The Oral–Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia
Abstract
1. Introduction
2. Overview on the Oral and Gut Microbiota Changes with Aging
2.1. Oral Microbiota Changes with Aging
2.2. Gut Microbiota Changes with Aging
3. Oral and Gut Microbiota Interactions
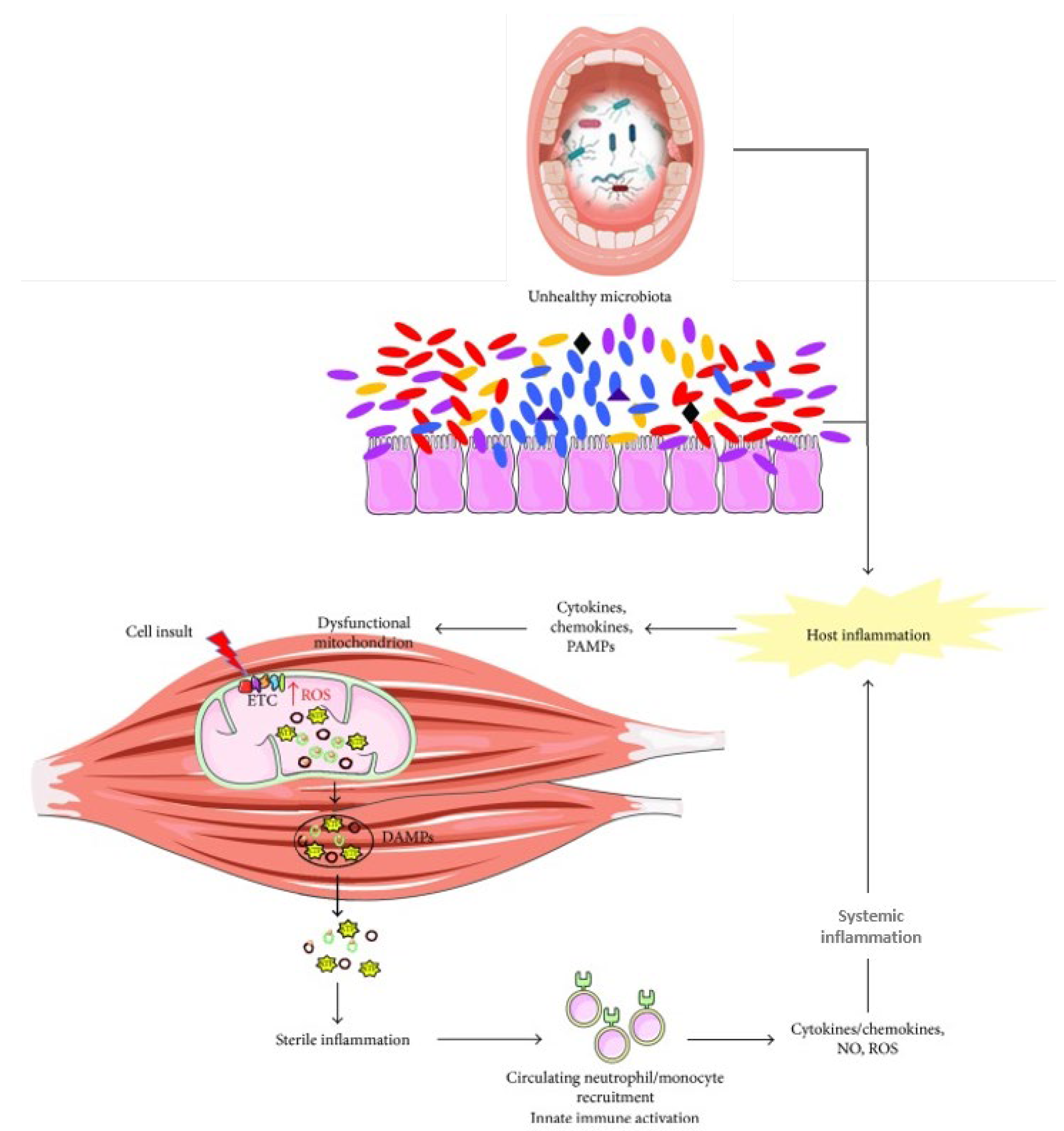
3.1. The Enteral Route
3.2. The Bloodstream Route
3.3. Fecal-Oral Route
4. Influence of the Oral–Gut Axis on Frailty and Sarcopenia
- (1)
- By enhancing LPS in the bloodstream, which subsequently induces the upregulation of flavin-containing dimethylaniline monooxygenase 3 expression (FMO3) and elevates circulating trimethylamine N-oxide (TMAO) concentrations, resulting in metabolic dysregulation, gut dysbiosis and inflammation [109,110];
- (2)
- By downregulating the expression of tight junction protein cytosolic zonula occludens 1 (ZO-1) and occludin in the small intestine, thereby increasing intestinal permeability [109].
- Disruption of intestinal barriers. P. gingivalis and K. pneumoniae and consequently gut inflammation, have been indicated as downregulating the expressions of tight junction protein 1 and occludin. Additionally, the secretion of gingipain proteases disrupts the mucus layer’s function and integrity by degrading intestinal mucus and inhibiting mucus shedding locally, as well as breaking down junction-associated proteins like the cytosolic ZO-1 [23].
- LPS-triggered inflammation. F. nucleatum, K. pneumoniae, and P. gingivalis can trigger the release of LPS [108,132]. LPS from P. gingivalis activates the NF-κB pathway and Caspase-1 inflammasome, resulting in increased IL-1β and IL-18 production [132], which drive intestinal inflammation and can cross the blood–brain barrier to promote neuroinflammation by activating microglia [138].
- T cell imbalances. F. nucleatum and Candida albicans can disrupt the balance between Th1/Th17 cells, further inducing inflammatory reactions [108]. P. gingivalis and F. nucleatum can trigger overproduction of pro-inflammatory cytokines such as IL-6, IL-8, IL-1β, TNF-α, IL-17, CXC motif chemokine ligand 10 (CXCL10), and IL-23 via TLR2, TLR4, Th17 cells and myeloid differentiation primary response 88 (MYD88) signaling [23,138,160,161]. In turn, the abnormal release of several of these pro-inflammatory cytokines and chemokines, including IL-6, TNF-α, and CXCL10, has been independently associated with frailty [162] and sarcopenia [163].
