From Fish Oil to Resolution: A Narrative Review on the Potential of SPM-Enriched Marine Oil for Exercise-Induced Muscle Damage Recovery
Abstract
1. Introduction
2. Materials and Methods
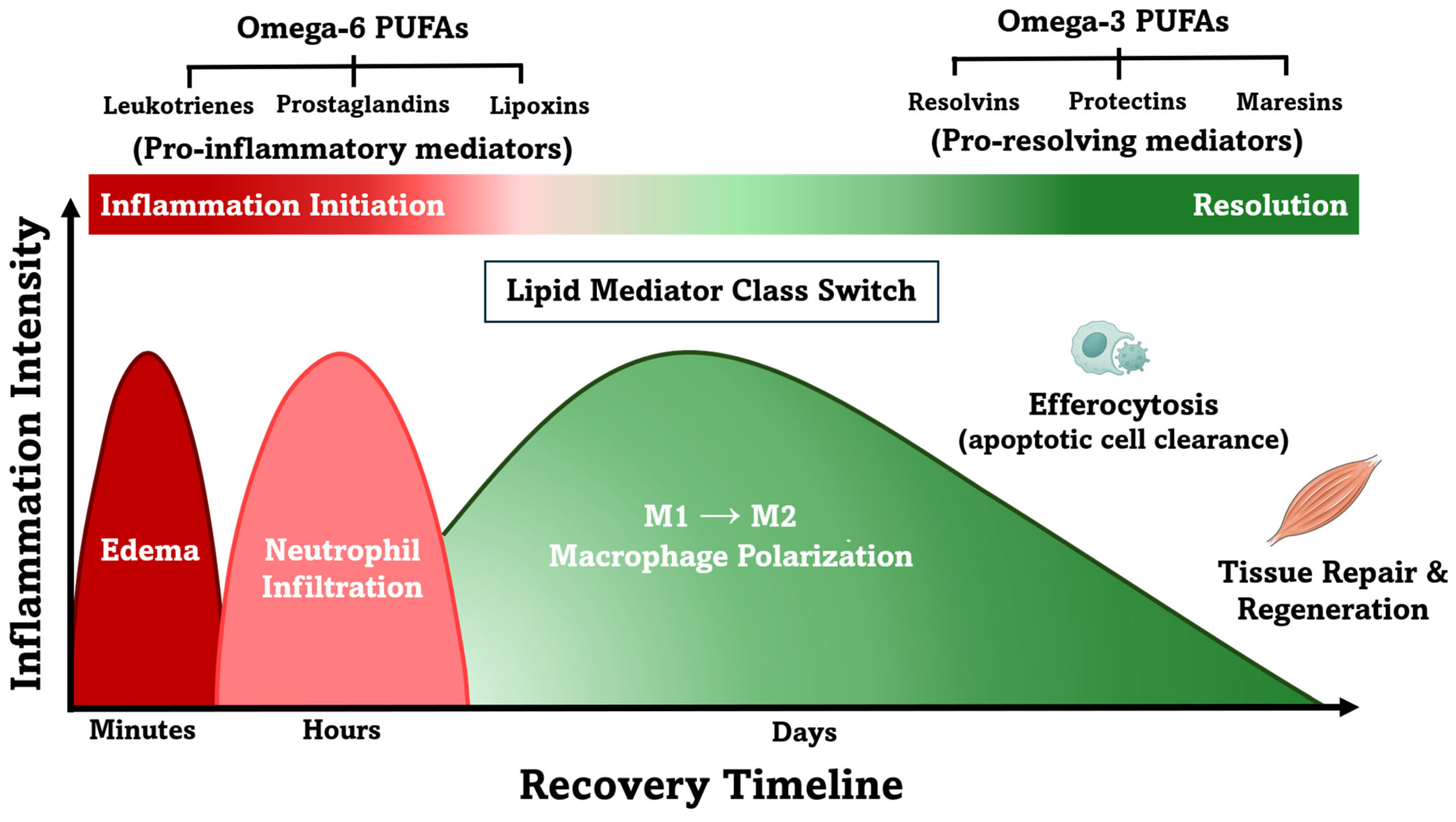
3. Omega-3 PUFAs and Specialized Pro-Resolving Mediators in Inflammation and Recovery
3.1. Role of Omega-3 Polyunsaturated Fatty Acids in Inflammation and Recovery
3.2. Oxylipins: The Broader Family of Lipid Mediators
3.3. The Discovery of Specialized Pro-Resolving Mediators
3.4. Roles of Specialized Pro-Resolving Mediators in Inflammation Resolution
3.5. Inflammation Resolution vs. Suppression: Distinct Mechanisms of SPMs and NSAIDs
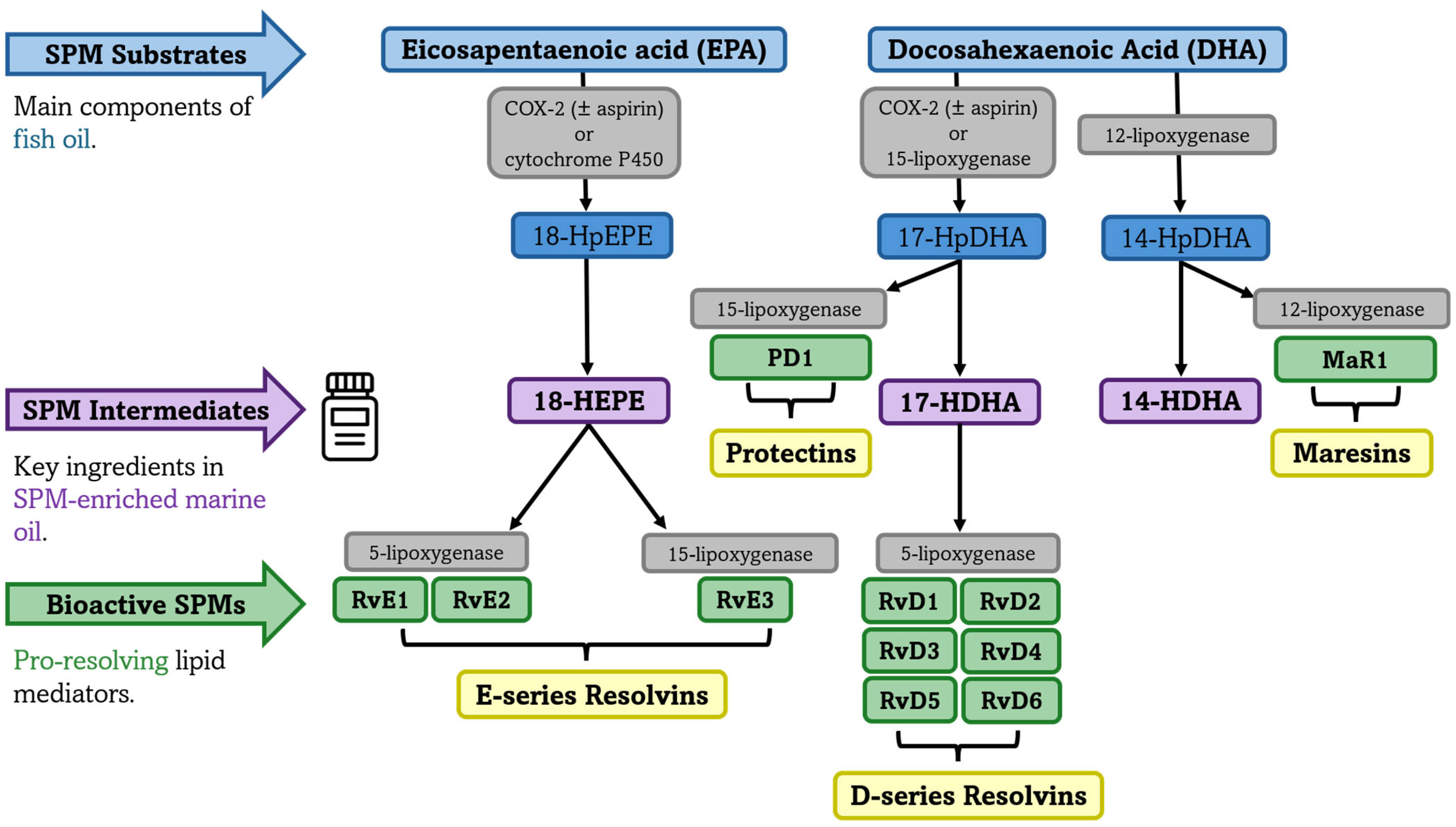
3.6. Synthesis of Specialized Pro-Resolving Mediators from Omega-3 PUFAs
4. Therapeutic Applications of Specialized Pro-Resolving Mediators (SPMs)
4.1. Therapeutic Applications of SPMs in In Vivo Preclinical Models
4.2. Identification and Quantification of SPMs and Their Pathway Intermediates in Humans
4.3. Bioavailability Challenges in the EPA-DHA Conversion to SPMs
4.4. Analgesic Properties of SPMs and Their Intermediates
5. Current Evidence, Applications, and Knowledge Gaps on SPM-Enriched Marine Oil
5.1. SPM-Enriched Marine Oil Supplementation in Humans
5.2. Dosage Variability in SPM-Enriched Marine Oils
5.3. SPM-Enriched Marine Oil and Recovery After Exercise-Induced Muscle Damage
5.4. Potential Applications in Physically Active and Trained Populations
5.5. Contextualizing SPM-Enriched Marine Oil Within the Broader Fish Oil Literature
5.6. Analytical Challenges and Methodological Considerations in Quantifying SPMs in Humans
6. Conclusions
- SPMs are endogenously synthesized from EPA and DHA via enzymatic conversion and actively coordinate inflammation resolution, distinguishing their role from agents that merely suppress inflammation, such as NSAIDs.
- SPM-enriched marine oil supplements contain low concentrations of EPA, DHA, and SPM pathway intermediates such as 14-HDHA, 17-HDHA, and 18-HEPE; however, optimal concentrations remain undefined, and it is unclear whether observed increases in plasma SPM concentrations are primarily driven by EPA+DHA content or by the direct contribution of the SPM pathway intermediates.
