The Association Between Prevotella copri and Advanced Fibrosis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Stool Collection
2.2. Liver Ultrasound Elastography
2.3. 16s rRNA Sequencing
2.4. Patient Metadata Analysis
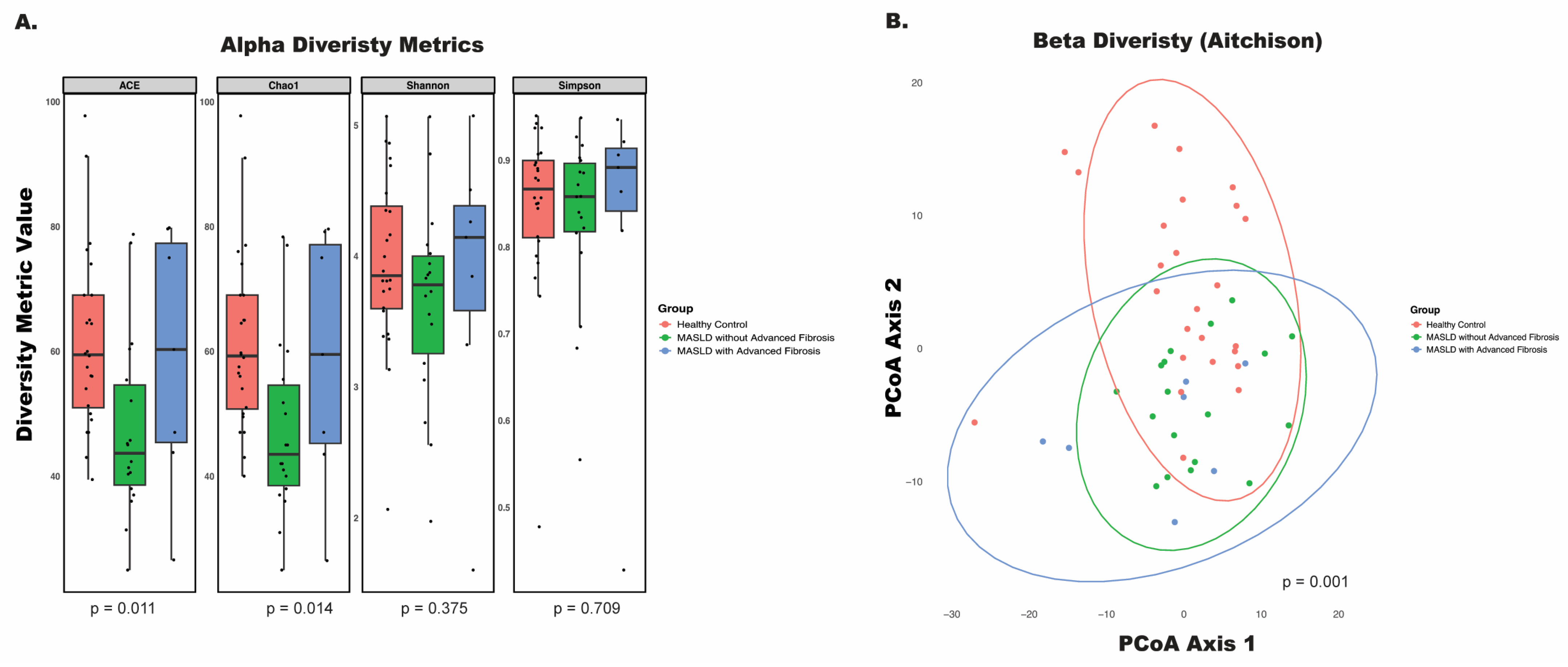
2.5. Diversity Analysis
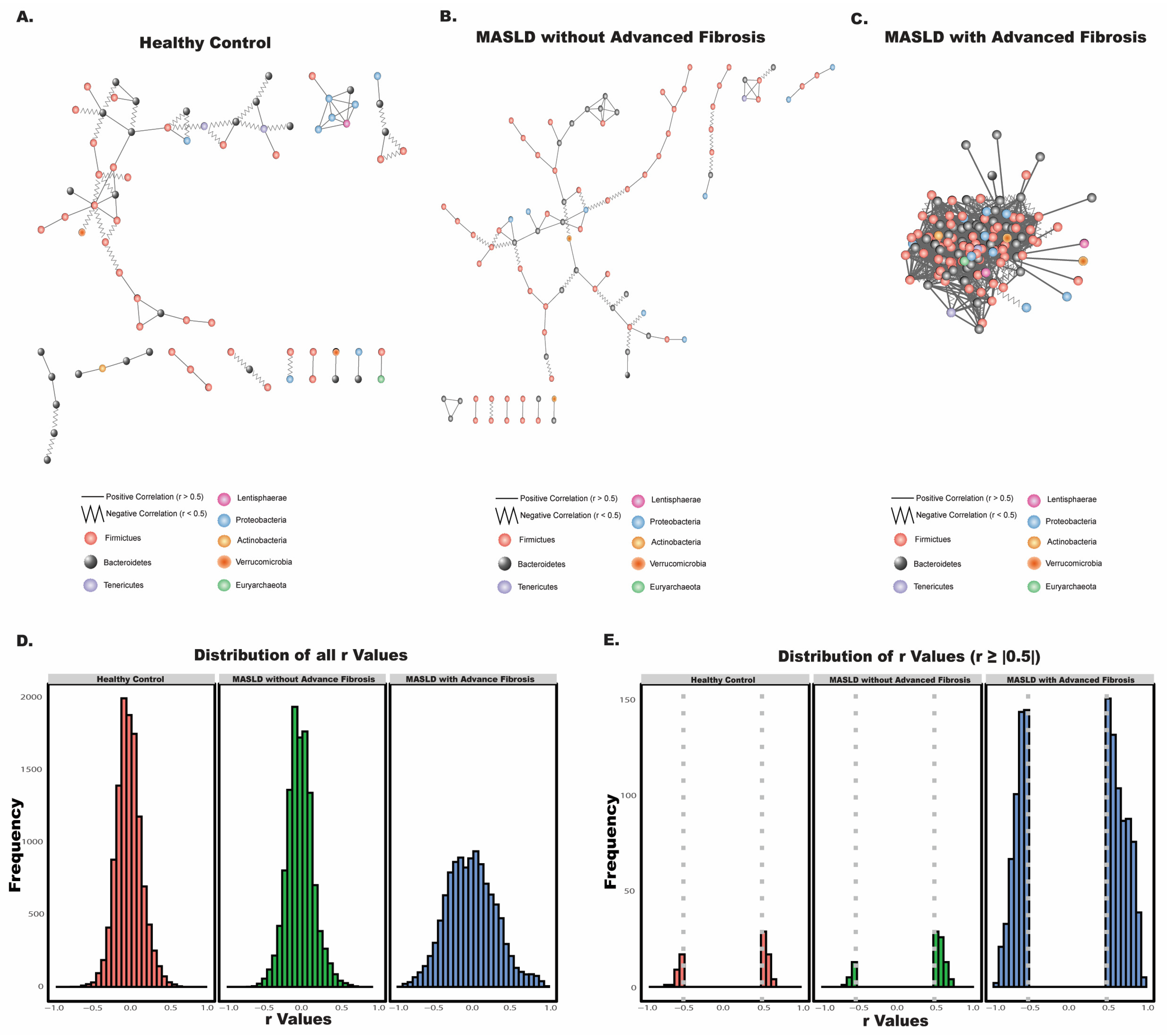
2.6. Bacterial Co-Occurrence Network
2.7. Differential Taxonomic Abundance Analysis
2.8. Mice
2.9. Oral Gavage
2.10. Hematoxylin and Eosin (H&E) Staining
2.11. Hepatic Triglyceride Quantification
2.12. Quantitative PCR (qPCR)
3. Results
3.1. Characterization of MASLD Patients and Healthy Controls
3.2. Disease Status and Fibrosis Stage Influence Microbial Profiles
3.3. Disease Status and Fibrosis Stage Influence Bacterial Interactions
3.4. Disease Status and Fibrosis Stage Influence Phylum- and Genus-Level Microbial Composition
3.5. P. copri Is More Abundant in MASLD Patients with Advanced Fibrosis
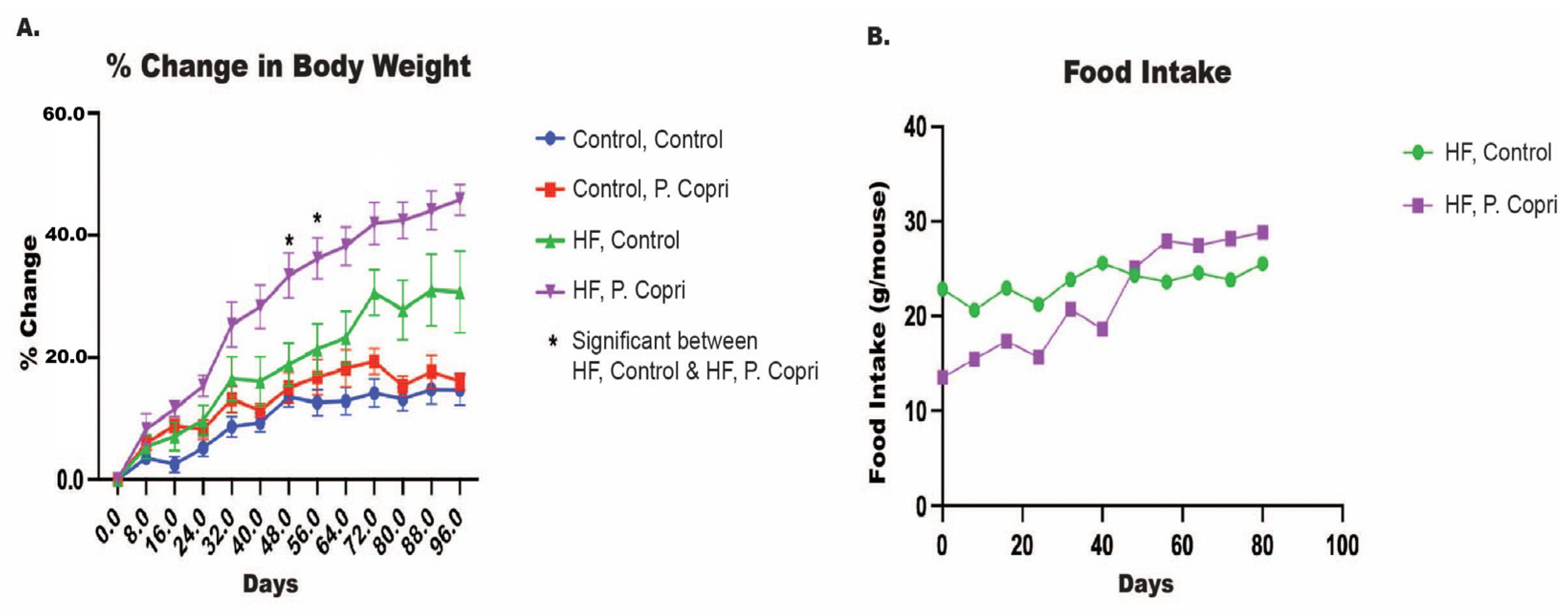
3.6. P. copri Colonization Increase Mice Weights on a High-Fat Diet
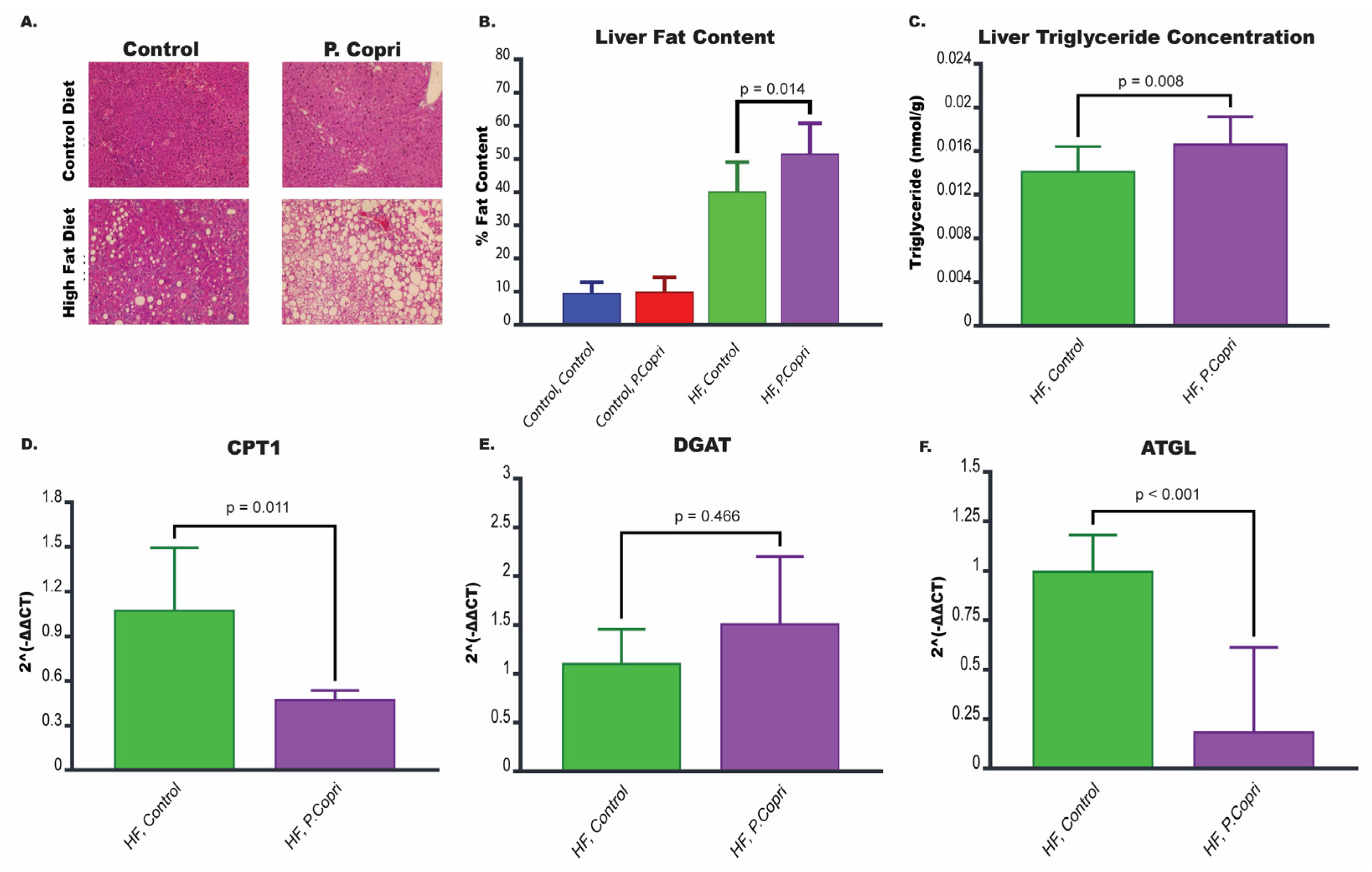
3.7. P. copri Colonization in Mice on a High-Fat Diet Increases Hepatic Lipid Content
3.8. P. copri Colonization in Mice on a High-Fat Diet Leads to Downregulation of Genes Involved in Lipid Metabolism in the Liver
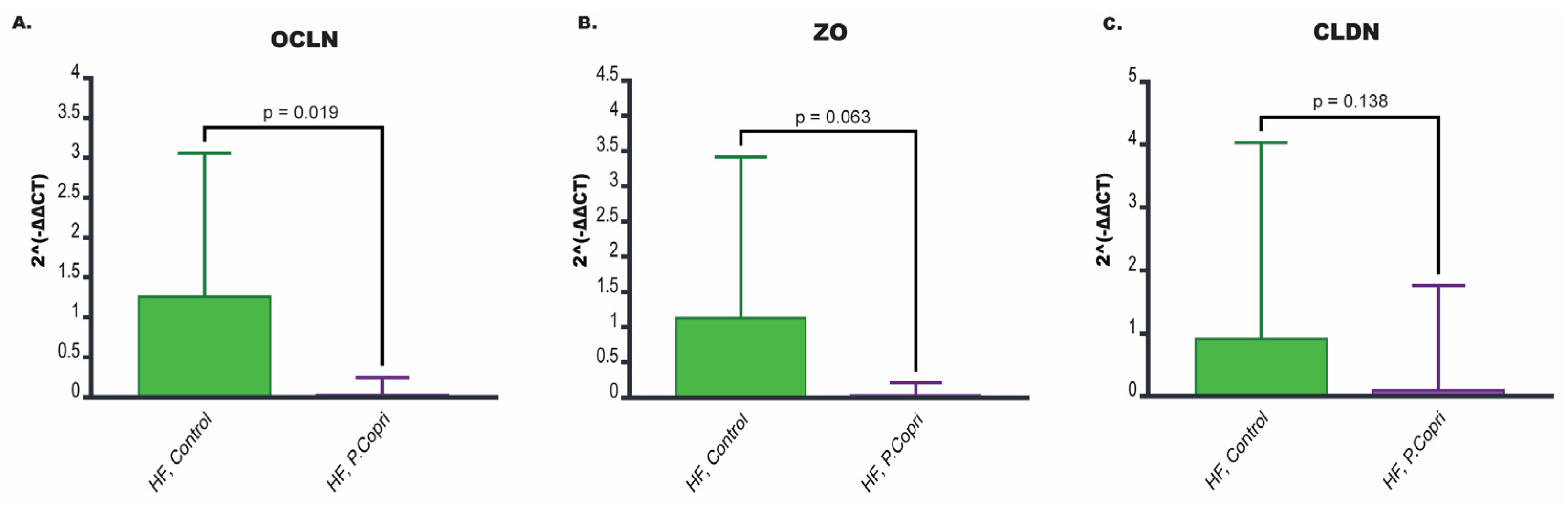
3.9. P. copri Colonization in Mice on a High-Fat Diet Leads to Downregulation of Genes Involved in Tight Junction Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| SCFA | Short-chain fatty acid |
| VA | Veterans affair |
