60 Years and Older—Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
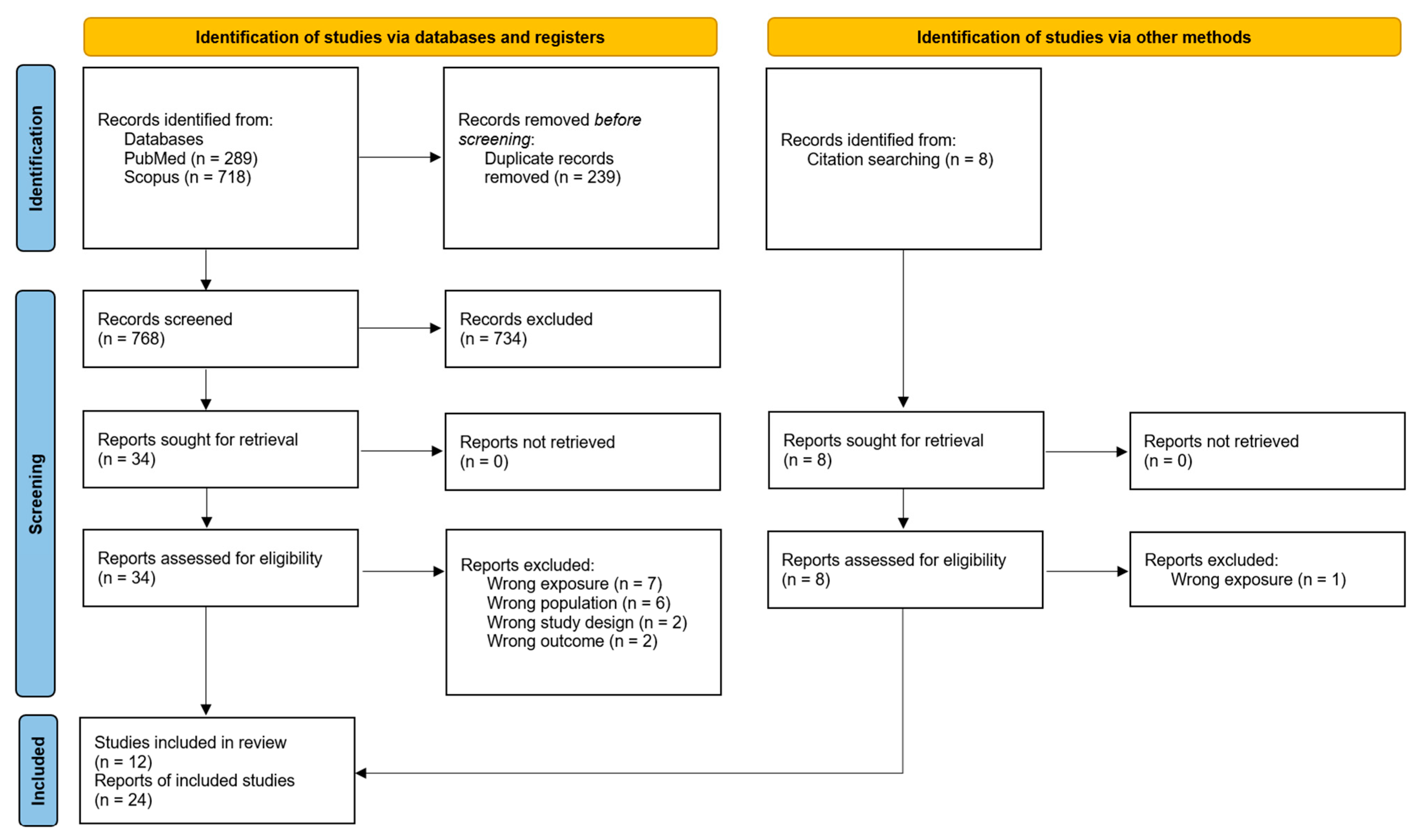
2.3. Article Selection
2.4. Data Collection
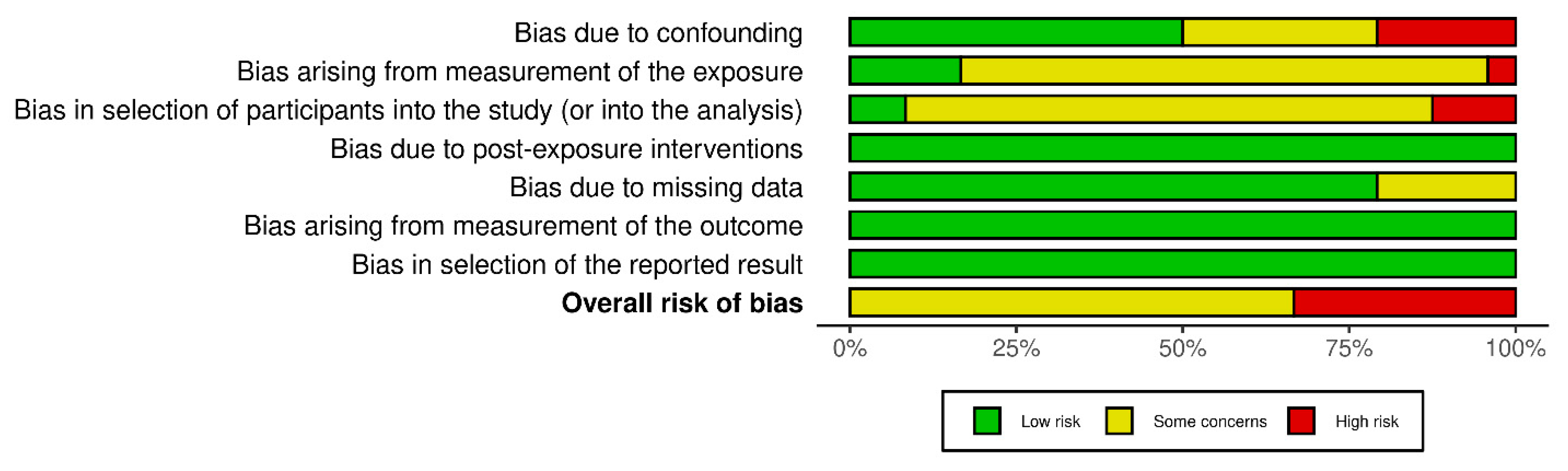
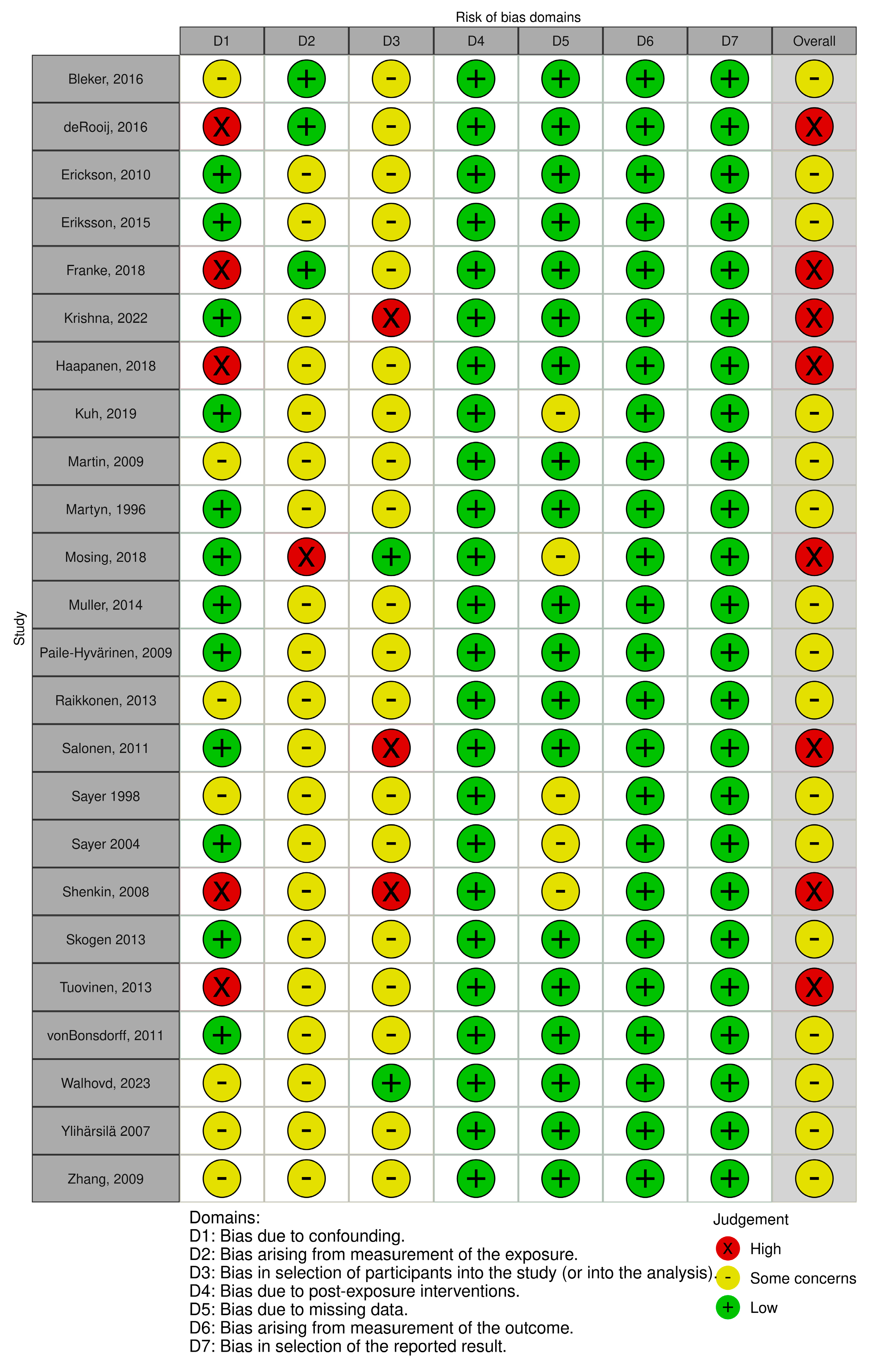
2.5. Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
3.1. Study Characteristics
3.2. Risk of Bias in Included Studies
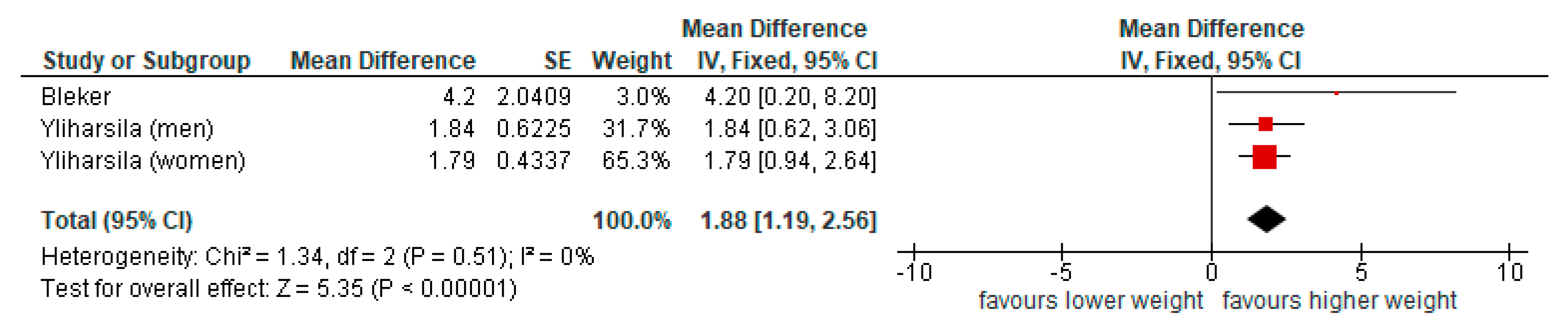
3.3. Summary of Findings
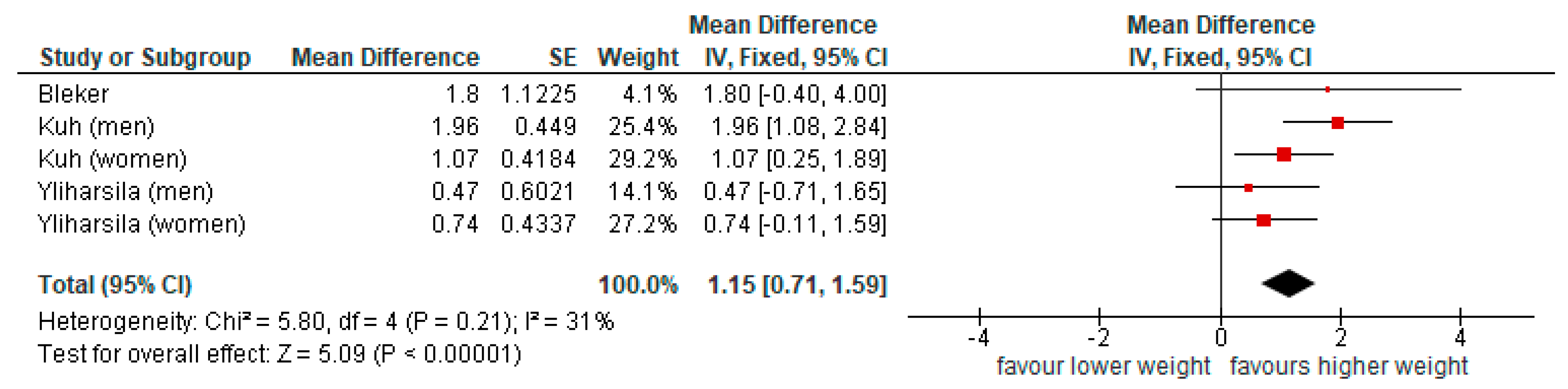
3.3.1. Physical Function
3.3.2. Cognitive Function
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Herman, D.R.; Taylor Baer, M.; Adams, E.; Cunningham-Sabo, L.; Duran, N.; Johnson, D.B.; Yakes, E. Life Course Perspective: Evidence for the role of nutrition. Matern. Child Health J. 2014, 18, 450–461. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Zhou, Y.; Ma, L. Intrinsic Capacity in Older Adults: Recent Advances. Aging Dis. 2022, 13, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Wang, S.F.; Shu, L.; Sheng, J.; Mu, M.; Wang, S.; Tao, X.Y.; Xu, S.J.; Tao, F.B. Birth weight and risk of coronary heart disease in adults: A meta-analysis of prospective cohort studies. J. Dev. Orig. Health Dis. 2014, 5, 408–419. [Google Scholar] [CrossRef]
- Whincup, P.H.; Kaye, S.J.; Owen, C.G.; Huxley, R.; Cook, D.G.; Anazawa, S.; Barrett-Connor, E.; Bhargava, S.K.; Birgisdottir, B.E.; Carlsson, S.; et al. Birth weight and risk of type 2 diabetes: A systematic review. JAMA 2008, 300, 2886–2897. [Google Scholar] [CrossRef]
- de Souza, L.V.; de Meneck, F.; Parizotto, G.P.; Franco, M. Low birth weight and its relation to physical fitness parameters in children: Its negative effect on muscle strength and cardiorespiratory endurance. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2022, 34, e23595. [Google Scholar] [CrossRef]
- Liu, J.; Au Yeung, S.L.; He, B.; Kwok, M.K.; Leung, G.M.; Schooling, C.M. The effect of birth weight on body composition: Evidence from a birth cohort and a Mendelian randomization study. PLoS ONE 2019, 14, e0222141. [Google Scholar] [CrossRef]
- Dodds, R.; Denison, H.J.; Ntani, G.; Cooper, R.; Cooper, C.; Sayer, A.A.; Baird, J. Birth weight and muscle strength: A systematic review and meta-analysis. J. Nutr. Health Aging 2012, 16, 609–615. [Google Scholar] [CrossRef]
- Silva, A.R.D.; Puglisi, M.L.; Pompéia, S.; Ploubidis, G.B.; Swardfager, W.; Cogo-Moreira, H. Birth weight, verbal cognition in early adolescence, and lexical and reading skills in late adolescence: A formal mediation analysis using a potential outcomes approach. J. Child Psychol. Psychiatry 2019, 60, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.S.; Mann, K.D.; Singh, G.; Sayers, S.M. Birth weight and cognitive function in early adulthood: The Australian Aboriginal birth cohort study. J. Dev. Orig. Health Dis. 2014, 5, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Hardy, R.; Kuh, D.; Wadsworth, M.E. Birth weight and cognitive function in the British 1946 birth cohort: Longitudinal population based study. BMJ 2001, 322, 199–203. [Google Scholar] [CrossRef]