- Dosages of 3 and 4.5 g of SPM-enriched supplements, but not 1.5 g, have been shown to elevate plasma SPM concentrations within two hours; however, whether this acute increase translates into meaningful exercise recovery benefits remains unknown.
- Individual responses to SPM supplementation vary significantly, but daily doses of 1–2 g, often initiated at 2 g and tapered to 1.5 or 1 g based on early pain improvement, have demonstrated analgesic effects in chronic pain conditions over treatment periods of 4 to 12 weeks.
- The efficacy of SPM supplementation for exercise recovery remains theoretical and requires validation through randomized controlled trials, with no studies directly comparing SPM-enriched marine oil to traditional fish oil (EPA+DHA) supplementation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 14-HDHA | 14-Hydroxy-Docosahexaenoic Acid |
| 17-HDHA | 17-Hydroxy-Docosahexaenoic Acid |
| 17(R)-HDHA | 17(R)-Hydroxy-docosahexaenoic acid |
| 18-HEPE | 18-Hydroxy-Eicosapentaenoic Acid |
| 14-HpDHA | 14-Hydroxyperoxydocosahexaenoic acid |
| 17-HpDHA | 17-Hydroxyperoxydocosahexaenoic acid |
| AA | arachidonic acid |
| ALA | alpha-linoleic acid |
| AT-RvD1 | Aspirin-Triggered Resolvin D1 |
| CD11b | Cluster of Differentiation 11b |
| CD16 | Cluster of Differentiation 16 |
| CD41 | Cluster of Differentiation 41 |
| CD163+ | Cluster of Differentiation 163-positive |
| COX | cyclooxygenase |
| COX-2 | cyclooxygenase-2 |
| CRP | C-Reactive Protein |
| DHA | docosahexaenoic acid |
| EIMD | exercise-induced muscle damage |
| ELISA | enzyme-linked immunosorbent assay |
| EPA | eicosapentaenoic acid |
| ESR | Erythrocyte Sedimentation Rate |
| IGF-1 | Insulin-Like Growth Factor 1 |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| LA | linoleic acid |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LOX | lipoxygenase |
| LTs | leukotrienes |
| M1 | macrophage phenotype 1 |
| M2 | macrophage phenotype 2 |
| MaR1 | Maresin 1 |
| NF-κB | nuclear factor kappa beta |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| n-3 PUFAs | omega-3 polyunsaturated fatty acids |
| n-6 PUFAs | omega-6 polyunsaturated fatty acids |
| Pax7+ | paired box 7-positive |
| PGI2/PGI3 | Prostacyclin I2/I3 |
| PGs | prostaglandins |
| PLA2 | Phospholipase A2 |
| PD1 | Protectin D1 |
| ROS | reactive oxygen species |
| RvD1-RvD6 | Resolvins D1 to D6 (DHA-derived) |
| RvE1-RvE3 | Resolvins E1 to E3 (EPA-derived) |
| SPMs | specialized pro-resolving mediators |
| TNF-α | tumor necrosis factor-alpha |
| TXs | thromboxanes |
References
- Clarkson, P.M.; Hubal, M.J. Exercise-Induced Muscle Damage in Humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef]
- Fridén, J.; Lieber, R.L. Structural and mechanical basis of exercise-induced muscle injury. Med. Sci. Sports Exerc. 1992, 24, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Heileson, J.L.; Harris, D.R.; Tomek, S.; Ritz, P.P.; Rockwell, M.S.; Barringer, N.D.; Forsse, J.S.; Funderburk, L.K. Long-Chain Omega-3 Fatty Acid Supplementation and Exercise-Induced Muscle Damage: EPA or DHA? Med. Sci. Sports Exerc. 2024, 56, 476–485. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; López-Román, F.J.; Miñarro, J.; Contreras, C.; Soto-Méndez, F.; Domingo Pedrol, J.C.; Luque-Rubia, A.J. Supplementation of Re-Esterified Docosahexaenoic and Eicosapentaenoic Acids Reduce Inflammatory and Muscle Damage Markers after Exercise in Endurance Athletes: A Randomized, Controlled Crossover Trial. Nutrients 2020, 12, 719. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Kerksick, C.M.; Mangine, G.T.; Holmes, A.J.; Lee, M.; Endito, M.R.; et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients 2020, 12, 2246. [Google Scholar] [CrossRef]
- Anthony, R.; Macartney, M.J.; Peoples, G.E. The Influence of Long-Chain Omega-3 Fatty Acids on Eccentric Exercise-Induced Delayed Muscle Soreness: Reported Outcomes Are Compromised by Study Design Issues. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 143–153. [Google Scholar] [CrossRef]
- Cannataro, R.; Abrego-Guandique, D.M.; Straface, N.; Cione, E. Omega-3 and Sports: Focus on Inflammation. Life 2024, 14, 1315. [Google Scholar] [CrossRef]
- Jannas-Vela, S.; Espinosa, A.; Candia, A.A.; Flores-Opazo, M.; Peñailillo, L.; Valenzuela, R. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Patel, J.; Staton, K.; Martindale, R.G.; Moore, F.A.; Upchurch, G.R. Can Specialized Pro-resolving Mediators Deliver Benefit Originally Expected from Fish Oil? Curr. Gastroenterol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016, 22, 25. [Google Scholar]
