Effect of Peanut Shell Extract and Luteolin on Gut Microbiota and High-Fat Diet-Induced Sequelae of the Inflammatory Continuum in a Metabolic Syndrome-like Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Collection
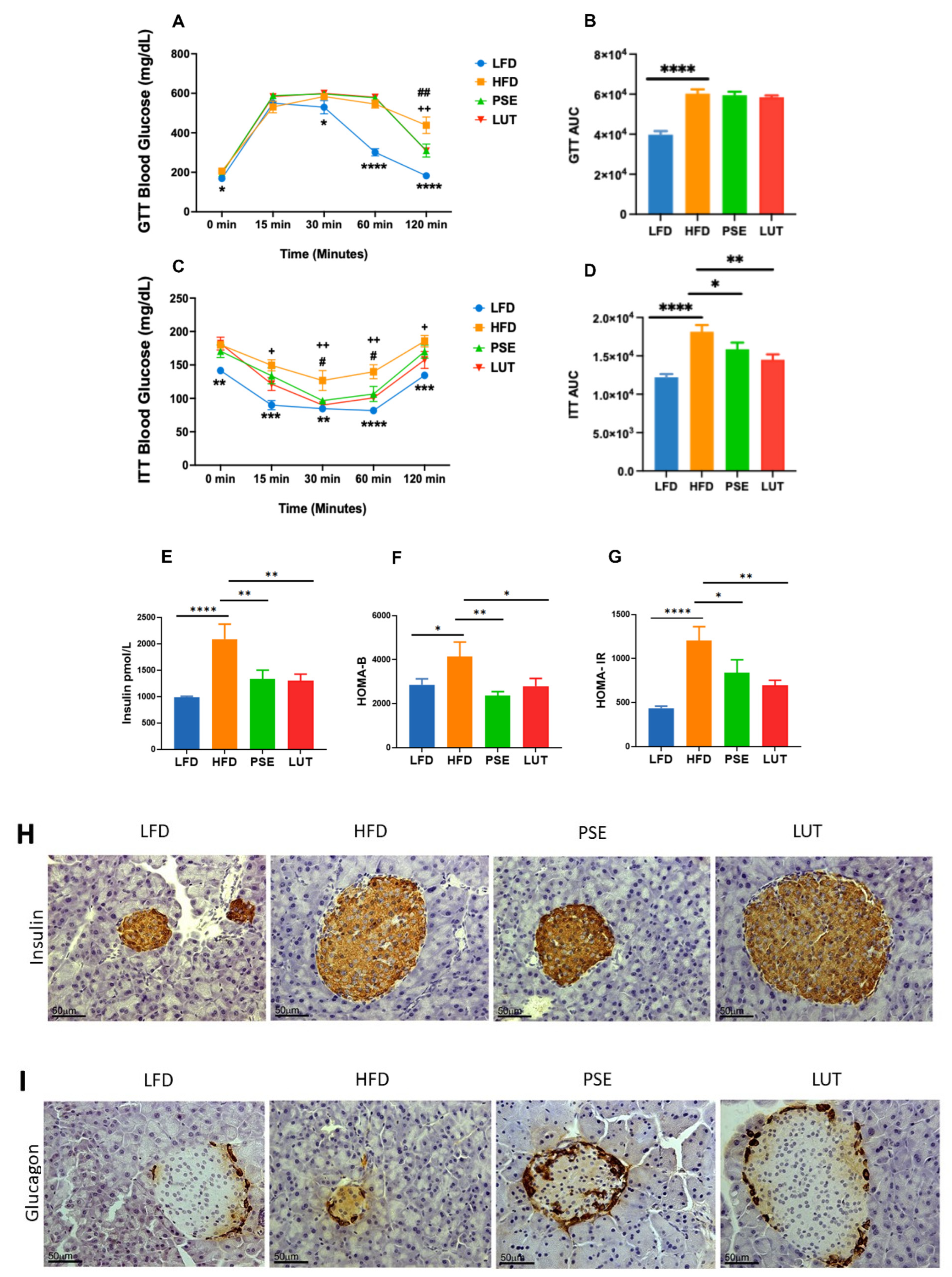
2.3. Glucose and Insulin Tolerance Tests
2.4. Serum Insulin and HOMA Assessment