- Kormos, C.E.; Wilkinson, A.J.; Davey, C.J.; Cunningham, A.J. Low birth weight and intelligence in adolescence and early adulthood: A meta-analysis. J. Public Health 2014, 36, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Jones, S.; Maden, M.; Du, B.; Mc, R.; Kumaran, K.; Karat, S.C.; Fall, C.H.D. Size at birth and cognitive ability in late life: A systematic review. Int. J. Geriatr. Psychiatry 2019, 34, 1139–1169. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 791–797. [Google Scholar] [CrossRef]
- Schroeder, R.W.; Martin, P.K.; Walling, A. Neuropsychological Evaluations in Adults. Am. Fam. Physician 2019, 99, 101–108. [Google Scholar] [PubMed]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane Handb. Syst. Rev. Interv. 2022, 241–284. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-10#section-10-10-4 (accessed on 10 March 2025).
- Bleker, L.S.; De Rooij, S.R.; Painter, R.C.; Van Der Velde, N.; Roseboom, T.J. Prenatal Undernutrition and Physical Function and Frailty at the Age of 68 Years: The Dutch Famine Birth Cohort Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1306–1314. [Google Scholar] [CrossRef]
- Kuh, D.; Hardy, R.; Blodgett, J.M.; Cooper, R. Developmental factors associated with decline in grip strength from midlife to old age: A British birth cohort study. BMJ Open 2019, 9, e025755. [Google Scholar] [CrossRef]
- Ylihärsilä, H.; Kajantie, E.; Osmond, C.; Forsén, T.; Barker, D.J.; Eriksson, J.G. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. 2007, 31, 1392–1399. [Google Scholar] [CrossRef]
- Erickson, K.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Birth weight and cognitive performance in older women: The rancho bernardo study. Arch. Women’s Ment. Health 2010, 13, 141–146. [Google Scholar] [CrossRef]
- Krishna, M.; Krishnaveni, G.V.; Sargur, V.; Kumaran, K.; Kumar, M.; Nagaraj, K.; Coakley, P.; Karat, S.C.; Chandak, G.R.; Varghese, M.; et al. Size at birth, lifecourse factors, and cognitive function in late life: Findings from the MYsore study of Natal effects on Ageing and Health (MYNAH) cohort in South India. Int. Psychogeriatr. 2022, 34, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Skogen, J.C.; Øverland, S.; Smith, A.D.; Mykletun, A.; Stewart, R. The impact of early life factors on cognitive function in old age: The Hordaland Health Study (HUSK). BMC Psychol. 2013, 1, 16. [Google Scholar] [CrossRef]
- von Hippel, P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Paile-Hyvärinen, M.; Räikkönen, K.; Kajantie, E.; Darby, D.; Ylihärsilä, H.; Salonen, M.K.; Osmond, C.; Eriksson, J.G. Impact of glucose metabolism and birth size on cognitive performance in elderly subjects. Diabetes Res. Clin. Pract. 2009, 83, 379–386. [Google Scholar] [CrossRef] [PubMed]
