Effect of Plant-Based Proteins on Recovery from Resistance Exercise-Induced Muscle Damage in Healthy Young Adults—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results
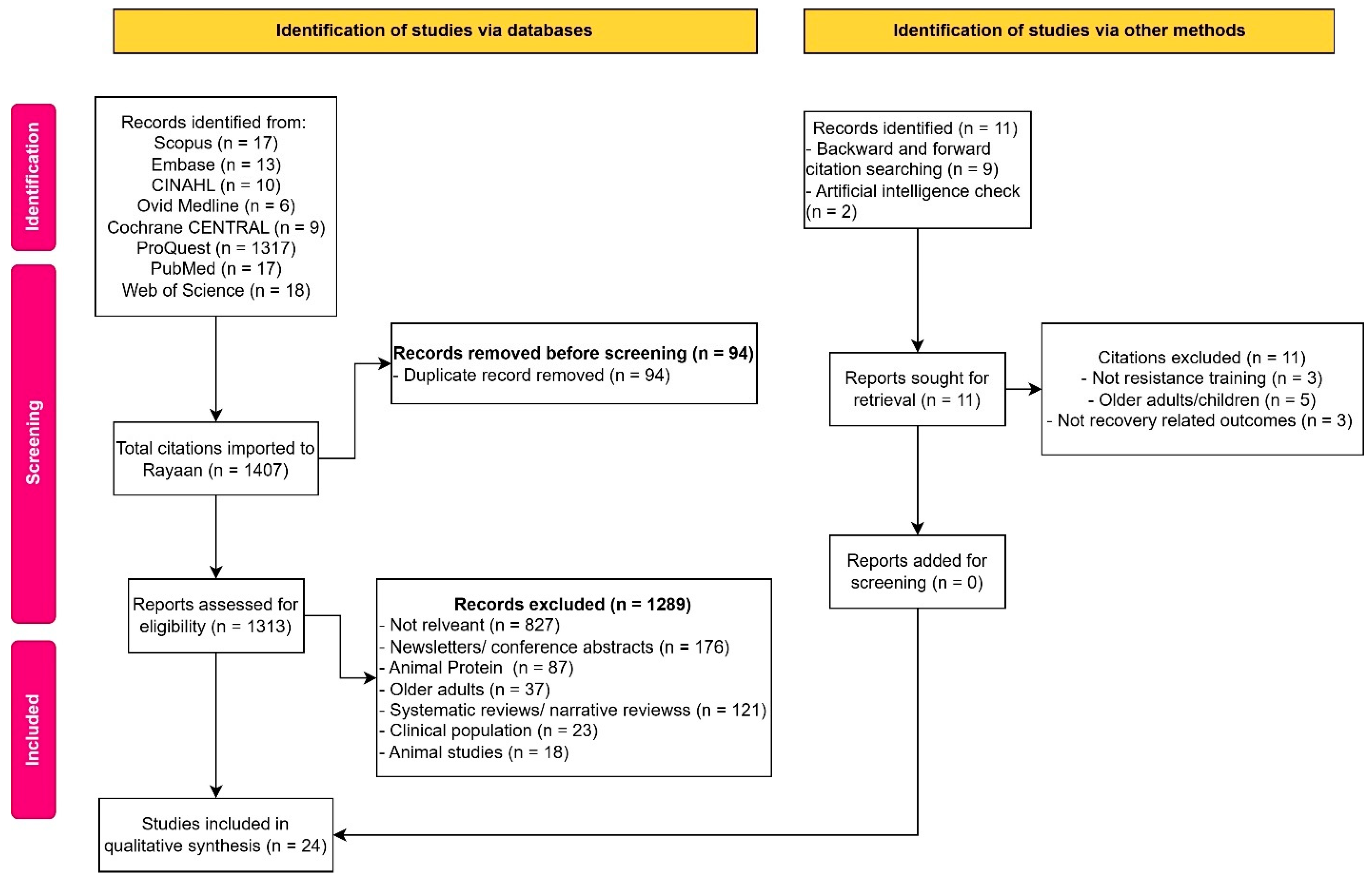
3.1. Study Selection and Characteristics
3.2. Baseline Characteristics of the Included Studies
3.3. Participant Characteristics
3.4. Intervention and Outcome Characteristics
3.5. Effectiveness of Plant-Based Proteins on Muscle Recovery
3.6. Dose–Response Relationship of Plant-Based Proteins on Muscle Recovery
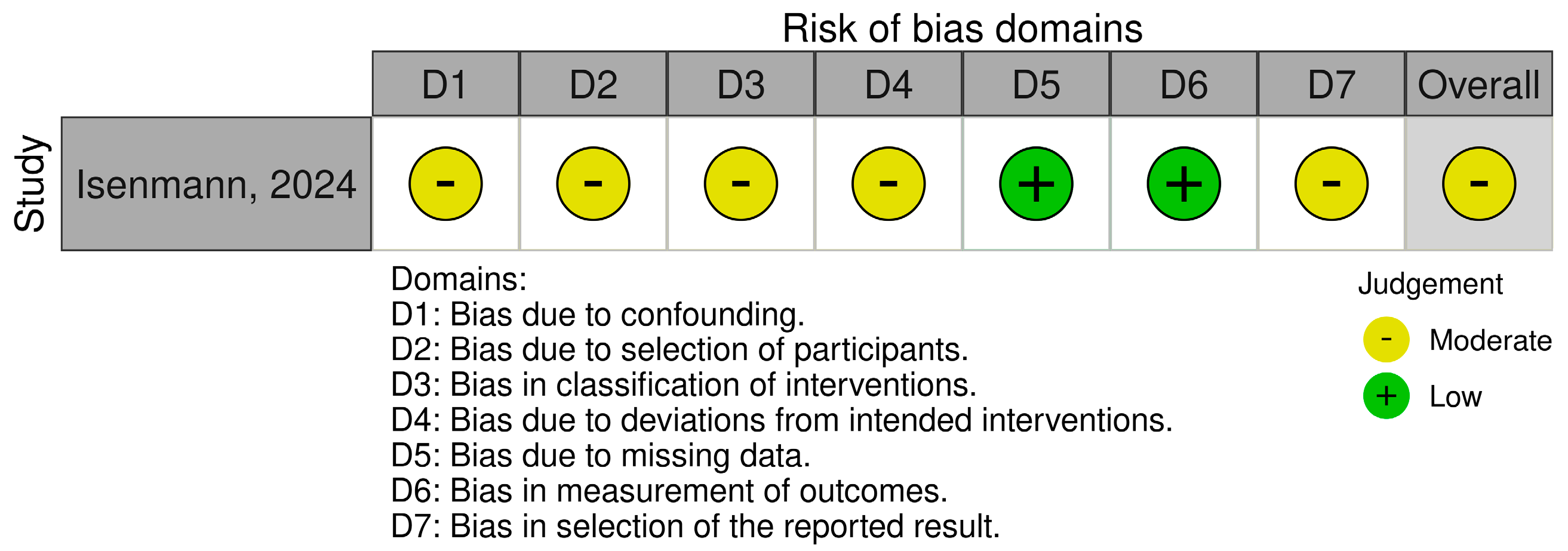
3.7. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| IL-6 | interleukin-6 |
References
- Sarmento, T.C.; Ferreira, R.D.S.; Franco, O.L. Plant-Based Diet and Sports Performance. ACS Omega 2024, 9, 47939–47950. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, Y.; Li, J.; Ning, Z. The Effect of Plant-Based Protein Ingestion on Athletic Ability in Healthy People—A Bayesian Meta-Analysis with Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2748. [Google Scholar] [CrossRef]
- Viroli, G.; Kalmpourtzidou, A.; Cena, H. Exploring Benefits and Barriers of Plant-Based Diets: Health, Environmental Impact, Food Accessibility and Acceptability. Nutrients 2023, 15, 4723. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Jagim, A.; Hagele, A.; Jäger, R. Plant Proteins and Exercise: What Role Can Plant Proteins Have in Promoting Adaptations to Exercise? Nutrients 2021, 13, 1962. [Google Scholar] [CrossRef]
- Goldman, D.M.; Warbeck, C.B.; Karlsen, M.C. Completely Plant-Based Diets that Meet Energy Requirements for Resistance Training Can Supply Enough Protein and Leucine to Maximize Hypertrophy and Strength in Male Bodybuilders: A Modeling Study. Nutrients 2024, 16, 1122. [Google Scholar] [CrossRef]
- Tumkur Anil Kumar, N.; Oliver, J.L.; Lloyd, R.S.; Pedley, J.S.; Radnor, J.M. The Influence of Growth, Maturation and Resistance Training on Muscle-Tendon and Neuromuscular Adaptations: A Narrative Review. Sports 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Med. 2017, 47, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Taguchi, T. Delayed onset muscle soreness: Involvement of neurotrophic factors. J. Physiol. Sci. 2016, 66, 43–52. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.G.; Hind, K.; Macnaughton, L.S. The impact of dietary protein supplementation on recovery from resistance exercise-induced muscle damage: A systematic review with meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 767–783. [Google Scholar] [CrossRef]