2.5. Histology on Pancreas Tissue
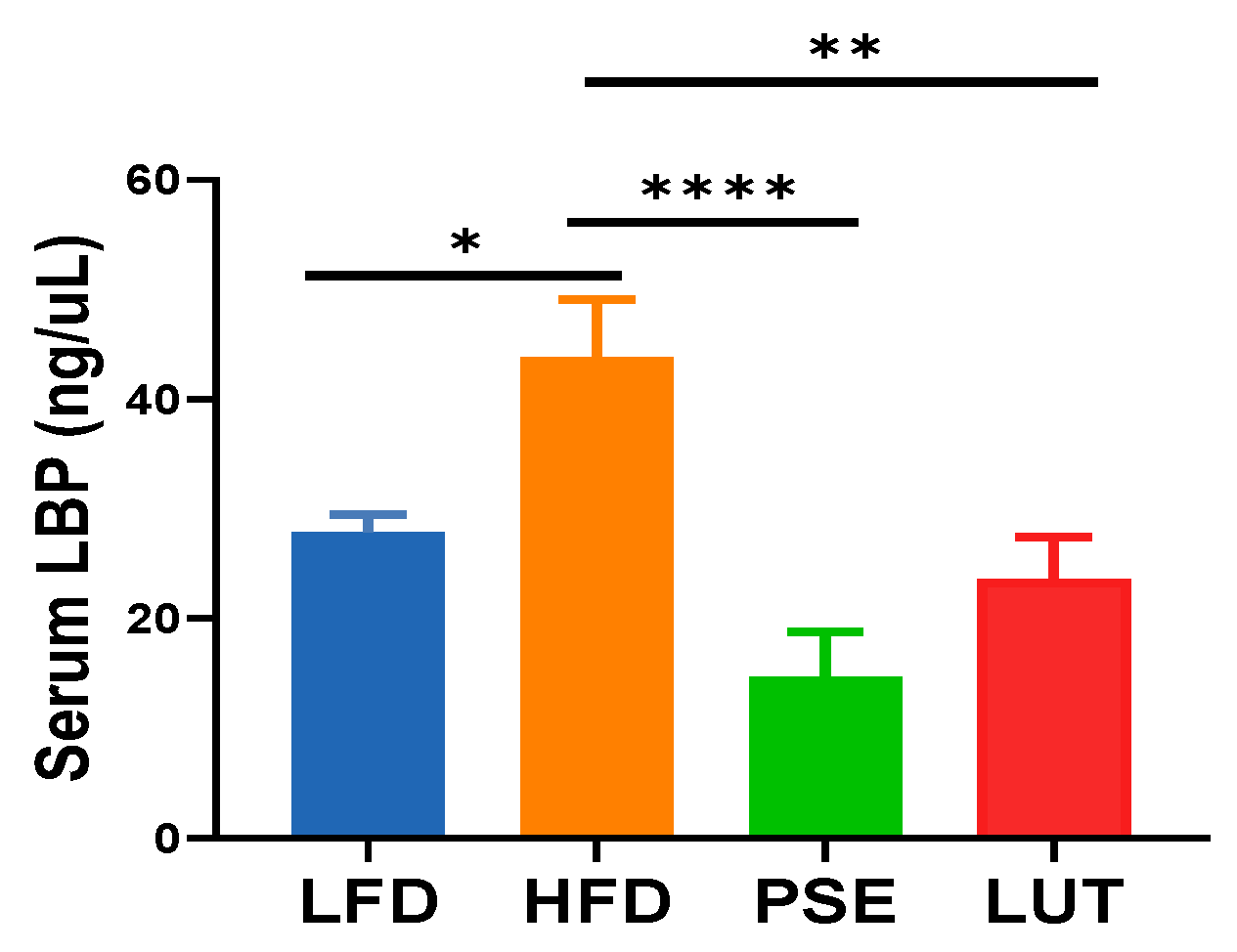
2.6. Measurement of Intestinal Permeability
2.7. Gene Profiling of Gut Microbiota and Data Analysis
2.8. RNA Extraction and qRT-PCR
2.9. Statistical Analysis
3. Results
3.1. PSE and LUT Improved Insulin Tolerance and Pancreatic Islet Function
3.2. PSE and LUT Decreased Intestinal Permeability