- Blaauw, R.; Calder, P.C.; Martindale, R.G.; Berger, M.M. Combining proteins with n-3 PUFAs (EPA + DHA) and their inflammation pro-resolution mediators for preservation of skeletal muscle mass. Crit. Care 2024, 28, 38. [Google Scholar] [CrossRef]
- Tomczyk, M.; Heileson, J.L.; Babiarz, M.; Calder, P.C. Athletes Can Benefit from Increased Intake of EPA and DHA—Evaluating the Evidence. Nutrients 2023, 15, 4925. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Floyd, C.; Larouche, J.; Castor-Macias, J.A.; Sugg, K.B.; Sarver, D.C.; Macpherson, P.C.D.; Davis, C.; et al. Resolvin D1 supports skeletal myofiber regeneration via actions on myeloid and muscle stem cells. JCI Insight 2020, 5, e137713. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, A.E.; Regan, J.; Buddenbaum, N.; Tharwani, S.; Drawdy, C.; Behee, M.; Sergin, S.; Fenton, J.I.; Maddipati, K.R.; Kane, S.; et al. Enriched Marine Oil Supplement Increases Specific Plasma Specialized Pro-Resolving Mediators in Adults with Obesity. J. Nutr. 2022, 152, 1783–1791. [Google Scholar] [CrossRef]
- Callan, N.; Hanes, D.; Bradley, R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J. Transl. Med. 2020, 18, 401. [Google Scholar] [CrossRef]
- Irún, P.; Carrera-Lasfuentes, P.; Sánchez-Luengo, M.; Belio, Ú.; Domper-Arnal, M.J.; Higuera, G.A.; Hawkins, M.; De La Rosa, X.; Lanas, A. Pharmacokinetics and Changes in Lipid Mediator Profiling after Consumption of Specialized Pro-Resolving Lipid-Mediator-Enriched Marine Oil in Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 16143. [Google Scholar] [CrossRef]
- Schaller, M.S.; Chen, M.; Colas, R.A.; Sorrentino, T.A.; Lazar, A.A.; Grenon, S.M.; Dalli, J.; Conte, M.S. Treatment With a Marine Oil Supplement Alters Lipid Mediators and Leukocyte Phenotype in Healthy Patients and Those With Peripheral Artery Disease. J. Am. Heart Assoc. 2020, 9, e016113. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Hirsh, S.; Bruce, D.; Bou-Sliman, T.; Joyal, S.; Swick, A.G. The effects of an SPM-enriched marine oil and bioavailable curcumin combination on inflammation-associated discomfort in generally healthy individuals: A virtual open-label pilot study. Transl. Med. Commun. 2022, 7, 25. [Google Scholar] [CrossRef]
- Gracia Aznar, A.; Moreno Egea, F.; Gracia Banzo, R.; Gutierrez, R.; Rizo, J.M.; Rodriguez-Ledo, P.; Nerin, I.; Regidor, P.-A. Pro-Resolving Inflammatory Effects of a Marine Oil Enriched in Specialized Pro-Resolving Mediators (SPMs) Supplement and Its Implication in Patients with Post-COVID Syndrome (PCS). Biomedicines 2024, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Möller, I.; Rodas, G.; Villalón, J.M.; Rodas, J.A.; Angulo, F.; Martínez, N.; Vergés, J. Randomized, double-blind, placebo-controlled study to evaluate the effect of treatment with an SPMs-enriched oil on chronic pain and inflammation, functionality, and quality of life in patients with symptomatic knee osteoarthritis: GAUDI study. J. Transl. Med. 2023, 21, 423. [Google Scholar] [CrossRef]
- Barden, A.E.; Moghaddami, M.; Mas, E.; Phillips, M.; Cleland, L.G.; Mori, T.A. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids 2016, 107, 24–29. [Google Scholar] [CrossRef]
- Barden, A.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014, 55, 2401–2407. [Google Scholar] [CrossRef]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood following n-3 Fatty Acid Supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef]
- Ostermann, A.I.; West, A.L.; Schoenfeld, K.; Browning, L.M.; Walker, C.G.; Jebb, S.A.; Calder, P.C.; Schebb, N.H. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: Results from a randomized controlled trial in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1251–1263. [Google Scholar] [CrossRef]
- Mozurkewich, E.L.; Greenwood, M.; Clinton, C.; Berman, D.; Romero, V.; Djuric, Z.; Qualls, C.; Gronert, K. Pathway Markers for Pro-resolving Lipid Mediators in Maternal and Umbilical Cord Blood: A Secondary Analysis of the Mothers, Omega-3, and Mental Health Study. Front. Pharmacol. 2016, 7, 274. [Google Scholar] [CrossRef]