- Van Der Heijden, I.N.O.; Monteyne, A.J.; West, S.A.M.; Morton, J.P.; Langan-Evans, C.; Hearris, M.A.; Abdelrahman, D.R.; Murton, A.J.; Stephens, F.B.; Wall, B.T. Plant Protein Blend Ingestion Stimulates Postexercise Myofibrillar Protein Synthesis Rates Equivalently to Whey in Resistance-Trained Adults. Med. Sci. Sports Exerc. 2024, 56, 1467–1479. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; van der Heijden, I.; Stephens, F.B.; Wall, B.T. Nutritional Considerations for the Vegan Athlete. Adv. Nutr. 2023, 14, 774–795. [Google Scholar] [CrossRef] [PubMed]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Ashtary-Larky, D. Are plant-based and omnivorous diets the same for muscle hypertrophy? A narrative review of possible challenges of plant-based diets in resistance-trained athletes. Nutrition 2025, 135, 112742. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sports Med. 2022, 56, 175–195. [Google Scholar] [CrossRef]
- Yao, M.; Mei, F.; Ma, Y.; Qin, X.; Huan, J.; Zou, K.; Li, L.; Sun, X. Including non-randomized studies of interventions in meta-analyses of randomized controlled trials changed the estimates in more than a third of the studies: Evidence from an empirical analysis. J. Clin. Epidemiol. 2025, 183, 111815. [Google Scholar] [CrossRef]
- Isenmann, E.; Trojak, I.; Lesch, A.; Schalla, J.; Havers, T.; Diel, P.; Geisler, S. The influence of a vegan diet on body composition, performance and the menstrual cycle in young, recreationally trained women—A 12-week controlled trial. J. Int. Soc. Sports Nutr. 2024, 21, 2413961. [Google Scholar] [CrossRef] [PubMed]
- Box, W.; Hill, S.; Disilvestro, R.A. Soy intake plus moderate weight resistance exercise: Effects on serum concentrations of lipid peroxides in young adult women. J. Sports Med. Phys. Fit. 2005, 45, 524–528. [Google Scholar]
- Mobley, C.B.; Haun, C.T.; Roberson, P.A.; Mumford, P.W.; Kephart, W.C.; Romero, M.A.; Osburn, S.C.; Vann, C.G.; Young, K.C.; Beck, D.T.; et al. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training. PLoS ONE 2018, 13, e0195203. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Kozior, M.; Lynch, A.E.; Bass, J.J.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial. Nutrients 2022, 14, 3688. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jäger, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Shaw, K.A.; Candow, D.G.; Farthing, J.P.; Chilibeck, P.D. Effects of hemp supplementation during resistance training in trained young adults. Eur. J. Appl. Physiol. 2024, 124, 1097–1107. [Google Scholar] [CrossRef]
- Moon, J.M.; Ratliff, K.M.; Blumkaitis, J.C.; Harty, P.S.; Zabriskie, H.A.; Stecker, R.A.; Currier, B.S.; Jagim, A.R.; Jäger, R.; Purpura, M.; et al. Effects of daily 24-gram doses of rice or whey protein on resistance training adaptations in trained males. J. Int. Soc. Sports Nutr. 2020, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guérin-Deremaux, L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients 2020, 12, 2382. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Hendriks, F.K.; Hermans, W.J.H.; Goessens, J.P.B.; Senden, J.M.; van Kranenburg, J.M.X.; Wodzig, W.; Snijders, T.; van Loon, L.J.C. Potato Protein Ingestion Increases Muscle Protein Synthesis Rates at Rest and during Recovery from Exercise in Humans. Med. Sci. Sports Exerc. 2022, 54, 1572–1581. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Smeets, J.S.J.; Kouw, I.W.K.; Goessens, J.P.B.; Gijsen, A.P.B.; de Groot, L.C.P.G.M.; Verdijk, L.B.; van Loon, L.J.C.; Snijders, T. Post-prandial muscle protein synthesis rates following the ingestion of pea-derived protein do not differ from ingesting an equivalent amount of milk-derived protein in healthy, young males. Eur. J. Nutr. 2024, 63, 893–904. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J. Appl. Physiol. (Bethesda, Md. 1985) 2014, 116, 1353–1364. [Google Scholar] [CrossRef]
- Shenoy, S.; Dhawan, M.; Jaspal Singh, S. Four Weeks of Supplementation with Isolated Soy Protein Attenuates Exercise-Induced Muscle Damage and Enhances Muscle Recovery in Well Trained Athletes: A Randomized Trial. Asian J. Sports Med. 2016, 7, e33528. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K. The Effect of a Plant Based Protein Source on the Skeletal Muscle Metabolic and Functional Response to Eccentric Exercise. Ph.D. Thesis, University of Exeter (United Kingdom), Exeter, UK, 2023. [Google Scholar]
- Bartholomae, E.; Incollingo, A.; Vizcaino, M.; Wharton, C.; Johnston, C.S. Mung Bean Protein Supplement Improves Muscular Strength in Healthy, Underactive Vegetarian Adults. Nutrients 2019, 11, 2423. [Google Scholar] [CrossRef]
- Born, K.A.; Dooley, E.E.; Cheshire, P.A.; McGill, L.E.; Cosgrove, J.M.; Ivy, J.L.; Bartholomew, J.B. Chocolate Milk versus carbohydrate supplements in adolescent athletes: A field based study. J. Int. Soc. Sports Nutr. 2019, 16, 6. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Domagalski, A.; Główka, N.; Kamińska, J.; Szymczak, D.; Podgórski, T. Effect of a Four-Week Vegan Diet on Performance, Training Efficiency and Blood Biochemical Indices in CrossFit-Trained Participants. Nutrients 2022, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Perreau, C.; Crowley, D.C.; Lewis, E.D.; Evans, M.; Moulin, M. Efficacy of Pea Protein Supplementation in Combination with a Resistance Training Program on Muscle Performance in a Sedentary Adult Population: A Randomized, Comparator-Controlled, Parallel Clinical Trial. Nutrients 2024, 16, 2017. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Solomon-Hill, G.; Volk, B.M.; Kupchak, B.R.; Looney, D.P.; Dunn-Lewis, C.; Comstock, B.A.; Szivak, T.K.; Hooper, D.R.; Flanagan, S.D.; et al. The effects of soy and whey protein supplementation on acute hormonal reponses to resistance exercise in men. J. Am. Coll. Nutr. 2013, 32, 66–74. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Zare, R.; Devrim-Lanpir, A.; Guazzotti, S.; Ali Redha, A.; Prokopidis, K.; Spadaccini, D.; Cannataro, R.; Cione, E.; Henselmans, M.; Aragon, A.A. Effect of Soy Protein Supplementation on Muscle Adaptations, Metabolic and Antioxidant Status, Hormonal Response, and Exercise Performance of Active Individuals and Athletes: A Systematic Review of Randomised Controlled Trials. Sports Med. 2023, 53, 2417–2446. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Church, D.D.; Ferrando, A.A.; Moughan, P.J. Consideration of the role of protein quality in determining dietary protein recommendations. Front. Nutr. 2024, 11, 1389664. [Google Scholar] [CrossRef]
- Karabulut, G.; Goksen, G.; Khaneghah, A. Plant-based protein modification strategies towards challenges. J. Agric. Food Res. 2024, 15, 101017. [Google Scholar] [CrossRef]
- Reid-McCann, R.J.; Brennan, S.F.; Ward, N.A.; Logan, D.; McKinley, M.C.; McEvoy, C.T. Effect of Plant Versus Animal Protein on Muscle Mass, Strength, Physical Performance, and Sarcopenia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, e1581–e1603. [Google Scholar] [CrossRef]
- Lim, M.T.; Pan, B.J.; Toh, D.W.K.; Sutanto, C.N.; Kim, J.E. Animal Protein versus Plant Protein in Supporting Lean Mass and Muscle Strength: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef]
- Nichele, S.; Phillips, S.M.; Boaventura, B.C.B. Plant-based food patterns to stimulate muscle protein synthesis and support muscle mass in humans: A narrative review. Appl. Physiol. Nutr. Metab. 2022, 47, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, Q.; Chun-Hsien, S. from Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Ann Reed, M.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]



| Author | Year | Country | Study Design | Participants | Sample Size | Plant Protein Type | Frequency | Resistance Training Dose (C/A) | Muscle Recovery | Fatigue Outcome | Primary Outcome | Secondary Outcome | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bartholomae [31] | 2019 | United States | RCT |
| 37 | Mung bean protein supplement | Eighteen grams/day for 8 weeks |
| Not directly measured | Not directly measured |
|
|
|
| Born [32] | 2019 | United States | RCT |
| 103 | Chocolate milk (CM) vs. carbohydrate (CHO) | Immediately post-exercise, 4 days per week during summer training |
| Not directly measured | Not assessed |
|
|
|
| Box [19] | 2005 | United States | RCT |
| 18 | Soy protein isolate (Supro® Soy Isolated Soy Protein) | Forty grams/day for 4 weeks |
| Indirectly assessed via creatine kinase levels | Not directly measured |
|
|
|
| Brooks Mobley [20] | 2017 | United States | RCT |