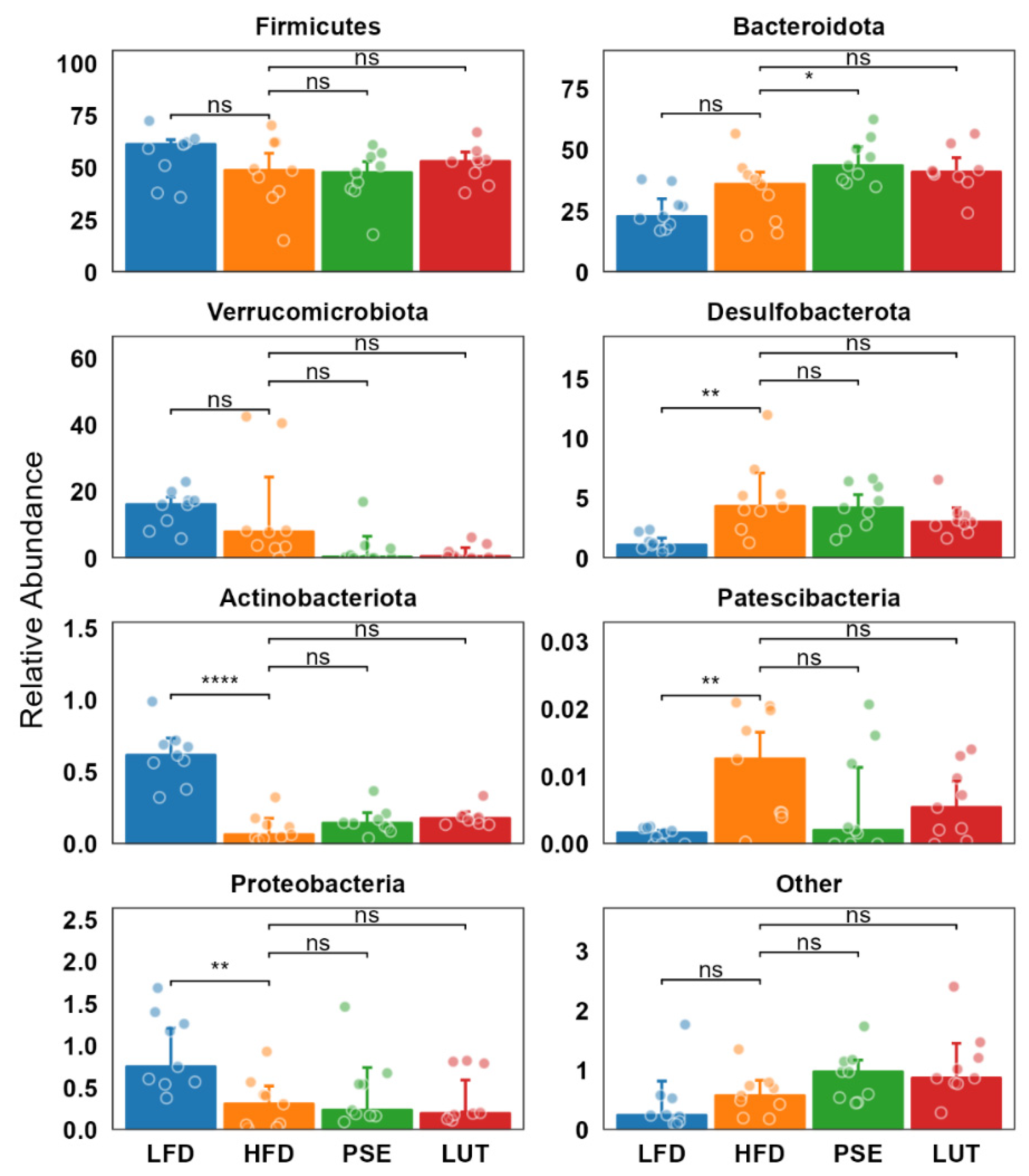
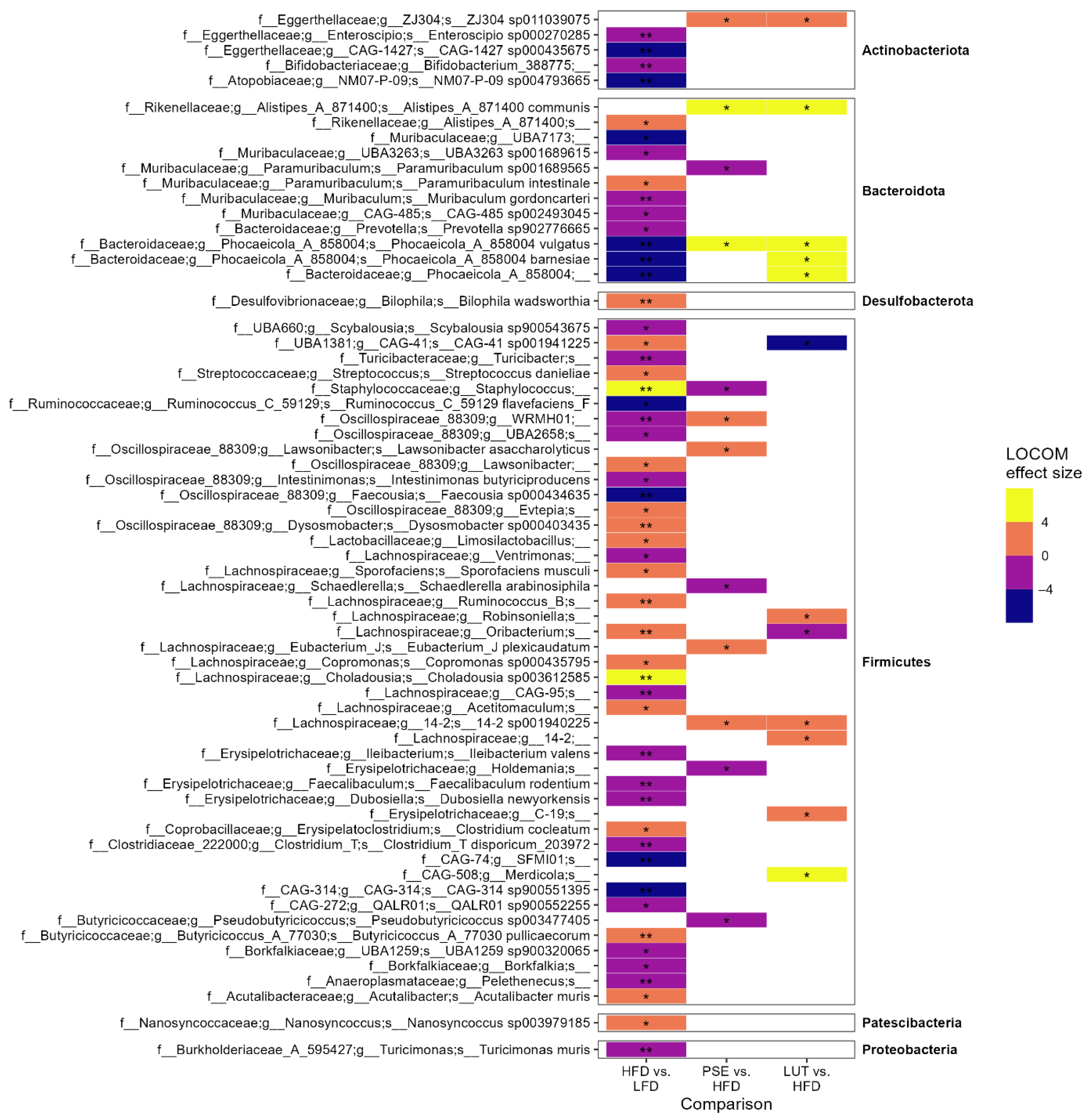
3.3. Gut Microbiome Analysis
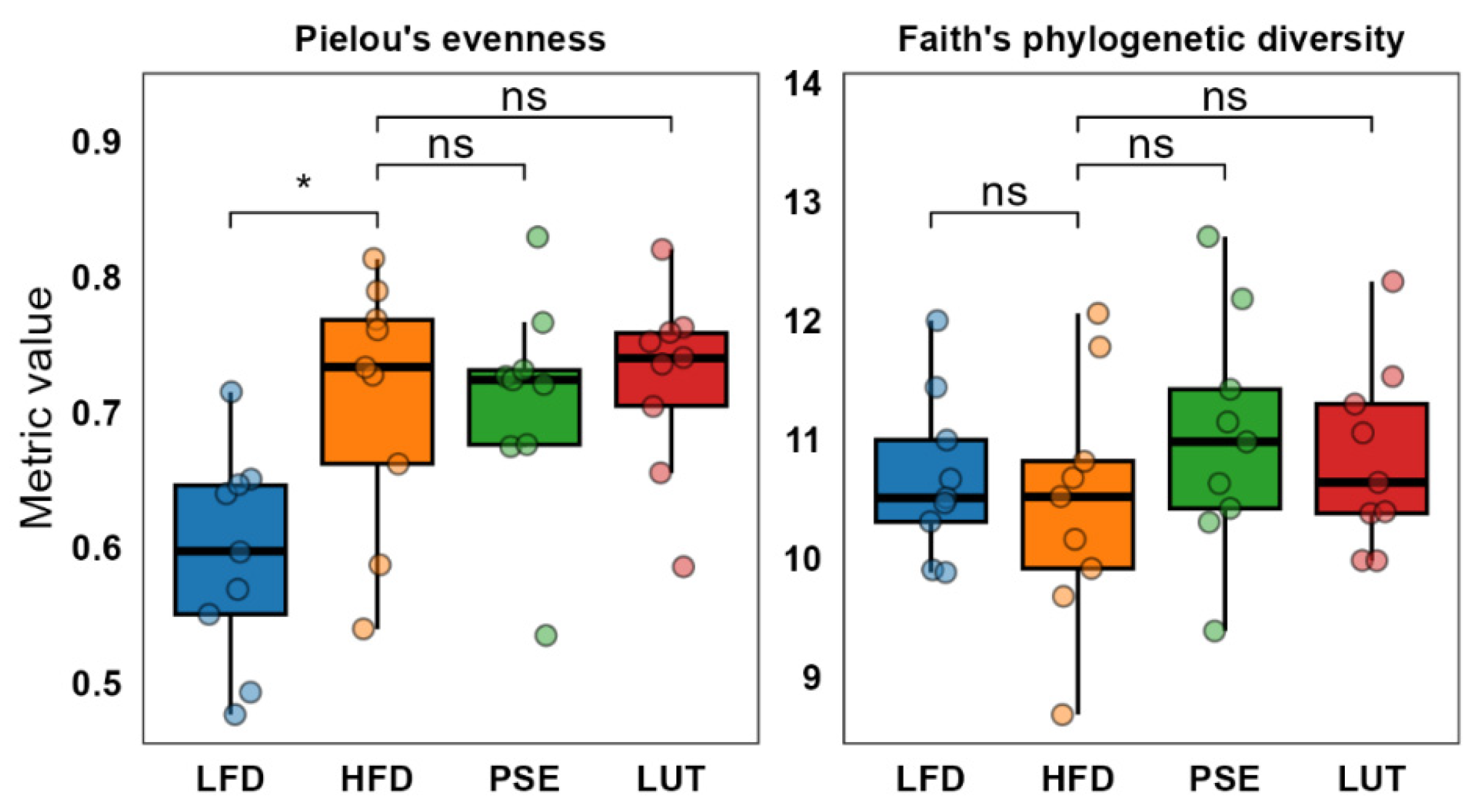
3.3.1. Microbiome Alpha Diversity
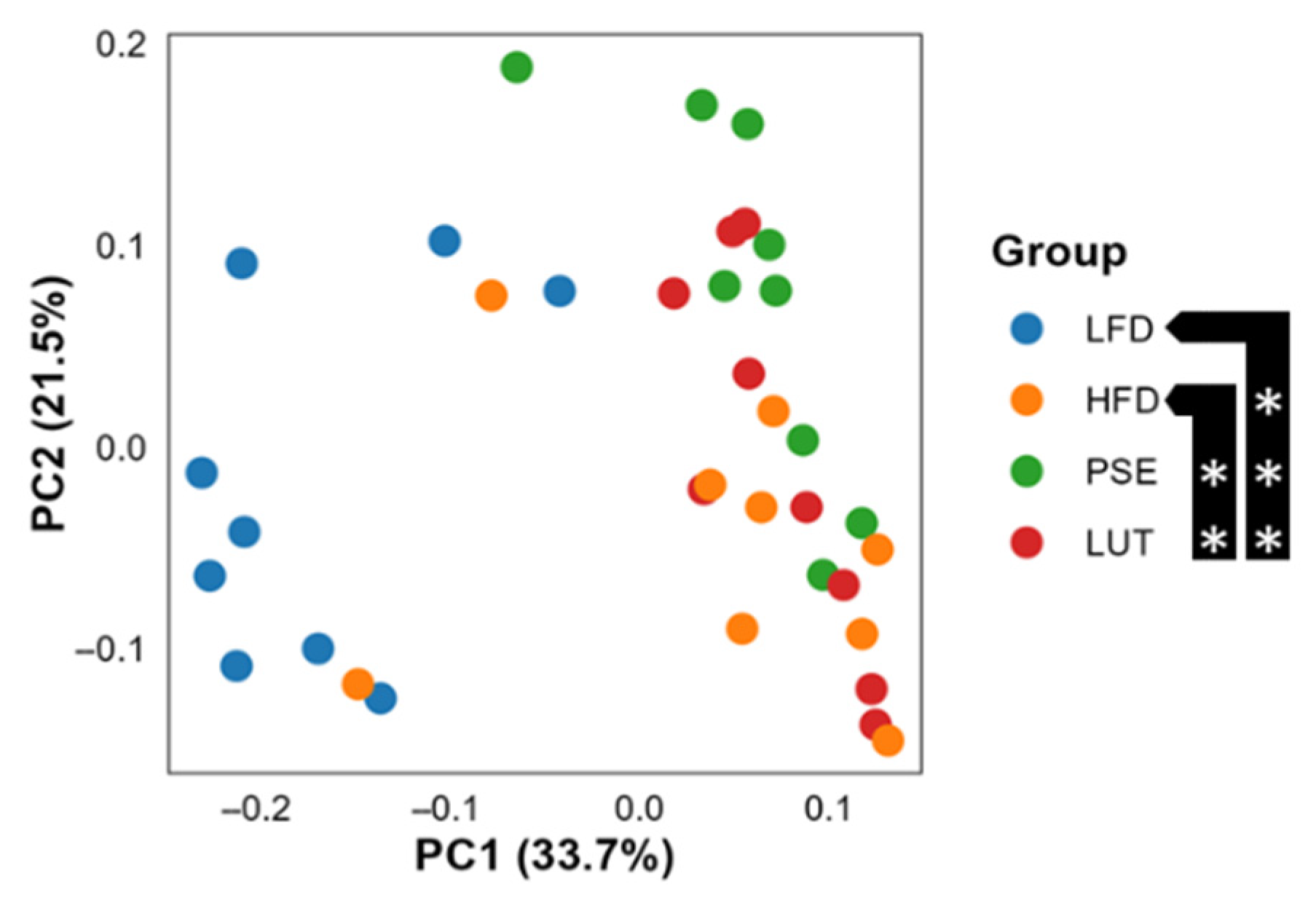
3.3.2. Microbiome Beta Diversity
3.3.3. Microbiome Composition Analysis
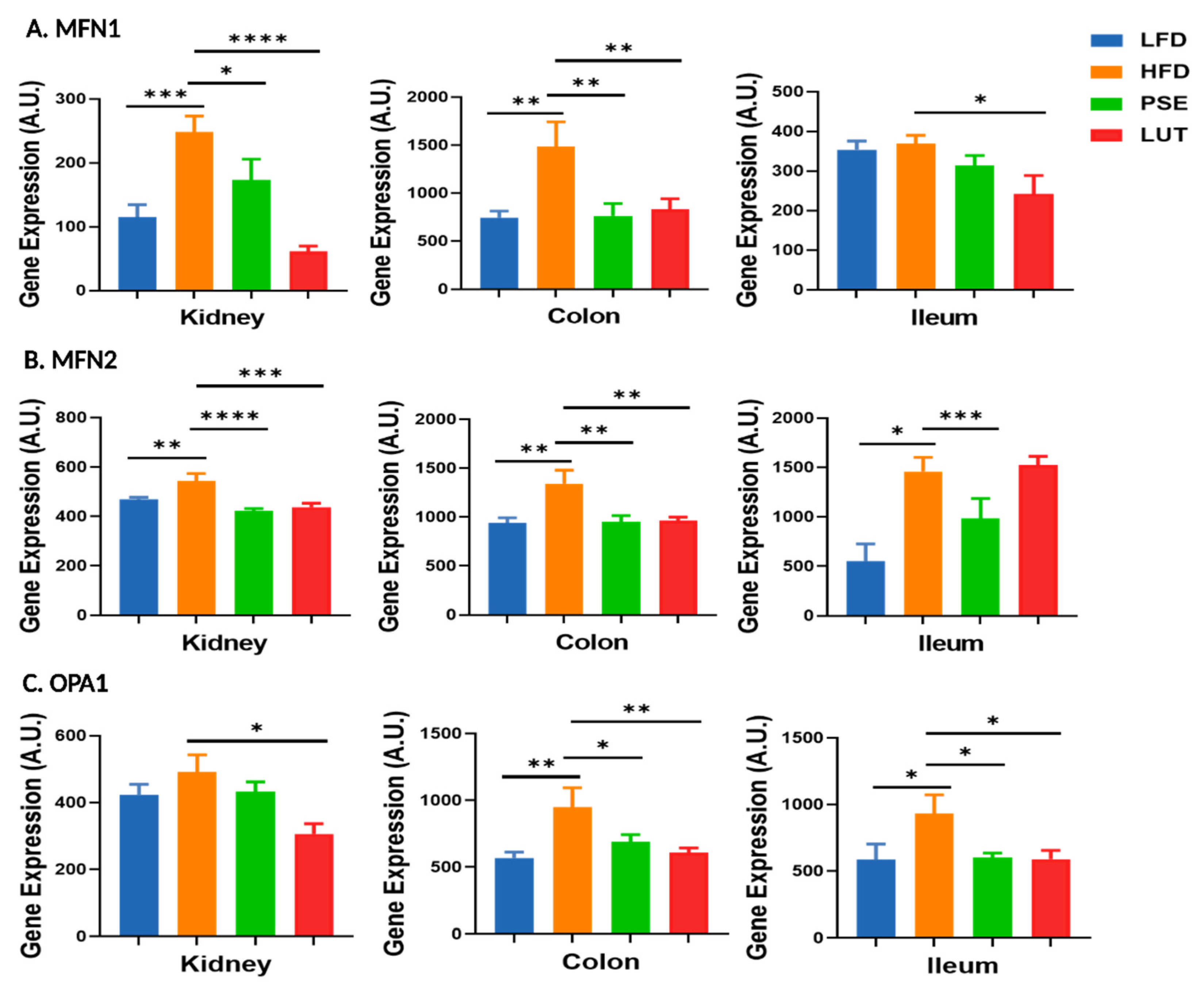