- von Bonsdorff, M.B.; Rantanen, T.; Sipilä, S.; Salonen, M.K.; Kajantie, E.; Osmond, C.; Barker, D.J.; Eriksson, J.G. Birth size and childhood growth as determinants of physical functioning in older age: The Helsinki Birth Cohort Study. Am. J. Epidemiol. 2011, 174, 1336–1344. [Google Scholar] [CrossRef]
- Salonen, M.K.; Kajantie, E.; Osmond, C.; Forsén, T.; Ylihärsilä, H.; Paile-Hyvärinen, M.; Barker, D.J.; Eriksson, J.G. Developmental origins of physical fitness: The Helsinki Birth Cohort Study. PLoS ONE 2011, 6, e22302. [Google Scholar] [CrossRef]
- Raikkonen, K.; Kajantie, E.; Pesonen, A.K.; Heinonen, K.; Alastalo, H.; Leskinen, J.T.; Nyman, K.; Henriksson, M.; Lahti, J.; Lahti, M.; et al. Early life origins cognitive decline: Findings in elderly men in the Helsinki Birth Cohort Study. PLoS ONE 2013, 8, e54707. [Google Scholar] [CrossRef]
- Tuovinen, S.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Osmond, C.; Barker, D.J.; Räikkönen, K. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: The Helsinki Birth Cohort Study. Am. J. Obs. Gynecol. 2013, 208, 200.e1–200.e9. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Osmond, C.; Perälä, M.M.; Salonen, M.K.; Simonen, M.; Pohjolainen, P.; Kajantie, E.; Rantanen, T.; von Bonsdorff, M.B. Prenatal and childhood growth and physical performance in old age—Findings from the Helsinki Birth Cohort Study 1934–1944. Age 2015, 37, 108. [Google Scholar] [CrossRef]
- Haapanen, M.J.; Perälä, M.M.; Salonen, M.K.; Kajantie, E.; Simonen, M.; Pohjolainen, P.; Eriksson, J.G.; von Bonsdorff, M.B. Early life determinants of frailty in old age: The Helsinki Birth Cohort Study. Age Ageing 2018, 47, 569–575. [Google Scholar] [CrossRef]
- de Rooij, S.R.; Caan, M.W.; Swaab, D.F.; Nederveen, A.J.; Majoie, C.B.; Schwab, M.; Painter, R.C.; Roseboom, T.J. Prenatal famine exposure has sex-specific effects on brain size. Brain 2016, 139, 2136–2142. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Roseboom, T.J.; Schwab, M.; de Rooij, S.R. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. NeuroImage 2018, 173, 460–471. [Google Scholar] [CrossRef]
- Martyn, C.N.; Gale, C.R.; Sayer, A.A.; Fall, C. Growth in utero and cognitive function in adult life: Follow up study of people born between 1920 and 1943. Br. Med. J. 1996, 312, 1393–1396. [Google Scholar] [CrossRef][Green Version]
- Sayer, A.A.; Cooper, C.; Evans, J.R.; Rauf, A.; Wormald, R.P.; Osmond, C.; Barker, D.J. Are rates of ageing determined in utero? Age Ageing 1998, 27, 579–583. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Gilbody, H.J.; Dennison, E.M.; Cooper, C. Does Sarcopenia Originate in Early Life? Findings From the Hertfordshire Cohort Study. J. Gerontol. Ser. A 2004, 59, M930–M934. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Aihie Sayer, A. Physical performance and physical activity in older people: Are developmental influences important? Gerontology 2009, 55, 186–193. [Google Scholar] [CrossRef]