| 75 | Soy protein concentrate | Two servings per day (~3 g leucine per serving) for 12 weeks |
| Indirectly assessed via changes in skeletal muscle satellite cell number | Not directly measured |
|
|
|
| Davies [21] | 2022 | Ireland | RCT |
| 16 | Fava bean protein (Vicia faba L.) | Post-exercise intake of 0.33 g/kg body mass |
| Not directly measured | Not directly measured |
|
|
|
| Durkalec-Michalski [33] | 2022 | Poland | RCT |
| 20 | Vegan diet (VegD) vs. mixed diet (MixD) | Diet adherence for 4 weeks, monitored daily |
| Not directly measured | Not directly measured |
|
|
|
| Goldman [5] | 2024 | Finland | Modeling study | Competitive male bodybuilders | 235 | Completely plant-based diet | Scaled to daily caloric intake of 4239 kcal |
| Not directly measured | Not measured |
|
|
|
| Isenmann [18] | 2024 | Germany | Non-randomized trial | Young, recreationally trained women | 10 | Vegan diet (no specific protein supplementation) | Eight-week vegan phase followed by 4-week omnivorous phase |
| Not directly measured | Not directly measured |
|
|
|
| Joy [22] | 2013 | United states | RCT | Twenty-four resistance-trained college-aged men | 24 | Rice protein isolate | Forty-eight grams of rice or whey protein isolate consumed post-exercise on training days for 8 weeks |
| soreness, perceived readiness to train, recovery scales) | Perceived readiness to train |
|
|
|
| Kaviani [23] | 2024 | Canada | RCT |
| 34 | Hemp protein powder (40 g protein, 9 g oil per day) | Sixty grams per day, divided into two doses |
| Indirectly assessed through muscle thickness | rate of torque development after fatigue test |
|
|
|
| Moon [24] | 2020 | United states | RCT |
| 24 | Rice protein concentrate | Twenty-four grams of rice protein concentrate daily for 8 weeks |
| Not directly assessed | Not directly assessed |
|
|
|
| Nieman [25] | 2020 | United States | RCT |
| 92 | Pea protein isolate (NUTRALYS® S85 Plus) | 0.9 g protein/kg per day divided into three doses for five days post-exercise |
| biomarkers (creatine kinase, myoglobin, lactate dehydrogenase) | Not directly measured |
|
|
|
| Pinckaers [26] | 2022 | Netherlands | RCT |
| 24 | Potato protein concentrate (Solanic 100) | Single ingestion of 30 g of potato protein post-exercise |
| Assessed through post-exercise MPS rates | Not directly measured |
|
|
|
| Pinckaers [27] | 2024 | Netherlands | RCT |
| 24 | Pea protein concentrate (Nutralys S85F) | Single ingestion of 30 g of pea protein post-exercise |
| Assessed through post-exercise MPS rates | Not directly measured |
|
|
|
| Reidy [28] | 2014 | United states | RCT |
| 16 | Soy-dairy protein blend (25% soy, 50% casein, 25% whey) vs. whey protein isolate | Single post-exercise ingestion (1 h after RT) |