- See, V.H.L.; Mas, E.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Barden, A.E.; Huang, R.-C.; Mori, T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: A double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017, 118, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, A.E.; Buddenbaum, N.; Shaikh, S.R. Polyunsaturated fatty acids, specialized pro-resolving mediators, and targeting inflammation resolution in the age of precision nutrition. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158936. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Lavie, C.J.; Marchioli, R.; Harris, W.S. Omega-3 Fatty Acids for Cardioprotection. Mayo Clin. Proc. 2008, 83, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Gerling, C.J.; Mukai, K.; Chabowski, A.; Heigenhauser, G.J.F.; Holloway, G.P.; Spriet, L.L.; Jannas-Vela, S. Incorporation of Omega-3 Fatty Acids into Human Skeletal Muscle Sarcolemmal and Mitochondrial Membranes Following 12 Weeks of Fish Oil Supplementation. Front. Physiol. 2019, 10, 348. [Google Scholar] [CrossRef]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, S1505–S1519S. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of conversion of α-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Galloway, S.D.R.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J.; Bowles, E.; Macgregor, L.J.; Prokopidis, K.; Campbell, C.; Barber, E. Fish oil supplementation fails to modulate indices of muscle damage and muscle repair during acute recovery from eccentric exercise in trained young males. Eur. J. Sport Sci. 2023, 23, 1666–1676. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef] [PubMed]
- Mcglory, C.; Gorissen, S.H.; Kamal, M.; Bahniwal, R.; Hector, A.J.; Baker, S.K. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019, 33, 4586–4597. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Love, K.; Winter, T.; Gauthier, J.; Taylor, C.G.; Blydt-Hansen, T.; Zahradka, P.; Aukema, H.M. Dietary Linoleic Acid and α-Linolenic Acid Differentially Affect Renal Oxylipins and Phospholipid Fatty Acids in Diet-Induced Obese Rats. J. Nutr. 2013, 143, 1421–1431. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Gronert, K.; Chiang, N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: A novel mechanism for NSAID and N-3 PUFA therapeutic actions. J. Physiol. Pharmacol. 2000, 51, 643–654. [Google Scholar]
- Dalli, J.; Gomez, E.A.; Serhan, C.N. Evidence for the presence and diagnostic utility of SPM in human peripheral blood. bioRxiv 2022. [Google Scholar] [CrossRef]
- Dalli, J.; Gomez, E.A. Reply to: Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Nat. Commun. 2023, 14, 7172. [Google Scholar] [CrossRef]
- Gakis, A.G.; Nomikos, T.; Philippou, A.; Antonopoulou, S. The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage. Physiologia 2023, 3, 305–328. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef]
- Shu, L.-Z.; Zhang, X.-L.; Ding, Y.-D.; Lin, H. From inflammation to bone formation: The intricate role of neutrophils in skeletal muscle injury and traumatic heterotopic ossification. Exp. Mol. Med. 2024, 56, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Characterization of Exercise-Induced Cytokine Release, the Impacts on the Body, the Mechanisms and Modulations. Int. J. Sports Exerc. Med. 2019, 5, 122. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef]
- Giannakis, N.; Sansbury, B.E.; Patsalos, A.; Hays, T.T.; Riley, C.O.; Han, X.; Spite, M.; Nagy, L. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat. Immunol. 2019, 20, 626–636. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, O.; Camell, C.D.; Bosurgi, L.; Nguyen, K.Y.; Youm, Y.-H.; Rothlin, C.V.; Dixit, V.D. IGF1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell Rep. 2017, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524®, a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 10. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Lilja, M.; Mandić, M.; Apró, W.; Melin, M.; Olsson, K.; Rosenborg, S.; Gustafsson, T.; Lundberg, T.R. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2018, 222, e12948. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Brennan, R.; Wazaify, M.; Shawabkeh, H.; Boardley, I.; McVeigh, J.; Van Hout, M.C. A Scoping Review of Non-Medical and Extra-Medical Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Drug Saf. 2021, 44, 917–928. [Google Scholar] [CrossRef]
- Pizza, F.; Cavender, D.; Stockard, A.; Baylies, H.; Beighle, A. Anti-Inflammatory Doses of Ibuprofen: Effect on Neutrophils and Exercise-Induced Muscle Injury. Int. J. Sports Med. 1999, 20, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Trappe, T.A.; Fluckey, J.D.; White, F.; Lambert, C.P.; Evans, W.J. Skeletal Muscle PGF2 α and PGE2 in Response to Eccentric Resistance Exercise: Influence of Ibuprofen and Acetaminophen. J. Clin. Endocrinol. Metab. 2001, 86, 5067–5070. [Google Scholar] [CrossRef][Green Version]