- Shenkin, S.D.; Deary, I.J.; Starr, J.M. Birth parameters and cognitive ability in older age: A follow-up study of people born 1921–1926. Gerontology 2008, 55, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mosing, M.A.; Lundholm, C.; Cnattingius, S.; Gatz, M.; Pedersen, N.L. Associations between birth characteristics and age-related cognitive impairment and dementia: A registry-based cohort study. PLoS Med. 2018, 15, e1002609. [Google Scholar] [CrossRef]
- Muller, M.; Sigurdsson, S.; Kjartansson, O.; Jonsson, P.V.; Garcia, M.; von Bonsdorff, M.B.; Gunnarsdottir, I.; Thorsdottir, I.; Harris, T.B.; van Buchem, M.; et al. Birth size and brain function 75 years later. Pediatrics 2014, 134, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Plassman, B.L.; Xu, Q.; Zahner, G.E.; Wu, B.; Gai, M.Y.; Wen, H.B.; Chen, X.; Gao, S.; Hu, D.; et al. Lifespan influences on mid- to late-life cognitive function in a Chinese birth cohort. Neurology 2009, 73, 186–194. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Krogsrud, S.K.; Amlien, I.K.; Sørensen, Ø.; Wang, Y.; Bråthen, A.C.S.; Overbye, K.; Kransberg, J.; Mowinckel, A.M.; Magnussen, F.; et al. Fetal influence on the human brain through the lifespan. eLife 2024, 12, RP86812. [Google Scholar] [CrossRef] [PubMed]
- Garay, J.L.; Barreira, T.V.; Wang, Q.; Brutsaert, T.D. Intra-uterine effects on adult muscle strength. Early Hum. Dev. 2021, 163, 105490. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Costa, A.J.; Kale, P.L.; Luiz, R.R.; De Moraes, S.A.; Mosley, T.H.; Szklo, M. Association between birthweight and cognitive function in middle age: The atherosclerosis risk in communities study. Ann. Epidemiol. 2011, 21, 851–856. [Google Scholar] [CrossRef]
- Chia, A.R.; Chen, L.W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Roseboom, T.J.; Painter, R.C.; van Abeelen, A.F.M.; Veenendaal, M.V.E.; de Rooij, S.R. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Maturitas 2011, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hrolfsdottir, L.; Gunnarsdottir, I.; Birgisdottir, B.E.; Hreidarsdottir, I.T.; Smarason, A.K.; Hardardottir, H.; Halldorsson, T.I. Can a Simple Dietary Screening in Early Pregnancy Identify Dietary Habits Associated with Gestational Diabetes? Nutrients 2019, 11, 1868. [Google Scholar] [CrossRef] [PubMed]

| Cohort, Country | Reference(s) | No. of Participants | Population | Outcome Type |
|---|---|---|---|---|
| Helsinki Birth Cohort, Finland | Paile-Hyvärinen et al. [27] von Bonsdorff et al. [28] Salonen et al. [29] Raikkonen et al. [30] Tuovinen et al. [31] Eriksson et al. [32] Ylihärsilä et al. [22] Haapanen et al. [33] | 13,345 | Men and women born in Helsinki between 1934 and 1944 and were still living in Finland in 1971. | Cognitive Physical |