| Assessed via MPS (amino acid synthesis) | Not directly measured |
|
|
|
| Shenoy [29] | 2016 | India | RCT |
| 40 | Isolated Soy Protein (ISP) | Twenty-five grams of ISP twice daily (mixed with water) for 4 weeks |
| Inflammatory markers, Myeloperoxidase and Isometric muscle strength | Visual Analog Scale (VAS) for muscle soreness |
|
|
|
| Ruma [34] | 2024 | Canada | RCT |
| 50 | Pea protein powder (NUTRALYS® S85 Plus) | Between 20 and 22.5 g per day, mixed with water and consumed post-exercise |
| Assessed via DOMS questionnaire at 24 h, 48 h, and 72 h post-exercise | Not directly measured |
|
|
|
| Tang [35] | 2009 | Canada | RCT |
| 18 | Soy protein isolate | Single ingestion |
| Evaluated through MPS measurement | Not directly measured |
|
|
|
| van der Heijden [11] | 2024 | United Kingdom and United states | RCT |
| 10 | Protein blend composed of pea (39.5%), brown rice (39.5%), and canola (21.0%) | Single ingestion (32 g of protein) post-exercise |
| Assessed via MPS | Not directly measured |
|
|
|
| Wilkinson [30] | 2023 | United Kingdom | RCT |
| 19 | Pea protein fortified with methionine | A combination of 25 g protein + 2.2 g leucine daily, post-exercise for 7 days |
| MPS, Soreness | Not directly measured |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindasamy, K.; Parpa, K.; Katanic, B.; Clark, C.C.T.; Elayaraja, M.; Kambitta Valappil, I.N.; Dulceanu, C.; Geantă, V.A.; Tolan, G.A.; Zouhal, H. Effect of Plant-Based Proteins on Recovery from Resistance Exercise-Induced Muscle Damage in Healthy Young Adults—A Systematic Review. Nutrients 2025, 17, 2571. https://doi.org/10.3390/nu17152571
Govindasamy K, Parpa K, Katanic B, Clark CCT, Elayaraja M, Kambitta Valappil IN, Dulceanu C, Geantă VA, Tolan GA, Zouhal H. Effect of Plant-Based Proteins on Recovery from Resistance Exercise-Induced Muscle Damage in Healthy Young Adults—A Systematic Review. Nutrients. 2025; 17(15):2571. https://doi.org/10.3390/nu17152571
Chicago/Turabian StyleGovindasamy, Karuppasamy, Koulla Parpa, Borko Katanic, Cain C. T. Clark, Masilamani Elayaraja, Ibnu Noufal Kambitta Valappil, Corina Dulceanu, Vlad Adrian Geantă, Gloria Alexandra Tolan, and Hassane Zouhal. 2025. "Effect of Plant-Based Proteins on Recovery from Resistance Exercise-Induced Muscle Damage in Healthy Young Adults—A Systematic Review" Nutrients 17, no. 15: 2571. https://doi.org/10.3390/nu17152571
APA StyleGovindasamy, K., Parpa, K., Katanic, B., Clark, C. C. T., Elayaraja, M., Kambitta Valappil, I. N., Dulceanu, C., Geantă, V. A., Tolan, G. A., & Zouhal, H. (2025). Effect of Plant-Based Proteins on Recovery from Resistance Exercise-Induced Muscle Damage in Healthy Young Adults—A Systematic Review. Nutrients, 17(15), 2571. https://doi.org/10.3390/nu17152571