| CAP | Controlled attenuation parameter |
| kPA | Kilopascals |
| ASVs | Amplicon sequence variants |
| ACE | Abundance based coverage estimator |
| SCNIC | Sparse Co-Occurrence Network Investigation for Compositional Data |
| SParCC | Sparse Correlations for Compositional Data |
| MaAsLin2 | Multivariate Association with Linear Models |
| TSS | Total sum scaling |
| LM | Linear model |
| HF | High fat |
| H&E | Hematoxylin and eosin |
| qPCR | Quantitative PCR |
| Cpt1 | Carnitine palmitoyltransferase 1 |
| Dgat | Diacylglycerol acyltransferase |
| Atgl | Adipose triglyceride lipase |
| Ocln | Occludin |
| Zo | Zonula occludens-1 |
| Cldn | Claudins |
References
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e641–e649, quiz e639–e640. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Setiawan, V.W.; Ng, C.H.; Lim, W.H.; Muthiah, M.D.; Tan, E.X.; Dan, Y.Y.; Roberts, L.R.; Loomba, R.; Huang, D.Q. Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH. Hepatology 2023, 77, 1150–1163. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e1606. [Google Scholar] [CrossRef]
- Juneja, P.; Tripathi, D.M.; Kaur, S. Revisiting the gut-liver axis: Gut lymphatic system in liver cirrhosis and portal hypertension. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G473–G479. [Google Scholar] [CrossRef]