| Dutch Famine Birth Cohort, The Netherlands | Bleker et al. [20] deRooij et al. [34] Franke et al. [35] | 2414 | Men and women born in the Wilhelmina Gashuis in Amsterdam between 1 November 1943 and 28 February 1947. | Cognitive Physical |
| Hertfordshire Cohort, England | Martyn et al. [36] Sayer et al. [37] Sayer et al. [38] Martin et al. [39] | 1576 1428 3207 1790 | Men and women born in Herefordshire between; 1920 and 1943. 1920 and 1930. 1931 and 1939. 1931 and 1939. | Cognitive Physical |
| MRC NSHD a England | Kuh et al. [21] | 5362 | Sample of all births (men and women) in the first week of March in 1946 in mainland Britain. | Cognitive Physical |
| Edinburgh, Scotland | Shenkin et al. [40] | 130 | Community-dwelling volunteers born in one hospital in Edinburgh between 1921 and 1926. | Cognitive |
| Rancho Bernardo Study, United States | Erickson et al. [23] | 2040 | Men and women living in Rancho Bernardo, California in 1972 to 1974. | Cognitive |
| MYNAH Cohort b, India | Krishna et al. [24] | 3427 | Men and women born in the Holdsworth Memorial Hospital in Mysore, South India between 1934 and 1966. | Cognitive |
| Swedish Twin Registry, Sweden | Mosing et al. [41] | 35,357 | All twins registered with the Swedish Twin Registry born between 1 January 1926 and 31 December 1960. | Cognitive |
| AGES c Cohort, Iceland | Muller et al. [42] | 5764 | Men and women born between 1907 and 1935 and living in Reykjavik in 1967. | Cognitive |
| HUSK d Cohort, Norway | Skogen et al. [25] | 2156 | Men and women of a previously established cohort living in Bergen/nearby Areas, born between 1925 and 1927. | Cognitive |
| Chinese Birth Cohort, China | Zhang et al. [43] | 2062 | Men and women born in Peking Union Medical College Hospital between 1921 and 1954. | Cognitive |
| UK Biobank, United Kingdom | Walhovd et al. [44] | 500,000 | Men and women living in the United Kingdom, aged between 40 to 69 years old in 2006 to 2010 and living close enough to an assessment center. |
| Author, Year | Participants (n)/Women (n) | Age (Mean, SD) Age Range | Exposure | Mean (SD)/Definition | Outcome/s | Confounders | |
|---|---|---|---|---|---|---|---|
| Physical function outcomes (n = 10) | |||||||
| von Bonsdorff, 2011 [28] | 1999/1072 | 61.6 (2.9) 57 to 70 | Low Birth Weight | <2.5 kg 2.5–2.9 kg vs. 3.0–3.4 kg | SF36 physical functioning questionnaire. | Sex, age, length of gestation, adult lean body mass, highest social class in childhood and adulthood, smoking status. | |
| Eriksson, 2015 [32] | 1078/603 | 71.3 (NA) 67 to 79 | Birth Weight Birth Length Birth BMI | Men 3.5 (0.5) 50.7 (2.0) 13.5 (1.3) | Women 3.4 (0.4) 50.1 (1.8) 13.4 (1.2) | SFT Score, Chair Stands, Arm Curls, Chair Sit and reach, 6 min Walk, Back Scratch | Age, sex, occupation, education, adult lifestyle variables, metabolic measures, adult anthropometry. |
| Salonen, 2011 [29] | 581/312 | 61.8 (2.8) NA | Birth Weight Birth Length Birth BMI | Men 3.5 (0.5) 50.8 (2.1) 13.5 (1.2) | Women 3.4 (0.4) 50.2 (1.8) 13.4 (1.2) | VO2max | Age, sex, adult lean body mass, adult social class, exercise and smoking habits. |