3.4. Mitochondrial Fusion Markers: MFN1, MFN2, OPA1
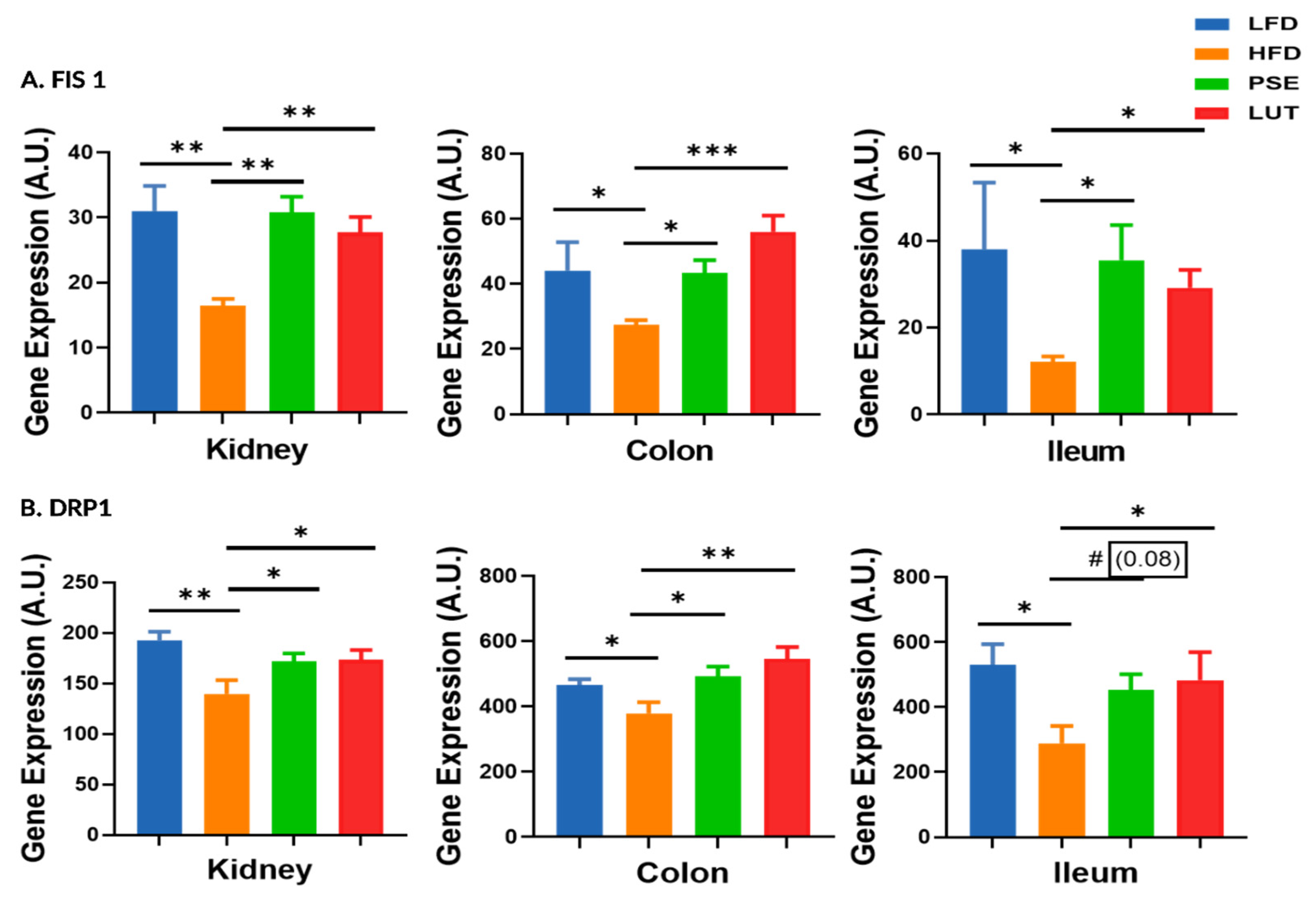
3.5. Mitochondrial Fission Markers: FIS1, DRP1
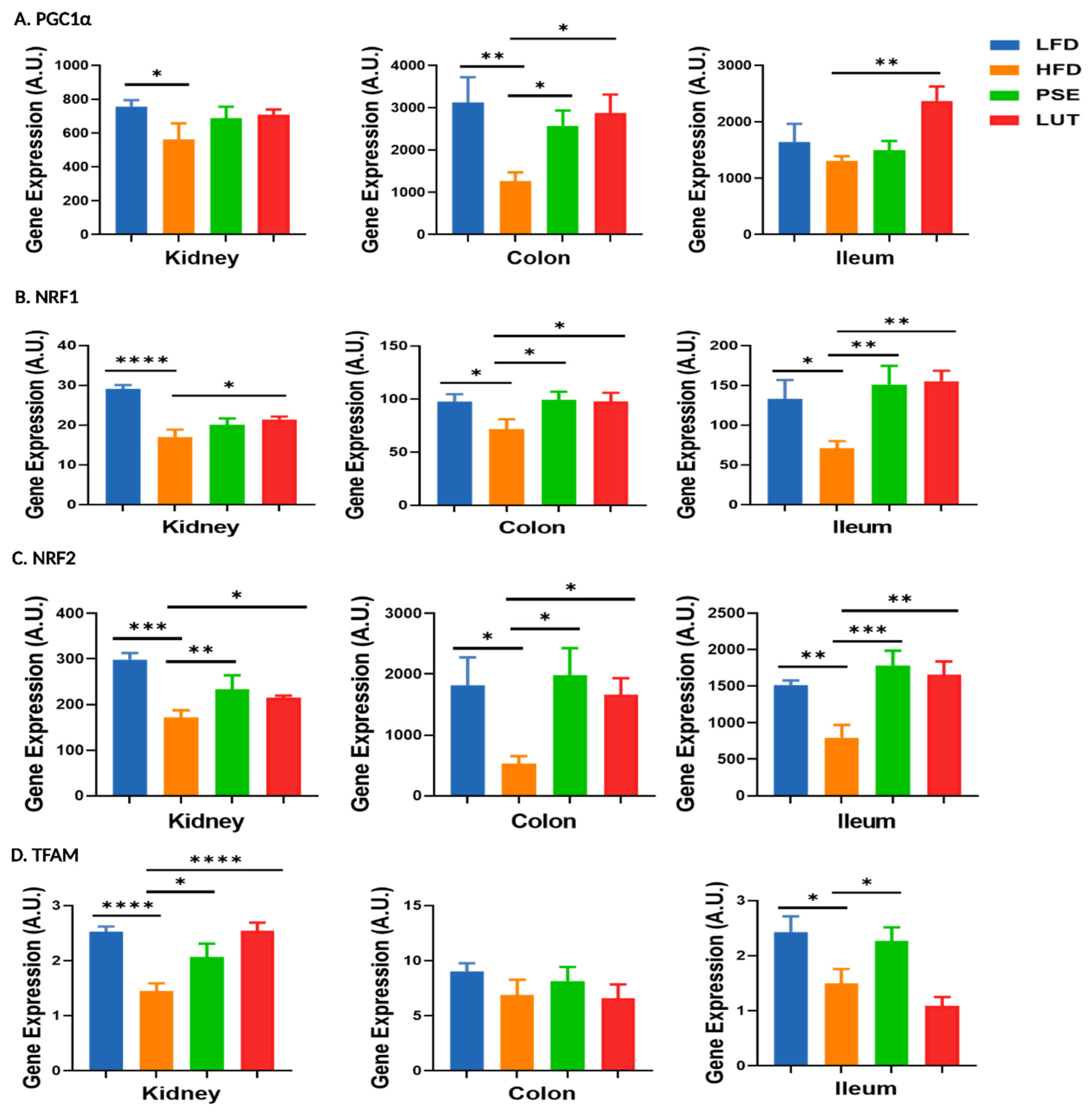
3.6. Mitochondrial Biosynthesis-Associated Markers: PGC1α, NRF1, NRF2, TFAM
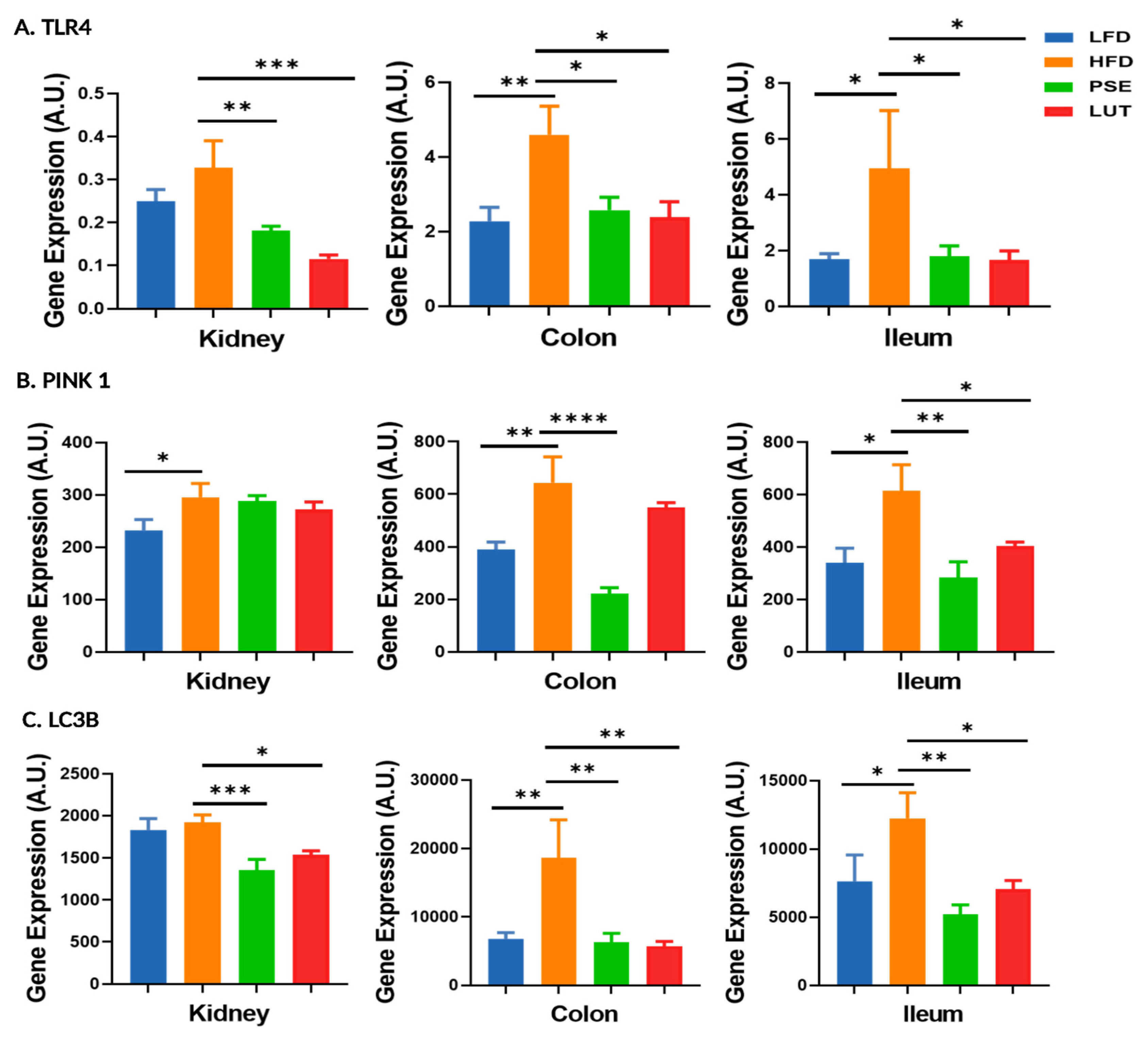
3.7. Mitophagy-Associated Markers: TLR4, PINK1, LC3B