- Inflammasome activation and immune dysregulation. Pathogenic microorganisms can also influence the oral–gut microbiota axis through immune pathways [24]. Imbalances in the oral microbiota can influence gut-associated immune cells, triggering immune responses negatively impacting both oral and gut health [24]. Oral pathogens, like Klebsiella and Enterobacter, when colonizing the gut, can activate the inflammasome and induce inflammation in colonic mononuclear phagocytes, disrupting the intestinal immune environment [24,164]. Klebsiella species also show adaptive capacity to distant mucosal sites such as the gut through sophisticated virulence strategies [165]. Streptococcus gordonii has been found to hinder macrophage-mediated destruction of Candida albicans, further contributing to immune system dysregulation [166]. Beyond reducing Th17 cell levels, oral dysbiosis can also reduce fecal immunoglobulin A (IgA), altering the M1/M2 macrophage balance, further promoting chronic inflammation. Oral microbiota dysbiosis can also be responsible for metabolic alterations by increasing lactate levels and reducing beneficial metabolites like succinate and n-butyrate, exacerbating gut dysbiosis [167]. The presence of oral bacteria in the gut can lead to mucosal and intestinal epithelial barrier damage by influencing lamina propria macrophages and increasing IL-1β levels through the overstimulation of the inflammasome [168]. This is particularly evident in periodontal disease, in which salivary-induced dysbiosis alters gut microbiota and exacerbates colitis with the consequent damage of the mucosal barrier [169]. Notably, it has been reported that about 30% of individuals with IBD show oral symptoms that may precede gastrointestinal manifestations, indicating a bidirectional relationship where systemic inflammation in IBD can alter oral microbiota and intensify oral inflammation [170].
5. Dietary and Exercise Strategies Targeting the Oral and Gut Microbiota and Their Effects on Frailty and Sarcopenia
5.1. Dietary Strategies
5.2. Exercise Strategies
6. Research Limitations and Future Perspectives
6.1. Research Limitations
6.2. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the gut and oral microbiome with obesity. Anaerobe 2021, 70, 102248. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Chang, K.; Albright, J.A.; Testa, E.J.; Balboni, A.B.; Daniels, A.H.; Cohen, E. Sarcopenia Is Associated with an Increased Risk of Postoperative Complications Following Total Hip Arthroplasty for Osteoarthritis. Biology 2023, 12, 295. [Google Scholar] [CrossRef]
- Cesari, M.; Bernabei, R.; Vellas, B.; Fielding, R.A.; Rooks, D.; Azzolino, D.; Mariani, J.; Oliva, A.A.; Bhasin, S.; Rolland, Y.; et al. Challenges in the Development of Drugs for Sarcopenia and Frailty—Report from the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force. J. Frailty Aging 2022, 11, 135–142. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metab. Clin. Exp. 2023, 149, 155597. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Canevelli, M.; Zhang, W.; Thiyagarajan, J.A.; Azzolino, D.; Cherubini, A.; Chhetri, J.K.; Dias, A.; Ferriolli, E.; Gentili, S.; et al. Enhancing the methodology of clinical trials in older people: A scoping review with global perspective. J. Nutr. Health Aging 2025, 29, 100582. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Ozkok, S. The Current Landscape of Pharmacotherapies for Sarcopenia. Drugs Aging 2024, 41, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Dao, M.M.; Cannon, K.; Desvarieux, M.; Miller, S.S.; Gimness, M.P.; Brandon, D.M.; Villareal, D.T.; Bruyere, O.; Bautmans, I.; et al. BIO101 in Sarcopenic Seniors at Risk of Mobility Disability: Results of a Double-Blind Randomised Interventional Phase 2b Trial. J. Cachexia Sarcopenia Muscle 2025, 16, e13750. [Google Scholar] [CrossRef]
- Attané, C.; Foussal, C.; Le Gonidec, S.; Benani, A.; Daviaud, D.; Wanecq, E.; Guzmán-Ruiz, R.; Dray, C.; Bezaire, V.; Rancoule, C.; et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes 2012, 61, 310–320. [Google Scholar] [CrossRef]
- Dray, C.; Knauf, C.; Daviaud, D.; Waget, A.; Boucher, J.; Buléon, M.; Cani, P.D.; Attané, C.; Guigné, C.; Carpéné, C.; et al. Apelin Stimulates Glucose Utilization in Normal and Obese Insulin-Resistant Mice. Cell Metab. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am. J. Transl. Res. 2015, 7, 1850–1859. [Google Scholar]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.-P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- De Spiegeleer, A.; Wynendaele, E.; Descamps, A.; Debunne, N.; Braeckman, B.P.; De Mey, M.; Coudenys, J.; Crombez, L.; Verbeke, F.; Janssens, Y.; et al. The bacterial quorum sensing peptide iAM373 is a novel inducer of sarcopenia. Clin. Transl. Med. 2022, 12, e1053. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Azzolino, D.; Felicetti, A.; Santacroce, L.; Lucchi, T.; Garcia-Godoy, F.; Passarelli, P.C. The emerging role of oral microbiota: A key driver of oral and systemic health. Am. J. Dent. 2025, 38, 111–116. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The oral-gut microbiome axis in health and disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, W.; Li, Y.; Cui, J.; Zhu, M.; Liu, Y.; Liu, Y. The oral-gut microbiota axis: A link in cardiometabolic diseases. NPJ Biofilms Microbiomes 2025, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Iqbal, U.; Sebastian, M.; Pasinetti, G.M. Chapter Eight—Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer’s disease. In Progress in Molecular Biology and Translational Science; Teplow, D.B., Ed.; Molecular Biology of Neurodegenerative Diseases: Visions for the Future, Part A; Academic Press: Cambridge, MA, USA, 2019; Volume 168, pp. 147–181. [Google Scholar]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Coperchini, F.; Greco, A.; Teliti, M.; Croce, L.; Chytiris, S.; Magri, F.; Gaetano, C.; Rotondi, M. Inflamm-ageing: How cytokines and nutrition shape the trajectory of ageing. Cytokine Growth Factor Rev. 2025, 82, 31–42. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Woo, J.; Tong, C.; Yu, R. Chewing Difficulty Should be Included as a Geriatric Syndrome. Nutrients 2018, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Maeda, K.; Wakabayashi, H. Prevalence of sarcopenia and association with oral health-related quality of life and oral health status in older dental clinic outpatients. Geriatr. Gerontol. Int. 2018, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A Lesson in coexistence. PLoS Pathog. 2018, 14, e1006719. [Google Scholar] [CrossRef]
- HOMD: Human Oral Microbiome Database. Available online: https://www.homd.org/ (accessed on 24 January 2023).