| Martin, 2009 [39] | 629/280 | 67.9 (2.5) NA | Birth Weight | Men 3.5 (0.5) | Women 3.4 (0.5) | 3-m walk, Chair Stands Balance | Age, height and weight adjusted for height. |
| Bleker, 2016 [20] | 150/83 | 68 (NA) NA | Birth Weight | 3.4 (0.5) | Grip Strength, SPPB, Frailty | Sex, BMI, SES, smoking, medication use, LTPA | |
| Kuh, 2019 [21] | 2098/1062 | 60.5 a (NA) 53 to 69 | Birth Weight | Men 3.5 (0.5) | Women 3.3 (0.5) | Grip Strength | Age term, adult height. |
| Sayer, 1998 [37] | 717/306 | 67.5 (2.3) NA | Birth Weight | Men 3.5 (0.5) | Women 3.4 (0.5) | Grip Strength | Age, sex, current social class, social class at birth and height |
| Sayer, 2004 [38] | 1403/673 | 64.9 (2.6) NA | Birth Weight | Men 3.5 (0.5) | Women 3.4 (0.5) | Grip Strength | Age, physical activity, social class, smoking, and alcohol. |
| Ylihärsila, 2007 [22] | 2003/1075 | 61.5 (2.9) 57 to 70 | Birth Weight | Men 3.5 (0.5) | Women 3.4 (0.5) | Grip Strength | Age and adult BMI. |
| Haapanen, 2018 [33] | 1078/603 | 71 (2.7) NA | Birth Weight Birth length Birth BMI | 3.4 (0.5) 50.3 (1.9) 13.4 (1.2) | Pre-frailty Frailty | Age, sex, gestational age, childhood and adult SES, adult BMI, smoking, hypertension and diabetes. | |
| Cognitive function outcomes (n = 14) | |||||||
| de Rooij, 2016 [34] | 118/66 | 67.5 (0.9) 65 to 69 | Birth Weight | 3.4 (0.5) | ICV, TBV | None reported. | |
| Franke, 2018 [35] | 52/0 | 67.5 (0.9) 65 to 69 | Birth Weight | 3.4 (0.5) | BrainAge Score | None reported. | |
| Martyn, 1996 [36] | 1576/Not reported | 60.9 (2.1) 48 to 74 | Birth Weight Birth Length Ponderal Index | 3.4 (0.5) 51.1 (2.5) 25.4 kg/m3 | Alice Heim intelligence test, Decline in Cognitive Function | Age, social class at birth and individual dataset. | |
| Shenkin, 2009 [40] | 128/90 | 78.4 (1.4) 75 to 81 | Birth Weight Birth Length | 3.3 (0.5) 50.6 (2.7) | National Adult Reading Test (NART), General cognitive factor (g), Raven’s Matrices Test, Murray House Test, Controlled Word Association, Logical Memory | Sex, gestational age, maternal Age, parity and social class | |
| Erickson, 2010 [23] | 292/292 | 71.1 (8.6) 55 to 89 | Birth Weight | Not reported | Buschke Total Recall, Buschke LTM, Buschke STMa, Heaton Visual Copying, Heaton Visual LTM, Heaton Visual STM, MMSE, Serial 7’s, World backward, Trails B, Category fluency, Total blessed | Age and education | |
| Krishna, 2022 [24] | 721/313 | 62.3 (5.3) 55 to 80 | Birth Weight Birth Length Ponderal Index | Men 2.8 (0.4) 48.2 (2.1) 25.4 (4.6) | Women 2.7 (0.4) 47.7 (2.9) 25.4 (5.1) | Global Cognition (SD) Verbal Fluency Score Immediate Recall Score Delayed Recall Score Composite Cognitive Score (SD) | Age, sex and sibship |