- Tellez, L.A.; Medina, S.; Han, W.; Ferreira, J.G.; Licona-Limon, P.; Ren, X.; Lam, T.T.; Schwartz, G.J.; de Araujo, I.E. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 2013, 341, 800–802. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The liver-brain-gut neural arc maintains the T(reg) cell niche in the gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N.; Delzenne, N. Targeting the Gut Microbiome to Treat Metabolic Dysfunction-Associated Fatty Liver Disease: Ready for Prime Time? Cells 2022, 11, 2718. [Google Scholar] [CrossRef] [PubMed]
- Decaris, M.L.; Li, K.W.; Emson, C.L.; Gatmaitan, M.; Liu, S.; Wang, Y.; Nyangau, E.; Colangelo, M.; Angel, T.E.; Beysen, C.; et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 2017, 65, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Angulo, P.; Machado, M.V.; Diehl, A.M. Fibrosis in nonalcoholic Fatty liver disease: Mechanisms and clinical implications. Semin. Liver Dis. 2015, 35, 132–145. [Google Scholar] [CrossRef]
- Dong, T.S.; Katzka, W.; Lagishetty, V.; Luu, K.; Hauer, M.; Pisegna, J.; Jacobs, J.P. A Microbial Signature Identifies Advanced Fibrosis in Patients with Chronic Liver Disease Mainly Due to NAFLD. Sci. Rep. 2020, 10, 2771. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; Gonzalez, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vazquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Forlano, R.; Martinez-Gili, L.; Takis, P.; Miguens-Blanco, J.; Liu, T.; Triantafyllou, E.; Skinner, C.; Loomba, R.; Thursz, M.; Marchesi, J.R.; et al. Disruption of gut barrier integrity and host-microbiome interactions underlie MASLD severity in patients with type-2 diabetes mellitus. Gut Microbes 2024, 16, 2304157. [Google Scholar] [CrossRef]