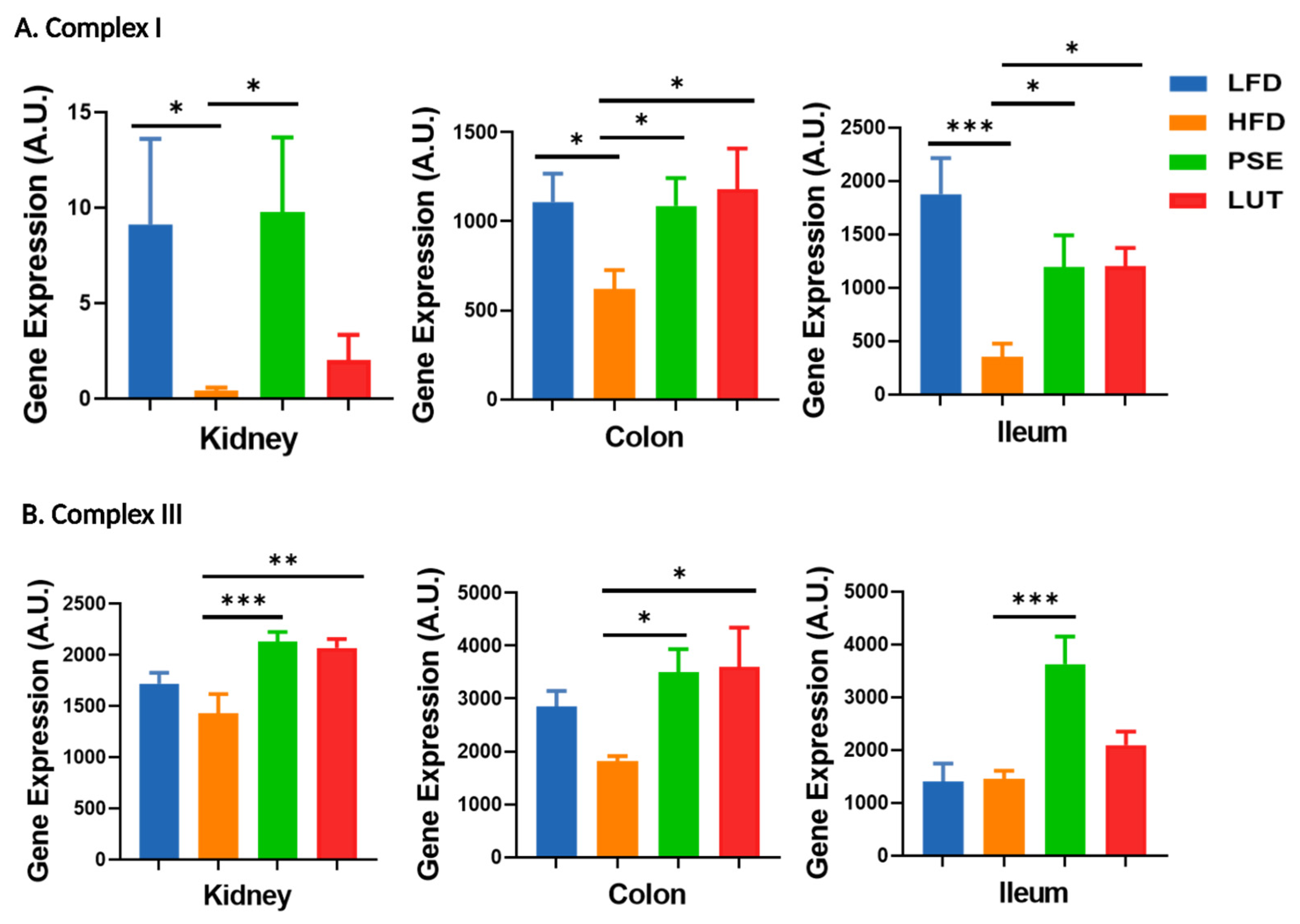
3.8. Mitochondrial Electron Transport Chain (ETC) Markers: Complex I, Complex III
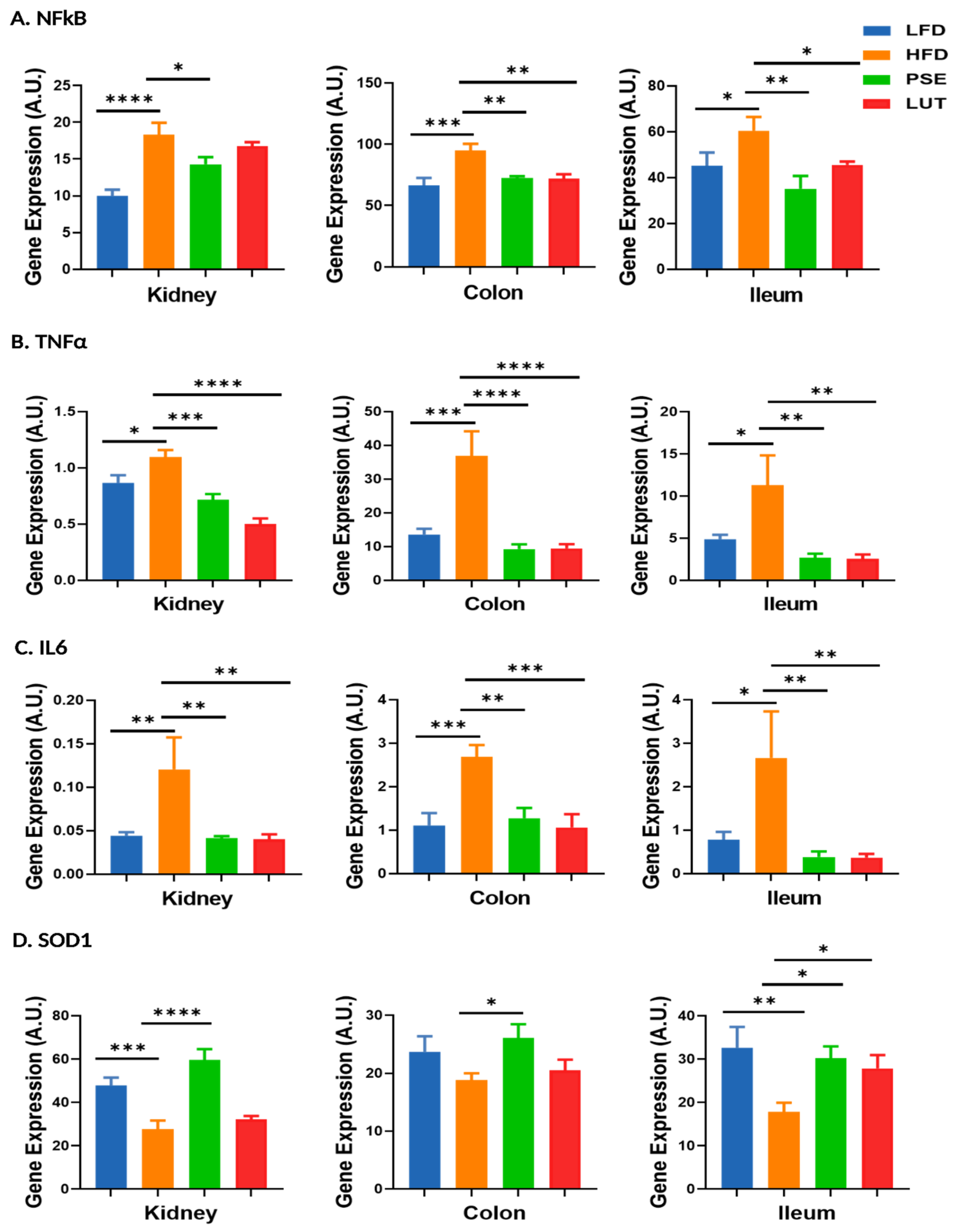
3.9. Proinflammatory Cytokines and Antioxidant Enzyme: NFκB, TNFα, IL6, SOD1
4. Discussion
4.1. Glucose Homeostasis and Islet Cell Function in the Slc6a14y/− Mouse Model
4.2. Gut Microbiome, Intestinal Barrier Function, and Metabolic Syndrome
4.3. Mitochondrial Function, Biogenesis, and Inflammatory Modulation by PSE and LUT
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Global Report on Diabetes 2023. Available online: https://www.who.int/health-topics/diabetes# (accessed on 1 April 2025).