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Wu, C. Human Microbiome, Actinobacteria in. In Encyclopedia of Metagenomics; Nelson, K.E., Ed.; Springer: New York, NY, USA, 2013; pp. 1–7. ISBN 978-1-4614-6418-1. [Google Scholar]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Sharma, G.; Garg, N.; Hasan, S.; Shirodkar, S. Prevotella: An insight into its characteristics and associated virulence factors. Microb. Pathog. 2022, 169, 105673. [Google Scholar] [CrossRef]
- Dashper, S.G.; Seers, C.A.; Tan, K.H.; Reynolds, E.C. Virulence Factors of the Oral Spirochete Treponema denticola. J. Dent. Res. 2011, 90, 691–703. [Google Scholar] [CrossRef]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3. mBio 2018, 9, e00202-18. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, T.; Ding, G.; Jiang, S. Findings and methodologies in oral phageome research: A systematic review. J. Oral Microbiol. 2024, 16, 2417099. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The pivotal role of oral microbiota in health and disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [CrossRef]
- Poehlein, A.; Seedorf, H. Draft Genome Sequences of Methanobrevibacter curvatus DSM11111, Methanobrevibacter cuticularis DSM11139, Methanobrevibacter filiformis DSM11501, and Methanobrevibacter oralis DSM7256. Genome Announc. 2016, 4, e00617-16. [Google Scholar] [CrossRef]
- Huynh, H.T.T.; Nkamga, V.D.; Drancourt, M.; Aboudharam, G. Genetic variants of dental plaque Methanobrevibacter oralis. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2015, 34, 1097–1101. [Google Scholar] [CrossRef]
- Bringuier, A.; Khelaifia, S.; Richet, H.; Aboudharam, G.; Drancourt, M. Real-Time PCR Quantification of Methanobrevibacter oralis in Periodontitis. J. Clin. Microbiol. 2013, 51, 993–994. [Google Scholar] [CrossRef]
- Eriksen, C.; Boustedt, K.; Sonne, S.B.; Dahlgren, J.; Kristiansen, K.; Twetman, S.; Brix, S.; Roswall, J. Early life factors and oral microbial signatures define the risk of caries in a Swedish cohort of preschool children. Sci. Rep. 2024, 14, 8463. [Google Scholar] [CrossRef]
- Sarafidou, K.; Alexakou, E.; Talioti, E.; Bakopoulou, A.; Anastassiadou, V. The oral microbiome in older adults –a state-of-the-art review. Arch. Gerontol. Geriatr. Plus 2024, 1, 100061. [Google Scholar] [CrossRef]
- Kazarina, A.; Kuzmicka, J.; Bortkevica, S.; Zayakin, P.; Kimsis, J.; Igumnova, V.; Sadovska, D.; Freimane, L.; Kivrane, A.; Namina, A.; et al. Oral microbiome variations related to ageing: Possible implications beyond oral health. Arch. Microbiol. 2023, 205, 116. [Google Scholar] [CrossRef]
- O’Donnell, L.E.; Robertson, D.; Nile, C.J.; Cross, L.J.; Riggio, M.; Sherriff, A.; Bradshaw, D.; Lambert, M.; Malcolm, J.; Buijs, M.J.; et al. The Oral Microbiome of Denture Wearers Is Influenced by Levels of Natural Dentition. PLoS ONE 2015, 10, e0137717. [Google Scholar] [CrossRef]
- Asakawa, M.; Takeshita, T.; Furuta, M.; Kageyama, S.; Takeuchi, K.; Hata, J.; Ninomiya, T.; Yamashita, Y. Tongue Microbiota and Oral Health Status in Community-Dwelling Elderly Adults. mSphere 2018, 3, e00332-18. [Google Scholar] [CrossRef]
- Sekundo, C.; Langowski, E.; Wolff, D.; Boutin, S.; Frese, C. Maintaining oral health for a hundred years and more?—An analysis of microbial and salivary factors in a cohort of centenarians. J. Oral Microbiol. 2022, 14, 2059891. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Aging of the human metaorganism: The microbial counterpart. Age Dordr. Neth. 2012, 34, 247–267. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Langille, M.G.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef]
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome—How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; et al. Gut microbiota of healthy and malnourished children in bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Yang, L.; Xi, Y.; Zhang, J.; Ding, Y.; Wu, Q. Gut microbiota as an antioxidant system in centenarians associated with high antioxidant activities of gut-resident Lactobacillus. NPJ Biofilms Microbiomes 2022, 8, 102. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, 1–18. [Google Scholar] [CrossRef]
- Wang, F.; Yu, T.; Huang, G.; Cai, D.; Liang, X.; Su, H.; Zhu, Z.; Li, D.; Yang, Y.; Shen, P.; et al. Gut Microbiota Community and Its Assembly Associated with Age and Diet in Chinese Centenarians. J. Microbiol. Biotechnol. 2015, 25, 1195–1204. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Brigidi, P.; O’Toole, P.W.; De Vos, W.M. Maintenance of a healthy trajectory of the intestinal microbiome during aging: A dietary approach. Mech. Ageing Dev. 2014, 136–137, 70–75. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Woodmansey, E.J.; McMurdo, M.E.T.; Macfarlane, G.T.; Macfarlane, S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol. 2004, 70, 6113–6122. [Google Scholar] [CrossRef]
- Kanimozhi, N.V.; Sukumar, M. Aging through the lens of the gut microbiome: Challenges and therapeutic opportunities. Arch. Gerontol. Geriatr. Plus 2025, 2, 100142. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Gong, T. The interplay between oral microbiota, gut microbiota and systematic diseases. J. Oral Microbiol. 2023, 15, 2213112. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Mammen, M.J.; Scannapieco, F.A.; Sethi, S. Oral-lung microbiome interactions in lung diseases. Periodontol. 2000 2020, 83, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. Landmark Ed. 2021, 26, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.F.F.A.; Correia-de-Sá, T.; Araujo, R.; Barbosa, F.; Burnet, P.W.J.; Ferreira-Gomes, J.; Sampaio-Maia, B. The oral-gut microbiota relationship in healthy humans: Identifying shared bacteria between environments and age groups. Front. Microbiol. 2024, 15, 1475159. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Tang, X.; Kudo, Y.; Baker, J.L.; LaBonte, S.; Jordan, P.A.; McKinnie, S.M.K.; Guo, J.; Huan, T.; Moore, B.S.; Edlund, A. Cariogenic Streptococcus mutans Produces Tetramic Acid Strain-Specific Antibiotics That Impair Commensal Colonization. ACS Infect. Dis. 2020, 6, 563–571. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chopra, A.; Karmakar, S.; Bhat, S.G. Periodontitis increases the risk of gastrointestinal dysfunction: An update on the plausible pathogenic molecular mechanisms. Crit. Rev. Microbiol. 2025, 51, 187–217. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, U.K. Oral Prevotella Species and Their Connection to Events of Clinical Relevance in Gastrointestinal and Respiratory Tracts. Front. Microbiol. 2021, 12, 798763. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Wang, J.; Liao, B.; Cheng, L.; Ren, B. The interactions between oral-gut axis microbiota and Helicobacter pylori. Front. Cell. Infect. Microbiol. 2022, 12, 914418. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R. Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920; quiz 1943. [Google Scholar] [CrossRef]
- Dosedělová, V.; Laštovičková, M.; Ayala-Cabrera, J.F.; Dolina, J.; Konečný, Š.; Schmitz, O.J.; Kubáň, P. Quantification and identification of bile acids in saliva by liquid chromatography-mass spectrometry: Possible non-invasive diagnostics of Barrett’s esophagus? J. Chromatogr. A 2022, 1676, 463287. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shansky, Y.; Bespyatykh, J. Bile Acids: Physiological Activity and Perspectives of Using in Clinical and Laboratory Diagnostics. Molecules 2022, 27, 7830. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; O’Keefe, S.J.D. Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 2021, 73, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Nehra, D.; Howell, P.; Williams, C.P.; Pye, J.K.; Beynon, J. Toxic bile acids in gastro-oesophageal reflux disease: Influence of gastric acidity. Gut 1999, 44, 598–602. [Google Scholar] [CrossRef]
- Kauer, W.K.H.; Peters, J.H.; DeMeester, T.R.; Feussner, H.; Ireland, A.P.; Stein, H.J.; Siewert, R.J. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery 1997, 122, 874–881. [Google Scholar] [CrossRef]
- Cleaver, L.M.; Carda-Diéguez, M.; Moazzez, R.; Carpenter, G.H. Novel bacterial proteolytic and metabolic activity associated with dental erosion-induced oral dysbiosis. Microbiome 2023, 11, 69. [Google Scholar] [CrossRef]
- Boisen, G.; Davies, J.R.; Neilands, J. Acid tolerance in early colonizers of oral biofilms. BMC Microbiol. 2021, 21, 45. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, F.; Charitos, I.A.; Di Cosola, M.; Cazzolla, A.P. Focus on the cariogenic process: Microbial and biochemical interactions with teeth and oral environment. J. Biol. Regul. Homeost. Agents 2021, 35, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Y.; Yi, J.; Fang, Y.; Guo, Q.; Cheng, L.; He, J.; Li, M. Imbalance of oral microbiome homeostasis: The relationship between microbiota and the occurrence of dental caries. BMC Microbiol. 2025, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chu, W.; Luo, J.; Yang, J.; He, L.; Li, J. Dental Materials for Oral Microbiota Dysbiosis: An Update. Front. Cell. Infect. Microbiol. 2022, 12, 900918. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Greytak, M.; Kessler, M.; Yadlapati, R. Pilot study evaluating salivary bile acids as a diagnostic biomarker of laryngopharyngeal reflux. Dis. Esophagus 2024, 37, doae021. [Google Scholar] [CrossRef]
- Vageli, D.P.; Doukas, S.G.; Doukas, P.G.; Judson, B.L. Bile reflux and hypopharyngeal cancer (Review). Oncol. Rep. 2021, 46, 244. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Snider, E.J.; Freedberg, D.E.; Abrams, J.A. Potential Role of the Microbiome in Barrett’s Esophagus and Esophageal Adenocarcinoma. Dig. Dis. Sci. 2016, 61, 2217–2225. [Google Scholar] [CrossRef]
- Grigor’eva, I.N.; Romanova, T.I. Gallstone Disease and Microbiome. Microorganisms 2020, 8, 835. [Google Scholar] [CrossRef]
- Ye, F.; Shen, H.; Li, Z.; Meng, F.; Li, L.; Yang, J.; Chen, Y.; Bo, X.; Zhang, X.; Ni, M. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS ONE 2016, 11, e0150519. [Google Scholar] [CrossRef]
- Bhandari, S.; Reddy, M.; Shahzad, G. Association between oral hygiene and ultrasound-confirmed gallstone disease in US population. Eur. J. Gastroenterol. Hepatol. 2017, 29, 861–862. [Google Scholar] [CrossRef]
- Haraga, H.; Sato, T.; Watanabe, K.; Hamada, N.; Tani-Ishii, N. Effect of the Progression of Fusobacterium nucleatum-induced Apical Periodontitis on the Gut Microbiota. J. Endod. 2022, 48, 1038–1045. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef]
- Wang, A.; Zhai, Z.; Ding, Y.; Wei, J.; Wei, Z.; Cao, H. The oral-gut microbiome axis in inflammatory bowel disease: From inside to insight. Front. Immunol. 2024, 15, 1430001. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Huang, L.; Zhou, X.; Zhao, D.; Wang, Y.; Min, H.; Song, S.; Sun, W.; Gao, Q.; Hu, Q.; et al. Experimental Periodontitis Deteriorated Atherosclerosis Associated With Trimethylamine N-Oxide Metabolism in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 820535. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Moreira Almeida, V.; Azevedo, J.; Leal, H.F.; de Queiroz, A.T.L.; da Silva Filho, H.P.; Reis, J.N. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020, 15, e0239664. [Google Scholar] [CrossRef]
- Éliás, A.J.; Barna, V.; Patoni, C.; Demeter, D.; Veres, D.S.; Bunduc, S.; Erőss, B.; Hegyi, P.; Földvári-Nagy, L.; Lenti, K. Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: A systematic review and meta-analysis. BMC Med. 2023, 21, 262. [Google Scholar] [CrossRef]