- Benede-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the barriers: The role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef]
- Ha, S.; Wong, V.W.; Zhang, X.; Yu, J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut 2024, 74, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Mladenic, K.; Lenartic, M.; Marinovic, S.; Polic, B.; Wensveen, F.M. The “Domino effect” in MASLD: The inflammatory cascade of steatohepatitis. Eur. J. Immunol. 2024, 54, e2149641. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.A.; Moreno-Fernandez, M.E.; Divanovic, S. IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Curr. Drug Targets 2015, 16, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The curious case of Prevotella copri. Gut Microbes 2023, 15, 2249152. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, X.; Wang, X.; Zhou, J.Y.; Park, S. Microbial Dysbiosis Linked to Metabolic Dysfunction-Associated Fatty Liver Disease in Asians: Prevotella copri Promotes Lipopolysaccharide Biosynthesis and Network Instability in the Prevotella Enterotype. Int. J. Mol. Sci. 2024, 25, 2183. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Cerqueda-Garcia, D.; Lopez-Contreras, B.; Larrieta-Carrasco, E.; Villamil-Ramirez, H.; Molina-Cruz, S.; Torres, N.; Sanchez-Tapia, M.; Hernandez-Pando, R.; Aguilar-Salinas, C.; et al. A metagenomic study identifies a Prevotella copri enriched microbial profile associated with non-alcoholic steatohepatitis in subjects with obesity. J. Gastroenterol. Hepatol. 2023, 38, 791–799. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Q.; Hu, R.; Yang, X.; Fang, C.; Yao, L.; Lv, J.; Wang, L.; Shi, M.; Zhang, W.; et al. Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes 2024, 16, 2340487. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Johnson, J.S.; Angeles, J.E.; Behling, C.; Belt, P.H.; Borecki, I.; Bross, C.; Durelle, J.; Goyal, N.P.; Hamilton, G.; et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157, 1109–1122. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Minehira, K.; Young, S.G.; Villanueva, C.J.; Yetukuri, L.; Oresic, M.; Hellerstein, M.K.; Farese, R.V., Jr.; Horton, J.D.; Preitner, F.; Thorens, B.; et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 2008, 49, 2038–2044. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites 2023, 14, 12. [Google Scholar] [CrossRef]
- Schreiber, R.; Xie, H.; Schweiger, M. Of mice and men: The physiological role of adipose triglyceride lipase (ATGL). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Vythoulkas-Biotis, N.; Adamou, A.; Zachariadou, T.; Kargioti, S.; Karampela, I.; Dalamaga, M. NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options. Metabolites 2024, 14, 366. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef]
- Paradis, T.; Begue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 25) | MASLD without Advanced Fibrosis (n = 18) | MASLD with Advanced Fibrosis (n = 7) | p-Value | |
|---|---|---|---|---|
| Age (yr) (SD) | 35.7 (3.5) | 57.2 (16.0) | 67.4 (8.0) | <0.001 |
| Male (%) (n = 50) | 52% (n = 13) | 83% (n = 15) | 100% (n = 7) | 0.018 |
| Charlson Comorbidity Index (SD) | N/A | 3.9 (2.9) | 5.7 (1.5) | 0.06 |