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M.Y. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–18. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicine 2023, 59, 561. [Google Scholar] [CrossRef] [PubMed]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBiomedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Chong, L.L.T.; Chong, C.K.; Jensen, S.L.; Lau, K.M. Gut microbiota in type 2 diabetes mellitus: A systematic review of compositional and functional alterations. Front. Endocrinol. 2024, 15, 104821. [Google Scholar] [CrossRef]
- Craciun, C.-I.; Neag, M.-A.; Catinean, A.; Mitre, A.-O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.-D.; Muntean, D.-M.; Craciun, A.-E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 1, 308. [Google Scholar] [CrossRef] [PubMed]
- Patloka, O.; Komprda, T.; Franke, G. Review of the Relationships Between Human Gut Microbiome, Diet, and Obesity. Nutrients 2024, 16, 3996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Li, X.J.; Shan, Q.Y.; Wu, X.; Miao, H.; Zhao, Y.Y. Gut microbiota regulates oxidative stress and inflammation: A double-edged sword in renal fibrosis. Cell Mol Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 1, 1582. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Ezenabor, E.H.; Adeyemi, A.A.; Adeyemi, O.S. Gut Microbiota and Metabolic Syndrome: Relationships and Mechanisms. Int. J. Endocrinol. 2024. [Google Scholar] [CrossRef]
- Cojocaru, K.-A.L.I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Todosenko, N.; Khaziakhmatova, O.; Malashchenko, V.; Yurova, K.; Bograya, M.; Beletskaya, M.; Vulf, M.; Gazatova, N.; Litvinova, L. Mitochondrial Dysfunction Associated with mtDNA in Metabolic Syndrome and Obesity. Int. J. Mol. Sci. 2023, 24, 12012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, S.A.; Ogawa, S.A.; Chau, L.; Whelan, K.A.; Hamilton, K.E.; Chen, J.; Tan, L.; Chen, E.Z.; Keilbaugh, S.; Fogt, F.; et al. Mitochondrial dysfunction in inflammatory bowel disease alters intestinal epithelial metabolism of hepatic acylcarnitines. J. Clin. Investig. 2021, 131, e133371. [Google Scholar] [CrossRef]
- Kabootari, M.; Habibi Tirtashi, R.; Amouzegar, A.; Masoumi, S.; Azizi, F.; Amouzegar, A. Changes in metabolic syndrome status and risk of chronic kidney disease over a decade of follow-up in the Iranian population. Sci. Rep. 2025, 15, 19041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshmukh, H.; Santos, J.M.; Bender, M.; Dufour, J.M.; Lovett, J.; Shen, C.L. Peanut Shell Extract Improves Mitochondrial Function in db/db Mice via Suppression of Oxidative Stress and Inflammation. Nutrients 2024, 16, 1977. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Santos, J.M.; Dufour, J.M.; Deshmukh, H.; Trasti, S.; Elmassry, M.M.; Shen, C.-L. Peanut Shell Extract Improves Markers of Glucose Homeostasis in Diabetic Mice by Modulating Gut Dysbiosis and Suppressing Inflammatory Immune Response. Nutrients 2024, 16, 4158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.M.; Chen, Z.S.; Deng, Q.P.; Zhu, S.J.; Wang, G. The roles of luteolin in peanut shell extract—Mediated protection of erythrocytes against hypoxanthine-xanthine oxidase-induced toxicity. Food Biosci. 2021, 39, 100826. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Kim, S.Y.; Choi, M.S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients 2018, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Liang, B.; Sun, Y.L. Luteolin ameliorates rat model of metabolic syndrome-induced cardiac injury by apoptosis suppression and autophagy promotion via NR4A2/p53 regulation. BMC Complement. Med. Ther. 2025, 25, 14. [Google Scholar] [CrossRef]
- Fikry, H.; Saleh, L.A.; Sadek, D.R.; Alkhalek, H.A.A. The possible protective effect of luteolin on cardiovascular and hepatic changes in metabolic syndrome rat model. Cell Tissue Res. 2025, 399, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.R.; Wang, L.H.; Zhang, J. Peanut Shell Extract and Luteolin Regulate Lipid Metabolism and Induce Browning in 3T3-L1 Adipocytes. Foods 2022, 11, 2696. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.-G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Kahksha; Alam, O.; Al-Keridis, L.A.; Khan, J.; Naaz, S.; Alam, A.; Ashraf, S.A.; Alshammari, N.; Adnan, M.; Beg, A. Evaluation of Antidiabetic Effect of Luteolin in STZ Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangeetha, R. Luteolin in the management of type 2 diabetes mellitus. Curr. Res. Nutr. Food Sci. J. 2019, 7, 393–398. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Long, Z.; Zhan, J. Immunometabolic alterations in type 2 diabetes mellitus revealed by single-cell RNA sequencing: Insights into subtypes and therapeutic targets. Front. Immunol. 2025, 15, 1537909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Sikder, M.O.; Ramalingam, L.; Kaur, G.; Dufour, J.M.; Moustaid-Moussa, N.; Wachtel, M.S.; Ganapathy, V. SLC6A14 deficiency is linked to obesity, fatty liver, and metabolic syndrome but only under conditions of a high-fat diet. BBA-Mol. Basis Dis. 2021, 1867, 166087. [Google Scholar] [CrossRef] [PubMed]
- Taweesap, P.; Potue, P.; Khamseekaew, J.; Iampanichakul, M.; Jan-O, B.; Pakdeechote, P.; Maneesai, P. Luteolin Relieves Metabolic Dysfunction-Associated Fatty Liver Disease Caused by a High-Fat Diet in Rats Through Modulating the AdipoR1/AMPK/PPARgamma Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, X.; Yang, Y.; Xu, J.; Zhang, P.; Deng, R.; Mao, Y.; He, J.; Chen, Y.; Zhang, Y.; Ding, J.; et al. Luteolin Exerts Neuroprotection via Modulation of the p62/Keap1/Nrf2 Pathway in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, A.X.; Xia, T.C.-X.; Peng, Z.-T.; Wu, Q.-Y.; Zhu, Y.; Dong, T.T.-X.; Tsim, K.W.-K. The ethanolic extract of peanut shell attenuates the depressive-like behaviors of mice through modulation of inflammation and gut microbiota. Food Res. Int. 2023, 168, 112765. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Wang, R.; Santos, J.M.; Elmassry, M.M.; Stephens, E.; Kim, N.; Neugebauer, V. Ginger alleviates mechanical hypersensitivity and anxio-depressive behavior in rats with diabetic neuropathy through beneficial actions on gut microbiome composition, mitochondria, and neuroimmune cells of colon and spinal cord. Nutr. Res. 2024, 124, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Deshmukh, H.; Elmassry, M.M.; Yakhnitsa, V.; Ji, G.; Kiritoshi, T.; Presto, P.; Antenucci, N.; Liu, X.; Neugebauer, V.; et al. Beneficial Effects of Ginger Root Extract on Pain Behaviors, Inflammation, and Mitochondrial Function in the Colon and Different Brain Regions of Male and Female Neuropathic Rats: A Gut-Brain Axis Study. Nutrients 2024, 16, 3563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar] [PubMed] [PubMed Central]