- Urso, M.L. Anti-inflammatory interventions and skeletal muscle injury: Benefit or detriment? J. Appl. Physiol. 2013, 115, 920–928. [Google Scholar] [CrossRef]
- Wang, P.-H.; Wang, Y.; Guo, Y.-Y.; Ma, Z.-H.; Wu, C.; Xing, L. Ibuprofen modulates macrophage polarization by downregulating poly (ADP-ribose) polymerase 1. Int. Immunopharmacol. 2024, 143, 113502. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.W.; Libreros, S.; De La Rosa, X.; Sansbury, B.E.; Norris, P.C.; Chiang, N.; Fichtner, D.; Keyes, G.S.; Wourms, N.; Spite, M.; et al. Frontline Science: Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukoc. Biol. 2018, 103, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Sakaguchi, C.A.; Omar, A.M.; Davis, K.L.; Shaffner, C.E.; Strauch, R.C.; Lila, M.A.; Zhang, Q. Blueberry intake elevates post-exercise anti-inflammatory oxylipins: A randomized trial. Sci. Rep. 2023, 13, 11976. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Kühn, H.; Kahnt, A.S.; Rund, K.M.; O’Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.-J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators—What is the Evidence so far? Front. Pharmacol. 2022, 13, 838782. [Google Scholar] [CrossRef]
- Arita, M.; Oh, S.F.; Chonan, T.; Hong, S.; Elangovan, S.; Sun, Y.-P.; Uddin, J.; Petasis, N.A.; Serhan, C.N. Metabolic Inactivation of Resolvin E1 and Stabilization of Its Anti-inflammatory Actions. J. Biol. Chem. 2006, 281, 22847–22854. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A.; Goguet-Surmenian, E.; Blackwood, A.; Andry, C.; Serhan, C.N.; Van Dyke, T.E. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. J. Immunol. 2007, 179, 7021–7029. [Google Scholar] [CrossRef]
- Turner, T.C.; Pittman, F.S.; Zhang, H.; Hymel, L.A.; Zheng, T.; Behara, M.; Anderson, S.E.; Harrer, J.A.; Link, K.A.; Ahammed, M.A.; et al. Improving Functional Muscle Regeneration in Volumetric Muscle Loss Injuries by Shifting the Balance of Inflammatory and Pro-Resolving Lipid Mediators. BioRxiv 2024. [Google Scholar] [CrossRef]
- Huang, J.; Burston, J.J.; Li, L.; Ashraf, S.; Mapp, P.I.; Bennett, A.J.; Ravipati, S.; Pousinis, P.; Barrett, D.A.; Scammell, B.E.; et al. Targeting the D Series Resolvin Receptor System for the Treatment of Osteoarthritis Pain. Arthritis Rheumatol. 2017, 69, 996–1008. [Google Scholar] [CrossRef]
- Lima-Garcia, J.; Dutra, R.; Da Silva, K.; Motta, E.; Campos, M.; Calixto, J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats: RvD series precursor and AT-RvD1 in arthritis. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef]
- Kutzner, L.; Rund, K.M.; Ostermann, A.I.; Hartung, N.M.; Galano, J.-M.; Balas, L.; Durand, T.; Balzer, M.S.; David, S.; Schebb, N.H. Development of an Optimized LC-MS Method for the Detection of Specialized Pro-Resolving Mediators in Biological Samples. Front. Pharmacol. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Massey, K.A.; Ness, C.; Kiehntopf, M.; Stepanow, S.; Platzer, M.; Grün, M.; Nicolaou, A.; Jahreis, G. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: Effects on circulating eicosanoids and cardiovascular risk factors. Clin. Nutr. 2013, 32, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050. [Google Scholar] [CrossRef]
- So, J.; Matthan, N.; Maddipati, K.; Lichtenstein, A.; Wu, D.; Lamon-Fava, S. Effects of EPA and DHA Supplementation on Plasma Specialized Pro-resolving Lipid Mediators and Blood Monocyte Inflammatory Response in Subjects with Chronic Inflammation (OR29-01-19). Curr. Dev. Nutr. 2019, 3, nzz031.OR29-01-19. [Google Scholar] [CrossRef]
- Marchand, N.E.; Choi, M.Y.; Oakes, E.G.; Cook, N.R.; Stevens, E.; Gomelskaya, N.; Kotler, G.; Manson, J.E.; Lasky-Su, J.; Mora, S.; et al. Over-the-counter fish oil supplementation and pro-resolving and pro-inflammatory lipid mediators in rheumatoid arthritis. Prostaglandins Leukot. Essent. Fatty Acids 2023, 190, 102542. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Gasper, W.J.; Khetani, S.A.; Zahner, G.J.; Hills, N.K.; Mitchell, P.T.; Sansbury, B.E.; Conte, M.S.; Spite, M.; Grenon, S.M. Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial). J. Surg. Res. 2019, 238, 164–174. [Google Scholar] [CrossRef]