| Caucasian (%) (n = 17) | 32% (n = 8) | 33.3% (n = 6) | 42.9% (n = 3) | 0.484 |
| African American (%) (n = 15) | 32% (n = 8) | 33.3% (n = 6) | 14.3% (n = 1) | |
| Hispanic (%) (n = 8) | 8% (n = 2) | 27.8% (n = 5) | 14.3% (n = 1) | |
| Asian (%) (n = 5) | 16% (n = 4) | 0% (n = 0) | 14.3% (n = 1) | |
| Other/Unknown (%) (n = 5) | 12% (n = 3) | 5.6% (n = 1) | 14.3% (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Leitman, M.; Pawar, S.; Shera, S.; Hernandez, L.; Jacobs, J.P.; Dong, T.S. The Association Between Prevotella copri and Advanced Fibrosis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2025, 17, 2145. https://doi.org/10.3390/nu17132145
Zhang D, Leitman M, Pawar S, Shera S, Hernandez L, Jacobs JP, Dong TS. The Association Between Prevotella copri and Advanced Fibrosis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients. 2025; 17(13):2145. https://doi.org/10.3390/nu17132145
Chicago/Turabian StyleZhang, David, Madelaine Leitman, Shrey Pawar, Simer Shera, Laura Hernandez, Jonathan P. Jacobs, and Tien S. Dong. 2025. "The Association Between Prevotella copri and Advanced Fibrosis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease" Nutrients 17, no. 13: 2145. https://doi.org/10.3390/nu17132145
APA StyleZhang, D., Leitman, M., Pawar, S., Shera, S., Hernandez, L., Jacobs, J. P., & Dong, T. S. (2025). The Association Between Prevotella copri and Advanced Fibrosis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients, 17(13), 2145. https://doi.org/10.3390/nu17132145