- Szajewska, H.; Scott, K.P.; de Meij, T.; Forslund-Startceva, S.K.; Knight, R.; Koren, O.; Little, P.; Johnston, B.C.; Łukasik, J.; Suez, J.; et al. Antibiotic-perturbed microbiota and the role of probiotics. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.N.; Hagner, M.; Nogueira, A.V.B.; Franke, A.; Jäger, A.; Deschner, J. Inflammatory bowel disease and oral health: Systematic review and a meta-analysis. J. Clin. Periodontol. 2017, 44, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Pinto, R.D.; Corridoni, D.; Rodriguez-Palacios, A.; Stefano, G.D.; Monaco, A.; Weinberg, A.; Cominelli, F. Occurrence of Spontaneous Periodontal Disease in the SAMP1/YitFc Murine Model of Crohn Disease. J. Periodontol. 2014, 85, 1799. [Google Scholar] [CrossRef]
- Teles, F.; Wang, Y.; Hajishengallis, G.; Hasturk, H.; Marchesan, J.T. Impact of systemic factors in shaping the perio-dontal microbiome. Periodontol. 2000 2021, 85, 126–160. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128.e4. [Google Scholar] [CrossRef]
- D’AIuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Stern, D.M.; Schmidt, A.M. Receptor for Advanced Glycation End Products, Inflammation, and Accelerated Periodontal Disease in Diabetes: Mechanisms and Insights Into Therapeutic Modalities. Ann. Periodontol. 2001, 6, 113–118. [Google Scholar] [CrossRef]
- Shaffer, M.; Lozupone, C. Prevalence and Source of Fecal and Oral Bacteria on Infant, Child, and Adult Hands. mSystems 2018, 3, e00192-17. [Google Scholar] [CrossRef]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.H.; Langendijk, J.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K.L. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016, 58, 32–40. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Kotwal, G.; Cannon, J.L. Environmental persistence and transfer of enteric viruses. Curr. Opin. Virol. 2014, 4, 37–43. [Google Scholar] [CrossRef]
- Tahaei, S.M.E.; Mohebbi, S.R.; Zali, M.R. Enteric hepatitis viruses. Gastroenterol. Hepatol. Bed Bench 2012, 5, 7–15. [Google Scholar] [PubMed]
- Wu, J.; Huang, F.; Ling, Z.; Liu, S.; Liu, J.; Fan, J.; Yu, J.; Wang, W.; Jin, X.; Meng, Y.; et al. Altered faecal microbiota on the expression of Th cells responses in the exacerbation of patients with hepatitis E infection. J. Viral Hepat. 2020, 27, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 2016, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, A.; Van Winckel, M.; Cornelis, K.; Moens, G.; Devlies, G.; De Backer, G. Helicobacter pylori infection: Further evidence for the role of feco-oral transmission. Helicobacter 2006, 11, 523–528. [Google Scholar] [CrossRef]
- Azzolino, D.; Coelho-Junior, H.J.; Proietti, M.; Manzini, V.M.; Cesari, M. Fatigue in older persons: The role of nutrition. Proc. Nutr. Soc. 2023, 82, 39–46. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Guo, X.; Shao, Y. Role of the oral-gut microbiota axis in pancreatic cancer: A new perspective on tumor pathophysiology, diagnosis, and treatment. Mol. Med. 2025, 31, 103. [Google Scholar] [CrossRef]
- Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines 2023, 11, 2749. [Google Scholar] [CrossRef]
- Wu, J.-T.; Sun, C.-L.; Lai, T.-T.; Liou, C.-W.; Lin, Y.-Y.; Xue, J.-Y.; Wang, H.-W.; Chai, L.M.X.; Lee, Y.-J.; Chen, S.-L.; et al. Oral short-chain fatty acids administration regulates innate anxiety in adult microbiome-depleted mice. Neuropharmacology 2022, 214, 109140. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, W.; Meng, H. A double-edged sword: Role of butyrate in the oral cavity and the gut. Mol. Oral Microbiol. 2021, 36, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Nakamura, Y.; Yamamura, M.; Ikezawa, H.; Namikawa, I. Quantitative analysis of the Epstein-Barr virus-inducing properties of short-chain fatty acids present in the culture fluids of oral bacteria. Arch. Virol. 1991, 119, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Adil, N.A.; Omo-Erigbe, C.; Yadav, H.; Jain, S. The Oral–Gut Microbiome–Brain Axis in Cognition. Microorganisms 2025, 13, 814. [Google Scholar] [CrossRef]
- Zheng, X.; He, J.; Wang, L.; Zhou, S.; Peng, X.; Huang, S.; Zheng, L.; Cheng, L.; Hao, Y.; Li, J.; et al. Ecological Effect of Arginine on Oral Microbiota. Sci. Rep. 2017, 7, 7206. [Google Scholar] [CrossRef]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, M.; Wang, Z.; Han, J.-D.J. Oral microbiota in aging and diseases. Life Med. 2024, 3, lnae024. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Chu, C.H.; Yu, O.Y.; He, J.; Li, M. Roles of Streptococcus mutans in human health: Beyond dental caries. Front. Microbiol. 2024, 15, 1503657. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Xin, S.; Wang, Y.; Xu, J. Insight into the Relationship between Oral Microbiota and the Inflammatory Bowel Disease. Microorganisms 2022, 10, 1868. [Google Scholar] [CrossRef]
- Fatani, H.; Olaru, A.; Stevenson, R.; Alharazi, W.; Jafer, A.; Atherton, P.; Brook, M.; Moran, G. Systematic review of sarcopenia in inflammatory bowel disease. Clin. Nutr. Edinb. Scotl. 2023, 42, 1276–1291. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Vieujean, S.; Caron, B.; Jairath, V.; Benetos, A.; Danese, S.; Louis, E.; Peyrin-Biroulet, L. Is it time to include older adults in inflammatory bowel disease trials? A call for action. Lancet Healthy Longev. 2022, 3, e356–e366. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Azzolino, D. Frailty. In Geriatric Medicine; Springer: Cham, Switzerland, 2024; pp. 323–340. ISBN 978-3-030-74720-6. [Google Scholar]
- Kitamoto, S.; Kamada, N. The oral-gut axis: A missing piece in the IBD puzzle. Inflamm. Regen. 2023, 43, 54. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Allela, O.Q.B.; Kareem, R.A.; Baldaniya, L.; Ballal, S.; Vashishth, R.; Parmar, M.; Sameer, H.N.; Hamad, A.K.; Athab, Z.H.; et al. Prognostic gene expression profile of colorectal cancer. Gene 2025, 955, 149433. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, Y.; Wu, L.; Zhang, C.; Han, B.; Cheng, Y.; Tong, Y.; Yan, D.; Wang, L. The association between inflammatory cytokines and sarcopenia-related traits: A bi-directional Mendelian randomization study. Eur. J. Clin. Nutr. 2024, 78, 1032–1040. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Zhao, X.; Yang, P.; Wang, X.; Chen, X.; Chen, N.; Chen, F. Standardized studies of the oral microbiome: From technology-driven to hypothesis-driven. iMeta 2022, 1, e19. [Google Scholar] [CrossRef]