- Skarke, C.; Alamuddin, N.; Lawson, J.A.; Li, X.; Ferguson, J.F.; Reilly, M.P.; FitzGerald, G.A. Bioactive products formed in humans from fish oils. J. Lipid Res. 2015, 56, 1808–1820. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2021, 164, 102219. [Google Scholar] [CrossRef]
- Sobrino, A.; Walker, M.E.; Colas, R.A.; Dalli, J. Protective activities of distinct omega-3 enriched oils are linked to their ability to upregulate specialized pro-resolving mediators. PLoS ONE 2020, 15, e0242543. [Google Scholar] [CrossRef]
- Valdes, A.M.; Ravipati, S.; Menni, C.; Abhishek, A.; Metrustry, S.; Harris, J.; Nessa, A.; Williams, F.M.K.; Spector, T.D.; Doherty, M.; et al. Association of the resolvin precursor 17-HDHA, but not D- or E- series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 2017, 7, 10748. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Kotlabova, K.; Lim, J.-H.; Mitroulis, I.; Ferreira, A.; Chen, L.-S.; Gercken, B.; Steffen, A.; Kemter, E.; Ameln, A.K.; et al. Developmental endothelial locus-1 modulates platelet-monocyte interactions and instant blood-mediated inflammatory reaction in islet transplantation. Thromb. Haemost. 2016, 115, 781–788. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Malan, L.; Zandberg, L.; Pienaar, C.; Nienaber, A.; Havemann-Nel, L. Regular moderate physical activity potentially accelerates and strengthens both the pro-inflammatory and pro-resolving lipid mediator response after acute exercise stress. Prostaglandins Leukot. Essent. Fatty Acids 2024, 202, 102642. [Google Scholar] [CrossRef]
- Hsu, F.-C.; Kritchevsky, S.B.; Liu, Y.; Kanaya, A.; Newman, A.B.; Perry, S.E.; Visser, M.; Pahor, M.; Harris, T.B.; Nicklas, B.J.; et al. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64A, 581–589. [Google Scholar] [CrossRef]
- Verdaet, D.; Dendale, P.; De Bacquer, D.; Delanghe, J.; Block, P.; De Backer, G. Association between leisure time physical activity and markers of chronic inflammation related to coronary heart disease. Atherosclerosis 2004, 176, 303–310. [Google Scholar] [CrossRef]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Zheng, J.-J.; Pena Calderin, E.; Hill, B.G.; Bhatnagar, A.; Hellmann, J. Exercise Promotes Resolution of Acute Inflammation by Catecholamine-Mediated Stimulation of Resolvin D1 Biosynthesis. J. Immunol. 2019, 203, 3013–3022. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Walker, M.E.; Serhan, C.N. Lipid Mediator Metabolomics Via LC-MS/MS Profiling and Analysis. Methods Mol. Biol. 2018, 1730, 59–72. [Google Scholar] [CrossRef]
- Hagn, G.; Meier-Menches, S.M.; Plessl-Walder, G.; Mitra, G.; Mohr, T.; Preindl, K.; Schlatter, A.; Schmidl, D.; Gerner, C.; Garhöfer, G.; et al. Plasma Instead of Serum Avoids Critical Confounding of Clinical Metabolomics Studies by Platelets. J. Proteome Res. 2024, 23, 3064–3075. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Schebb, N.H.; Milne, G.L.; Murphy, M.P.; Thomas, C.P.; Steinhilber, D.; Gelhaus, S.L.; Kühn, H.; Gelb, M.H.; Jakobsson, P.-J.; et al. Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Nat. Commun. 2023, 14, 7172. [Google Scholar] [CrossRef]
- Fedirko, V.; McKeown-Eyssen, G.; Serhan, C.N.; Barry, E.L.; Sandler, R.S.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Robertson, D.J.; Anderson, C.W.; et al. Plasma lipoxin A 4 and resolvin D1 are not associated with reduced adenoma risk in a randomized trial of aspirin to prevent colon adenomas. Mol. Carcinog. 2017, 56, 1977–1983. [Google Scholar] [CrossRef]
- Oliveira Perucci, L.; Pereira Santos, T.A.; Campi Santos, P.; Ribeiro Teixeira, L.C.; Nessralla Alpoim, P.; Braga Gomes, K.; Pires Sousa, L.; Sant’Ana Dusse, L.M.; Talvani, A. Pre-eclampsia is associated with reduced resolvin D1 and maresin 1 to leukotriene B4 ratios in the plasma. Am. J. Reprod. Immunol. 2020, 83, e13206. [Google Scholar] [CrossRef]
- Navarini, L.; Bisogno, T.; Margiotta, D.P.E.; Piccoli, A.; Angeletti, S.; Laudisio, A.; Ciccozzi, M.; Afeltra, A.; Maccarrone, M. Role of the Specialized Proresolving Mediator Resolvin D1 in Systemic Lupus Erythematosus: Preliminary Results. J. Immunol. Res. 2018, 2018, 5264195. [Google Scholar] [CrossRef]
- Aydogmus, E. Comparison of Resolvin D1 Levels in Patients with Aneurysmal Subarachnoid Haemorrhage with Those in Healthy Controls. South. Clin. Istanb. Eurasia 2021, 32, 273–279. [Google Scholar] [CrossRef]