| Mosing, 2018 [41] | 4000/2124 | 68.4 (2.6) 55 to 89 | Birth Weigh Birth Length Low Birth Weight Small for Gestational Age | 2.8 (0.5) ≤2.5 kg vs. >2.5 kg 2 SD below mean for a given GA and sex. | Cognitive Impairment | Age, sex, age of mother, parity, birth SES and education. | |
| Muller, 2014 [42] | 1254/715 | 75 (5) 69 to 82 | Birth Weight Birth Length Ponderal Index | 3.7 (0.5) | ICV, TBV, WMV, GMV CSFV, Ln WMLV Memory, Processing speed, Executive function | Sex, age, education, midlife weight, height, ICV. | |
| Paile Hyvärinen, 2009 [27] | 1243/658 | 63.9 (2.9) NA | Birth Weight | 3.4 (0.5) | Divided Attention Task (DA) reaction time, Associate Learning Task (AL) hit rate | Age, sex, gestational age, CHD, DM, depressive symptoms and education. | |
| Raikkonen, 2013 [30] | 931/0 | 67.9 (2.5) 51 to 70 | Birth Weight Birth Length Ponderal Index | 3.5 (0.5) 50.7 (2.0) 26.6 (2.1) | Standardized Cognitive Ability (CA) Test Score, Decline in CA, Top third CA Score (vs. middle/bottom) | Length of gestation, father’s occupational status in childhood, parity, mother’s age and height at delivery, history of breastfeeding, age, educational level, stroke and CAD. | |
| Tuovinen, 2013 [31] | 876/377 | 69.3 (3.1) NA | Birth Weight | 3.4 (0.5) | Complaints of cognitive failure, Dysexecutive functioning | Not reported. | |
| Skogen, 2013 [25] | 346/186 | 72.3 72 to 74 | Birth Weight Birth Length Ponderal Index | 3.5 (0.5) 50.3 (2.1) 27.3 (0.2) | Composite score, MMS, Digit symbol, KOLT, COWAT, TMA, Block design | Age and sex | |
| Zhang, 2009 [43] | 2062/1051 | 62.5 a (NA) 50 to 82 | Ponderal Index | 25.8 kg/m2 | Lower cognition | Sex, gestational age, prenatal and early life factors | |
| Walhovd, 2024 [44] | 1759/1009 | 62 (7.1) 47 to 80.3 | Birth Weight | 3.4 (0.6) | Cortical volume | None reported. | |
| Outcomes (Grouped) | Author [Ref] | Birth Size Exposure | |||||
|---|---|---|---|---|---|---|---|
| Birth Weight | Birth Length | Birth BMI | |||||
| n * | n * | n * | |||||
| Grip strength | Bleker [20], Kuh [21], Sayer [37], Sayer [38], Ylihärsila [22] | 5 | ↑ (n = 4) ↔ (n = 1) | 0 | 0 | ||
| Performance score | Bleker [20], Eriksson [32] | 2 | ↑ (n = 1) ↔ (n = 1) | 1 | ↑ (n = 1) | 1 | ↑ (n = 1) |
| Walking | Martin [39], Eriksson [32] | 2 | ↔ (n = 2) | ↔ (n = 2) | ↔ (n = 2) | ||
| Chair rise | Martin [39], Eriksson [32] | 2 | ↔ (n = 1) ↔ (m a)/↓ (w b) (n = 1) | 1 | ↔ (n = 1) | 1 | ↔ (n = 1) |
| Balance | Martin [39] | 1 | ↑ (m a)/↔ (w b) (n = 1) | ||||
| Arm curl | Eriksson [32] | 1 | ↔ (n = 1) | 1 | ↑ (n = 1) | 1 | ↔ (n = 1) |
| Lower flexibility (sit and reach) | Eriksson [32] | 1 | ↑ (n = 1) | 1 | ↑ (n = 1) | 1 | ↔ (n = 1) |
| Upper flexibility (back scratch) | Eriksson [32] | 1 | ↔ (n = 1) | 1 | ↔ (n = 1) | 1 | ↑ (n = 1) |
| VO2max | Salonen [29] | 1 | ↔ (n = 1) | 1 | ↔ (n = 1) | 1 | ↔ (n = 1) |
| Self-reported function | von Bonsdorff [28] | 1 | ↑ (n = 1) | ||||
| Frailty | Haapanen [33] | 1 | ↑ (n = 1) | 1 | ↑ (n = 1) | 1 | ↑ (n = 1) |