- Guo, Y.; Kitamoto, S.; Caballero-Flores, G.; Kim, Y.; Watanabe, D.; Sugihara, K.; Núñez, G.; Alteri, C.J.; Inohara, N.; Kamada, N. Oral pathobiont Klebsiella chaperon usher pili provide site-specific adaptation for the inflamed gut mucosa. Gut Microbes 2024, 16, 2333463. [Google Scholar] [CrossRef]
- Salvatori, O.; Kumar, R.; Metcalfe, S.; Vickerman, M.; Kay, J.G.; Edgerton, M. Bacteria Modify Candida albicans Hypha Formation, Microcolony Properties, and Survival within Macrophages. mSphere 2020, 5, e00689-20. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Lu, J.; Huang, Y.; Wang, M.; Chen, B.; Bao, J.; Wang, L.; Cui, D.; Luo, B.; Yan, F. Periodontitis Salivary Microbiota Worsens Colitis. J. Dent. Res. 2022, 101, 559–568. [Google Scholar] [CrossRef]
- Tanwar, H.; Gnanasekaran, J.M.; Allison, D.; Chuang, L.-S.; He, X.; Aimetti, M.; Baima, G.; Costalonga, M.; Cross, R.K.; Sears, C.; et al. Unravelling the Oral-Gut Axis: Interconnection Between Periodontitis and Inflammatory Bowel Disease, Current Challenges, and Future Perspective. J. Crohns Colitis 2024, 18, 1319–1341. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol. 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Crombez, L.; Descamps, A.; Hirmz, H.; Lambert, M.; Calewaert, J.; Siluk, D.; Markuszewski, M.; Biesemans, M.; Petrella, G.; Cicero, D.; et al. The Saliva and Muscle Study (SaMu): Rationale and Protocol for Associations between Salivary Microbiome and Accelerated Muscle Ageing. J. Frailty Aging 2024, 13, 331–340. [Google Scholar] [CrossRef]
- Ogawa, T.; Hirose, Y.; Honda-Ogawa, M.; Sugimoto, M.; Sasaki, S.; Kibi, M.; Kawabata, S.; Ikebe, K.; Maeda, Y. Composition of salivary microbiota in elderly subjects. Sci. Rep. 2018, 8, 414. [Google Scholar] [CrossRef]
- Wells, P.M.; Sprockett, D.D.; Bowyer, R.C.E.; Kurushima, Y.; Relman, D.A.; Williams, F.M.K.; Steves, C.J. Influential factors of saliva microbiota composition. Sci. Rep. 2022, 12, 18894. [Google Scholar] [CrossRef]
- Zhurakivska, K.; Troiano, G.; Caponio, V.C.A.; Dioguardi, M.; Laino, L.; Maffione, A.B.; Lo Muzio, L. Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Front. Physiol. 2019, 10, 1029. [Google Scholar] [CrossRef]
- Gómez-Rubio, P.; Trapero, I.; Cauli, O.; Buigues, C. Salivary IL-6 Concentration Is Associated with Frailty Syndrome in Older Individuals. Diagnostics 2022, 12, 117. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Bohn, B.; Ladanyi, A.; Pinsino, A.; Braghieri, L.; Carey, M.R.; Clerkin, K.; Sayer, G.T.; Latif, F.; Koji, T.; et al. Alterations in the sarcopenia index are associated with inflammation, gut, and oral microbiota among heart failure, left ventricular assist device, and heart transplant patients. J. Heart Lung Transplant. 2024, 43, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, S.; Wang, C.; Wang, Y.; Shi, Y.; Lin, J.; Wang, N.; Su, L.; Yang, F.; Wang, H.; et al. Association of Dental Caries with Muscle Mass, Muscle Strength, and Sarcopenia: A Community-Based Study. J. Nutr. Health Aging 2023, 27, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; McLellan, G.; Lennon, L.T.; Papacosta, A.O.; Weyant, R.J.; Kapila, Y.; Mathers, J.C.; Wannamethee, S.G.; Whincup, P.H.; Ramsay, S.E. Association between oral health markers and decline in muscle strength and physical performance in later life: Longitudinal analyses of two prospective cohorts from the UK and the USA. Lancet Healthy Longev. 2022, 3, e777–e788. [Google Scholar] [CrossRef]
- Eremenko, M.; Pink, C.; Biffar, R.; Schmidt, C.O.; Ittermann, T.; Kocher, T.; Meisel, P. Cross-sectional association between physical strength, obesity, periodontitis and number of teeth in a general population. J. Clin. Periodontol. 2016, 43, 401–407. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Rantanen, T.; Keskinen, M.; Meurman, J.H. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology 2004, 21, 155–160. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Lennon, L.T.; Ramsay, S.E.; Papacosta, O.; Shaper, A.G.; Wannamethee, S.G.; Whincup, P.H. Cohort Profile Update: The British Regional Heart Study 1978–2014: 35 years follow-up of cardiovascular disease and ageing. Int. J. Epidemiol. 2015, 44, 826–826g. [Google Scholar] [CrossRef]
- Castrejón-Pérez, R.C.; Jiménez-Corona, A.; Bernabé, E.; Villa-Romero, A.R.; Arrivé, E.; Dartigues, J.-F.; Gutiérrez-Robledo, L.M.; Borges-Yáñez, S.A. Oral Disease and 3-Year Incidence of Frailty in Mexican Older Adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2017, 72, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-F.; Lee, W.-F.; Salamanca, E.; Yao, W.-L.; Su, J.-N.; Wang, S.-Y.; Hu, C.-J.; Chang, W.-J. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Weyant, R.J.; Garcia, M.E.; Harris, T.; Launer, L.J.; Satterfield, S.; Simonsick, E.M.; Yaffe, K.; Newman, A.B. Adverse oral health and cognitive decline: The health, aging and body composition study. J. Am. Geriatr. Soc. 2013, 61, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.-M.; Zhou, L.-X.; Tsang, M.-W.; Tai, W.C.-S.; Wong, S.-C.C. The Oral Microbial Ecosystem in Age-Related Xerostomia: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12815. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The role of the oral microbiome in obesity and metabolic disease: Potential systemic implications and effects on taste perception. Nutr. J. 2023, 22, 28. [Google Scholar] [CrossRef]
- Konturek, P.C.; Haziri, D.; Brzozowski, T.; Hess, T.; Heyman, S.; Kwiecien, S.; Konturek, S.J.; Koziel, J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 483–491. [Google Scholar]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.H.J.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Moroni, A.; Perna, S.; Azzolino, D.; Gasparri, C.; Zupo, R.; Micheletti Cremasco, M.; Rondanelli, M. Discovering the Individualized Factors Associated with Sarcopenia and Sarcopenic Obesity Phenotypes—A Machine Learning Approach. Nutrients 2023, 15, 4536. [Google Scholar] [CrossRef]