- Yoon, H.; Jeong, D.K.; Lee, K.S.; Kim, H.S.; Moon, A.E.; Park, J. Relationship between metabolic syndrome and metabolic syndrome score and beta cell function by gender in Korean populations with obesity. Endocr. J. 2016, 63, 785–793. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.-M.; Ye, H.-Q.; Liu, J.-B.; Wu, L.; Lin, D.-B.; Yu, Y.-L.; Gao, F. Assessment of anti-diabetic activity of peanut shell polyphenol extracts. J. Zhejiang Univ. B 2018, 19, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Shehnaz, S.I.; Roy, A.; Vijayaraghavan, R.; Sivanesan, S. Luteolin Mitigates Diabetic Dyslipidemia in Rats by Modulating ACAT-2, PPARalpha, SREBP-2 Proteins, and Oxidative Stress. Appl. Biochem. Biotechnol. 2023, 195, 4893–4914. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Chen, M.Y.; Han, Y.P.; Liu, L.J.; Yan, J.L.; Qian, L.B. The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-suppressed autophagy. Food Funct. 2023, 14, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Leandro, A.; Azul, L.; Figueirinha, A.; Seiça, R.; Sena, C.M. Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 13671. [Google Scholar] [CrossRef]
- Morales-Ferra, D.L.; Zavala-Sanchez, M.A.; Jimenez-Ferrer, E.; Gonzalez-Cortazar, M.; Zamilpa, A. Effect of Tecoma stans (L.) Juss. ex Kunth in a Murine Model of Metabolic Syndrome. Plants 2022, 11, 1794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hudish, L.I.; Reusch, J.E.B.; Sussel, L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Fändriks, L. Roles of the gut in the metabolic syndrome: An overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef]
- Sun, W.-L.; Yang, J.-W.; Dou, H.-Y.; Li, G.-Q.; Li, X.-Y.; Shen, L.; Ji, H.-F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef]
- Sinha, A.K.; Laursen, M.F.; Brinck, J.E.; Rybtke, M.L.; Hjørne, A.P.; Procházková, N.; Pedersen, M.; Roager, H.M.; Licht, T.R. Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat. Microbiol. 2024, 9, 1964–1978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Yang, S.; Duan, H.; Yan, Z.; Xue, C.; Niu, T.; Cheng, W.; Zhang, Y.; Zhao, X.; Hu, J.; Zhang, L. Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism. Nutrients 2025, 17, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, H.; Ali, W.; Deng, K.; Chen, Y.; Sun, J.; Wang, T.; Ma, Y.; Liu, Z. The protective effect of luteolin on cadmium induced liver intestinal toxicity in chicken by Gut-liver axis regulation. Poult. Sci. 2024, 103, 104242. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, R.; Li, Z.; Xiao, R.; Lv, P.; Sun, X.; Olson, M.A.; Gong, Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chen, P.; Yang, J.; Li, D.; Liu, X.; Zhang, Y.; Xia, Q.; Li, Y.; Chen, G.; Li, Y.; et al. alleviates diet-induced metabolic dysfunction-associated steatotic liver disease progression by downregulating histone acetylation level via 3-HPAA. Gut Microbes 2024, 16, 2309683. [Google Scholar] [CrossRef]
- Gao, S.Y.; Gao, Y.J.; Cai, L.F.; Qin, R. Luteolin attenuates Staphylococcus aureus-induced endometritis through inhibiting ferroptosis and inflammation via activating the Nrf2/GPX4 signaling pathway. Microbiol. Spectr. 2024, 12, e0327923. [Google Scholar] [CrossRef]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2008, 29, 3595–3602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Losón, O.C.; Song, Z.Y.; Chen, H.C.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell. 2013, 24, 659–667. [Google Scholar] [CrossRef]
- Shpilka, T.; Haynes, C.M. The mitochondrial UPR: Mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Koyano, F.; Yamano, K.; Kosako, H.; Kimura, Y.; Kimura, M.; Fujiki, Y.; Tanaka, K.; Matsuda, N. Parkin-mediated ubiquitylation redistributes MITOL/March5 from mitochondria to peroxisomes. Embo Rep. 2019, 20, e47728. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Vongthip, W.; Nilkhet, S.; Boonruang, K.; Sukprasansap, M.; Tencomnao, T.; Baek, S.J. Neuroprotective mechanisms of luteolin in glutamate-induced oxidative stress and autophagy-mediated neuronal cell death. Sci. Rep. 2024, 14, 7707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, H.; Mendóza, R.; Santos, J.M.; Sivaprakasam, S.; Elmassry, M.M.; Miranda, J.M.; Pham, P.Q.; Driver, Z.; Bender, M.; Dufour, J.M.; et al. Effect of Peanut Shell Extract and Luteolin on Gut Microbiota and High-Fat Diet-Induced Sequelae of the Inflammatory Continuum in a Metabolic Syndrome-like Murine Model. Nutrients 2025, 17, 2290. https://doi.org/10.3390/nu17142290
Deshmukh H, Mendóza R, Santos JM, Sivaprakasam S, Elmassry MM, Miranda JM, Pham PQ, Driver Z, Bender M, Dufour JM, et al. Effect of Peanut Shell Extract and Luteolin on Gut Microbiota and High-Fat Diet-Induced Sequelae of the Inflammatory Continuum in a Metabolic Syndrome-like Murine Model. Nutrients. 2025; 17(14):2290. https://doi.org/10.3390/nu17142290
Chicago/Turabian StyleDeshmukh, Hemalata, Roberto Mendóza, Julianna M. Santos, Sathish Sivaprakasam, Moamen M. Elmassry, Jonathan M. Miranda, Patrick Q. Pham, Zarek Driver, Matthew Bender, Jannette M. Dufour, and et al. 2025. "Effect of Peanut Shell Extract and Luteolin on Gut Microbiota and High-Fat Diet-Induced Sequelae of the Inflammatory Continuum in a Metabolic Syndrome-like Murine Model" Nutrients 17, no. 14: 2290. https://doi.org/10.3390/nu17142290
APA StyleDeshmukh, H., Mendóza, R., Santos, J. M., Sivaprakasam, S., Elmassry, M. M., Miranda, J. M., Pham, P. Q., Driver, Z., Bender, M., Dufour, J. M., & Shen, C.-L. (2025). Effect of Peanut Shell Extract and Luteolin on Gut Microbiota and High-Fat Diet-Induced Sequelae of the Inflammatory Continuum in a Metabolic Syndrome-like Murine Model. Nutrients, 17(14), 2290. https://doi.org/10.3390/nu17142290