| Outcomes (Grouped) | Author [Ref] | Birth Size Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth Weight | Birth Length | Ponderal Index | SGA a | LBW b | |||||||
| n * | n * | n * | n * | n * | |||||||
| Cognitive impairment | Tuovinen [31], Mosing [41], Skogen [25], Erickson [23] | 6 | ↔ (n = 6) | 2 | ↔ (n = 3) | 2 | ↔ (n = 2) | 1 | ↑ (n = 1) | 1 | ↔ (n = 1) |
| Executive function | Skogen [25], Erickson [23], Muller [42] | 2 | ↔ (n = 1) | 1 | ↔ (n = 1) | 2 | ↔ (n = 2) | 0 | 0 | ||
| Word fluency | Krishna [24], Erickson [23], Shenkin [40] | 4 | ↑ (n = 1) ↔ (n = 3) | 3 | ↔ (n = 3) | 2 | ↔ (n = 2) | 0 | 0 | ||
| Cognition | Krishna [24], Shenkin [40], Skogen [25], Zhang [43] | 5 | ↑ (n = 1) ↔ (n = 3) | 4 | ↑ (n = 1) ↔ (n = 3) | ↔ (n = 3) | 0 | 0 | |||
| Verbal memory | Krishna [24], Erickson [23], Shenkin [40], Muller [42] | 4 | ↑ (n = 2) ↔ (n = 2) | 3 | ↑ (n = 1) ↔ (n = 2) | 3 | ↔ (n = 3) | 0 | 0 | ||
| Brain volume | Muller [42], de Rooij [34], Franke [35], Walhovd [44] | 10 | ↑ (n = 4) ↔ (n = 6) | 7 | ↑ (n = 1) ↔ (n = 6) | 7 | ↑ (n = 5) ↔ (n = 2) | 0 | 0 | ||
| Processing speed | Muller [42], Skogen [25], Paile-Hyvärinen [27] | 3 | ↔ (n = 1) ↓ (n = 2) | 1 | ↔ (n = 1) | 2 | ↔ (n = 2) | 0 | 0 | ||
| Cognitive decline | Raikkonen [30], Martyn [36] | 2 | ↔ (n = 2) | 2 | ↑ (n = 1) ↔ (n = 1) | 2 | ↔ (n = 2) | 0 | 0 | ||
| Simple attention | Skogen [25], Erickson [23] | 4 | ↑ (n = 1) ↔ (n = 3) | 1 | ↔ (n = 1) | 1 | ↔ (n = 1) | 0 | 0 | ||
| Intelligence | Martyn [36], Raikkonen [30], Shenkin [40] | 4 | ↑ (n = 1) ↔ (n = 3) | 4 | ↑ (n = 1) ↔ (n = 2) | 2 | ↔ (n = 2) | 0 | 0 | ||
| Premorbid ability | Shenkin [40] | 1 | ↔ (n = 1) | 0 | ↑ (n = 1) | 0 | 0 | 0 | |||
| Visual memory | Erickson [23] | 1 | ↔ (n = 1) | 0 | 0 | 0 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilmundardottir, V.K.; Thorisdottir, B.; Ramel, A.; Geirsdóttir, Ó.G. Associations of Birth Size with Physical and Cognitive Function in Men and Women 60 Years and Older—Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2583. https://doi.org/10.3390/nu17162583
Vilmundardottir VK, Thorisdottir B, Ramel A, Geirsdóttir ÓG. Associations of Birth Size with Physical and Cognitive Function in Men and Women 60 Years and Older—Systematic Review and Meta-Analysis. Nutrients. 2025; 17(16):2583. https://doi.org/10.3390/nu17162583
Chicago/Turabian StyleVilmundardottir, Vilborg Kolbrun, Birna Thorisdottir, Alfons Ramel, and Ólöf Guðný Geirsdóttir. 2025. "Associations of Birth Size with Physical and Cognitive Function in Men and Women 60 Years and Older—Systematic Review and Meta-Analysis" Nutrients 17, no. 16: 2583. https://doi.org/10.3390/nu17162583
APA StyleVilmundardottir, V. K., Thorisdottir, B., Ramel, A., & Geirsdóttir, Ó. G. (2025). Associations of Birth Size with Physical and Cognitive Function in Men and Women 60 Years and Older—Systematic Review and Meta-Analysis. Nutrients, 17(16), 2583. https://doi.org/10.3390/nu17162583