- Azzolino, D.; Passarelli, P.C.; D’Addona, A.; Cesari, M. Nutritional strategies for the rehabilitation of COVID-19 patients. Eur. J. Clin. Nutr. 2021, 75, 728–730. [Google Scholar] [CrossRef]
- O’Connor, J.-L.P.; Milledge, K.L.; O’Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2020, 78, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Borges-Yáñez, S.A.; Gutiérrez-Robledo, L.M.; Ávila-Funes, J.A. Oral health conditions and frailty in Mexican community-dwelling elderly: A cross sectional analysis. BMC Public Health 2012, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Spolidoro, G.C.I.; Saporiti, E.; Luchetti, C.; Agostoni, C.; Cesari, M. Musculoskeletal Changes Across the Lifespan: Nutrition and the Life-Course Approach to Prevention. Front. Med. 2021, 8, 697954. [Google Scholar] [CrossRef]
- Alvarez, J.O. Nutrition, tooth development, and dental caries. Am. J. Clin. Nutr. 1995, 61, 410S–416S. [Google Scholar] [CrossRef] [PubMed]
- Enwonwu, C.O.; Phillips, R.S.; Falkler, W.A. Nutrition and oral infectious diseases: State of the science. Compend. Contin. Educ. Dent. 2002, 23, 431–434, 436, 438 passim; quiz 448. [Google Scholar]
- Jurtshuk, P. Bacterial Metabolism. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Bowden, G.H.; Li, Y.H. Nutritional influences on biofilm development. Adv. Dent. Res. 1997, 11, 81–99. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Darwish, S.; Baroudi, K. Oral Microbial Shift: Factors affecting the Microbiome and Prevention of Oral Disease. J. Contemp. Dent. Pract. 2016, 17, 90–96. [Google Scholar] [CrossRef]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 59. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database J. Biol. Databases Curation 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Samaranayake, L.; Matsubara, V.H. Normal Oral Flora and the Oral Ecosystem. Dent. Clin. N. Am. 2017, 61, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 2018, 8, 5847. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Dahhan, R.; Freudenheim, J.L.; Hovey, K.M.; Li, L.; McSkimming, D.I.; Andrews, C.A.; Buck, M.J.; LaMonte, M.J.; Kirkwood, K.L.; et al. Dietary carbohydrate intake is associated with the subgingival plaque oral microbiome abundance and diversity in a cohort of postmenopausal women. Sci. Rep. 2022, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, P.; Gasparini, G.; Manicone, P.F.; Passarelli, P.C.; Azzolino, D.; Rella, E.; De Rosa, G.; Papi, P.; Pompa, G.; De Angelis, S.; et al. The Effect of an Optimized Diet as an Adjunct to Non-Surgical Periodontal Therapy in Subjects with Periodontitis: A Prospective Study. Healthcare 2022, 10, 583. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B.; Mølbak, L.; de Bruijn, A.; Schierbeek, H.; Boye, M.; Boehm, G.; Renes, I.; van Goudoever, J.; Burrin, D. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G300–G310. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Allison, C.; Gibson, S.A.; Cummings, J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988, 64, 37–46. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H.; Macfarlane, S.; Gibson, G.R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989, 67, 521–527. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Carlisle, E.; Alverdy, J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Farsijani, S.; Cauley, J.A.; Cawthon, P.M.; Langsetmo, L.; Orwoll, E.S.; Kado, D.M.; Kiel, D.P.; Newman, A.B. Associations Between Walking Speed and Gut Microbiome Composition in Older Men From the MrOS Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2024, 79, glae030. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Fluitman, K.S.; van den Broek, T.; Reinders, I.; Wijnhoven, H.A.H.; Nieuwdorp, M.; Visser, M.; IJzerman, R.G.; Keijser, B.J.F. The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial. Nutrients 2023, 15, 4567. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Taylor, G.W.; Manz, M.C.; Yoshihara, A.; Sato, M.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Oral health status: Relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent. Oral Epidemiol. 2014, 42, 441–450. [Google Scholar] [CrossRef]
- Kim, E.-J.; Jin, B.-H. Comparison of oral health status and daily nutrient intake between elders who live alone and elders who live with family: Based on the Korean National Health and Nutrition Examination Survey (KNHANES VI) (2013–2015). Gerodontology 2018, 35, 129–138. [Google Scholar] [CrossRef]
- Marshall, T.A.; Warren, J.J.; Hand, J.S.; Xie, X.-J.; Stumbo, P.J. Oral health, nutrient intake and dietary quality in the very old. J. Am. Dent. Assoc. 2002, 133, 1369–1379. [Google Scholar] [CrossRef]
- Bomfim, R.A.; de Souza, L.B.; Corrente, J.E. Tooth loss and its relationship with protein intake by elderly Brazilians—A structural equation modelling approach. Gerodontology 2018, 35, 51–58. [Google Scholar] [CrossRef]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Dis. Mon. 2019, 65, 147–154. [Google Scholar] [CrossRef]
- Farsijani, S.; Cauley, J.A.; Peddada, S.D.; Langsetmo, L.; Shikany, J.M.; Orwoll, E.S.; Ensrud, K.E.; Cawthon, P.M.; Newman, A.B. Relation Between Dietary Protein Intake and Gut Microbiome Composition in Community-Dwelling Older Men: Findings from the Osteoporotic Fractures in Men Study (MrOS). J. Nutr. 2022, 152, 2877–2887. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans1. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef]
- Li, N.; Cen, Z.; Zhao, Z.; Li, Z.; Chen, S. BCAA dysmetabolism in the host and gut microbiome, a key player in the development of obesity and T2DM. Med. Microecol. 2023, 16, 100078. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, Z.; Chen, L.; Zhang, Y.; Cheng, Q.; Nüssler, A.K.; Bao, W.; Liu, L.; Yang, W. Prospective Views for Whey Protein and/or Resistance Training Against Age-related Sarcopenia. Aging Dis. 2019, 10, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization (Eds.) Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO food and nutrition, paper; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A.; Perna, S.; Allieri, F.; Castellazzi, A.M. Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef]
- Saraswati, S.; Sitaraman, R. Aging and the human gut microbiota—From correlation to causality. Front. Microbiol. 2015, 5, 764. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Slomka, V.; Herrero, E.R.; Boon, N.; Bernaerts, K.; Trivedi, H.M.; Daep, C.; Quirynen, M.; Teughels, W. Oral prebiotics and the influence of environmental conditions in vitro. J. Periodontol. 2018, 89, 708–717. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.-M.; Botelho, C.; et al. Functional Dynamics of the Gut Microbiome in Elderly People during Probiotic Consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Azzolino, D.; Bertoni, C.; De Cosmi, V.; Spolidoro, G.C.I.; Agostoni, C.; Lucchi, T.; Mazzocchi, A. Omega-3 polyunsatured fatty acids and physical performance across the lifespan: A narrative review. Front. Nutr. 2024, 11, 1414132. [Google Scholar] [CrossRef]
- Lee, C.-T.; Tribble, G.D. Roles of specialized pro-resolving mediators and omega-3 polyunsaturated fatty acids in periodontal inflammation and impact on oral microbiota. Front. Oral Health 2023, 4, 1217088. [Google Scholar] [CrossRef]
- Awoyemi, A.; Trøseid, M.; Arnesen, H.; Solheim, S.; Seljeflot, I. Effects of dietary intervention and n-3 PUFA supplementation on markers of gut-related inflammation and their association with cardiovascular events in a high-risk population. Atherosclerosis 2019, 286, 53–59. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Stańdo-Retecka, M.; Piatek, P.; Namiecinska, M.; Bonikowski, R.; Lewkowicz, P.; Lewkowicz, N. Clinical and microbiological outcomes of subgingival instrumentation supplemented with high-dose omega-3 polyunsaturated fatty acids in periodontal treatment—A randomized clinical trial. BMC Oral Health 2023, 23, 290. [Google Scholar] [CrossRef] [PubMed]
- Shama, S.; Liu, W. Omega-3 Fatty Acids and Gut Microbiota: A Reciprocal Interaction in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2020, 65, 906–910. [Google Scholar] [CrossRef]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Oettlé, G.J. Effect of moderate exercise on bowel habit. Gut 1991, 32, 941–944. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Lavilla-Lerma, M.L.; Aibar-Almazán, A.; Martínez-Amat, A.; Jiménez-García, J.D.; Hita-Contreras, F. Moderate-intensity continuous training and high-intensity interval training modulate the composition of the oral microbiota of elderly adults: Randomized controlled trial. Maturitas 2024, 185, 107973. [Google Scholar] [CrossRef]
- Dubois, M.; Delcourt, B.; Ortis, M.; Bougault, V.; Doglio, A.; Bertrand, M.-F. Correlation Between the Oral Microbiota and Sports Practice: A Systematic Review. Cureus 2025, 17, e78168. [Google Scholar] [CrossRef]
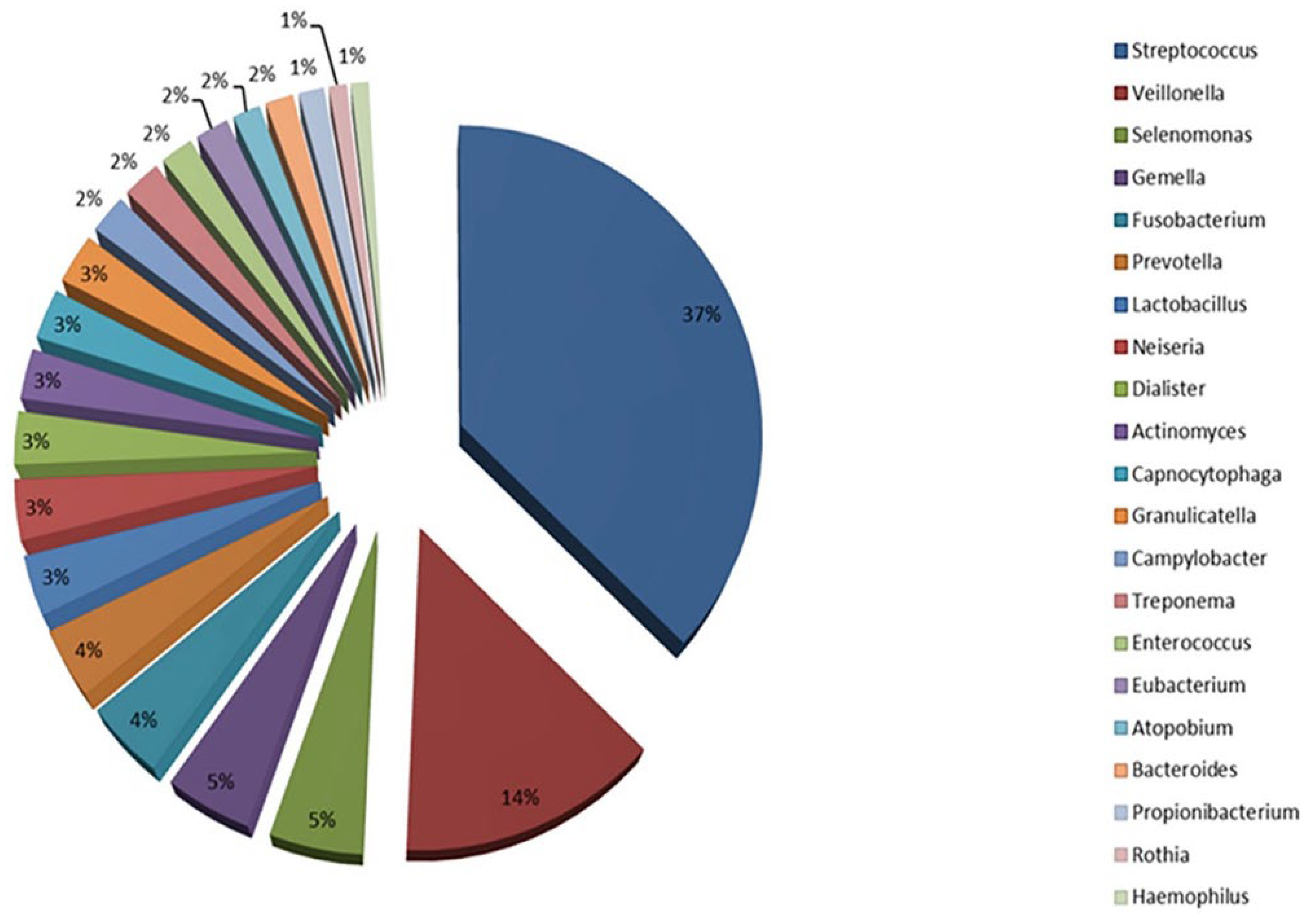

| Oral Microbiota | Gut Microbiota |
|---|---|
| Older people | |
| ↑ Lactobacillaceae, Streptococcus anginosus, and Gemella sanguinis ↓ Neisseria | ↓ Bacillota/Bacteroidota ratio, Bifidobacteriaceae ↑ Pseudomonadota (Escherichia coli, Klebsiella, Acquabacterium) |
| Denture users: ↑ Bacillota and Actinomycetota | - |
| Edentulous: ↑ Prevotella histicola, Veillonella atypica, Streptococcus salivarius, and Streptococcus parasanguinis | - |
| Centenarians | |
| Toothy centenarians Dental plaque and saliva: ↑ Spirochaetota and Synergistota (at phylum level), Aggregatibacter spp., Prevotella spp., Campylobacter spp., Anaeroglobus spp., Selenomonas spp., Fusobacterium spp., and Porphyromonas endodontalis (at genus level) Dental plaque: ↑ Bifidobacterium and Scardovia (at genus level), Porphyromonas gingivalis, Tannerella forsythia, and Prevotella intermedia (at species level) Edentulous Dental plaque and saliva: ↑ Bacillota and Actinomycetota (at phylum level), Streptococcus spp. (at genus level) | ↑ Pseudomonadota (Escherichia coli et rel., Haemophilus spp., Klebsiella pneumoniae et rel., Leminorella spp., Proteus et rel., Pseudomonas, Serratia spp., Vibrio spp., and Yersinia et rel.), Bacillota (Bacillus spp., Staphylococcus spp.) ↑ Methanobrevibacter smithii, Bifidobacterium adolescentis, Clostridium leptum ↑ Lactic acid species (Lactobacillaceae) ↓ Bacillota/>Bacteroidota ratio ↓ Faecalibacterium prausnitzii, Agathobacter rectalis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolino, D.; Carnevale-Schianca, M.; Bottalico, L.; Colella, M.; Felicetti, A.; Perna, S.; Terranova, L.; Garcia-Godoy, F.; Rondanelli, M.; Passarelli, P.C.; et al. The Oral–Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia. Nutrients 2025, 17, 2408. https://doi.org/10.3390/nu17152408
Azzolino D, Carnevale-Schianca M, Bottalico L, Colella M, Felicetti A, Perna S, Terranova L, Garcia-Godoy F, Rondanelli M, Passarelli PC, et al. The Oral–Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia. Nutrients. 2025; 17(15):2408. https://doi.org/10.3390/nu17152408
Chicago/Turabian StyleAzzolino, Domenico, Margherita Carnevale-Schianca, Lucrezia Bottalico, Marica Colella, Alessia Felicetti, Simone Perna, Leonardo Terranova, Franklin Garcia-Godoy, Mariangela Rondanelli, Pier Carmine Passarelli, and et al. 2025. "The Oral–Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia" Nutrients 17, no. 15: 2408. https://doi.org/10.3390/nu17152408
APA StyleAzzolino, D., Carnevale-Schianca, M., Bottalico, L., Colella, M., Felicetti, A., Perna, S., Terranova, L., Garcia-Godoy, F., Rondanelli, M., Passarelli, P. C., & Lucchi, T. (2025). The Oral–Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia. Nutrients, 17(15), 2408. https://doi.org/10.3390/nu17152408