- Poreba, M.; Mostowik, M.; Siniarski, A.; Golebiowska-Wiatrak, R.; Malinowski, K.P.; Haberka, M.; Konduracka, E.; Nessler, J.; Undas, A.; Gajos, G. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 50. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Anderson Berry, A.; Van Ormer, M.; Zoucha, S.; Elliott, E.; Johnson, R.; McGinn, E.; Cave, C.; Rilett, K.; Weishaar, K.; et al. Omega-3 Fatty Acid Supplementation, Pro-Resolving Mediators, and Clinical Outcomes in Maternal-Infant Pairs. Nutrients 2019, 11, 98. [Google Scholar] [CrossRef]
- Singh, K.P.; Lawyer, G.; Muthumalage, T.; Maremanda, K.P.; Khan, N.A.; McDonough, S.R.; Ye, D.; McIntosh, S.; Rahman, I. Systemic biomarkers in electronic cigarette users: Implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. 2019, 5, 00182–02019. [Google Scholar] [CrossRef]
- Morshedzadeh, N.; Saedisomeolia, A.; Djalali, M.; Eshraghian, M.R.; Hantoushzadeh, S.; Mahmoudi, M. Resolvin D1 impacts on insulin resistance in women with polycystic ovary syndrome and healthy women. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Su, M.-C.; Chin, C.-H.; Lin, I.-C.; Hsu, P.-Y.; Liou, C.-W.; Huang, K.-T.; Wang, T.-Y.; Lin, Y.-Y.; Zheng, Y.-X.; et al. Formyl peptide receptor 1 up-regulation and formyl peptide receptor 2/3 down-regulation of blood immune cells along with defective lipoxin A4/resolvin D1 production in obstructive sleep apnea patients. PLoS ONE 2019, 14, e0216607. [Google Scholar] [CrossRef] [PubMed]

| Injury Model | Administration | Molecule | Regimen | Timeline | Key Outcomes |
|---|---|---|---|---|---|
| Acute Muscle Injury (Mice) [17] | Systemic | RvD1 | 100 ng/day | 5 min post-injury to 14 days | ↓ IL-6 expression ↓ TNF-α expression ↓ Neutrophil infiltration ↑ Myofiber CSA ↑ Muscle strength recovery ↑ M2 macrophages |
| Muscle Regeneration Impairment (Mice) [59] | Intramuscular | RvD2 | 4 µg/kg | Single dose, 3 days post-injury | ↑ M2 macrophages ↑ Force recovery ↑ Muscle mass |
| Volumetric Muscle Loss (Mice) [78] | Topical | AT-RvD1 | 100 ng/day | Following injury (24 h gradual release) | ↑ Maximal isometric torque recovery |
| Arthritis (Rats) [80] | Systemic | 17[R]-HDHA or AT-RvD1 | 300 ng/day | 4–5 days treatment (17[R]-HDHA once daily for 5 days; AT-RvD1 twice daily for 4 days) | ↓ Pain ↓ Joint stiffness ↓ IL-1β expression ↓ TNF-α expression |
| Osteoarthritis (Rats) [79] | Systemic | 17[R]-HDHA | 300 ng/day | Single dose and repeated treatment every other day for 2 weeks | ↓ Pain (rapid onset within 1 h, lasting up to 7 days post-treatment) |
| Periodontitis (Rabbits) [77] | Topical | RvE1 | 4 µg/site | 3 times per week for 6 weeks | ↓ IL-1β expression ↓ CRP expression ↑ Bone and soft tissue restoration |
| Ref. | Sample (n) | Population | Dosage | Duration | Circulating SPMs | Immune Biomarkers | Pain Outcomes | Limitations |
|---|---|---|---|---|---|---|---|---|
| [22] | 20 | Healthy + patients with PAD | 1.5, 3, and 6 g | 5 days + 9 days washout | ↑ | Enhanced phagocytosis Reduced Biomarkers (TNF-α and MCP-1) | - | Open label |
| [18] | 22 | Healthy adults | 1.5, 3 and 4 g | Single dose | ↑ | Reduced adhesion molecule expression + increased phagocytosis | - | - |
| [20] | 44 | Adults with chronic pain | W 0–2: 1.5 g W 3–4: 1 g or 2 g (based on pain scores improvement) | 4 weeks | - | No change (hs-CRP and ESR) | ↓ pain intensity ↓ pain interference (PROMIS-43) | Open label, no placebo |
| [19] | 23 | Adults with obesity | 2 g | 28–30 days | ↑ | IgG concentrations decreased upon B-cell activation | - | No placebo |
| [23] | 29 | Adults with mild or moderate pain | 0.5 g of enriched marine oil + 0.5 g curcumin | 60 days | - | - | ↓ total pain ↓ pain intensity ↓ pain severity (SF-MPQ) | No placebo |
| [21] | 10 | Healthy adults | 6 g (60 mL) | Single dose | ↑ | - | - | - |
| [25] | 51 | Adults with knee OA | W 0–6: 2 g W 7–12: 1 g | 12 weeks | - | - | ↓ pain (OMERACT-OARSI score) | - |
| [24] | 53 | Adults with post-COVID syndrome | 0.5, 1.5, and 3 g | 12 weeks | ↑ * | - | - | Lack of clinical outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, L.C.; Moris, J.M.; Gordon, P.M.; Heileson, J.L.; Funderburk, L.K. From Fish Oil to Resolution: A Narrative Review on the Potential of SPM-Enriched Marine Oil for Exercise-Induced Muscle Damage Recovery. Nutrients 2025, 17, 2014. https://doi.org/10.3390/nu17122014
de Souza LC, Moris JM, Gordon PM, Heileson JL, Funderburk LK. From Fish Oil to Resolution: A Narrative Review on the Potential of SPM-Enriched Marine Oil for Exercise-Induced Muscle Damage Recovery. Nutrients. 2025; 17(12):2014. https://doi.org/10.3390/nu17122014
Chicago/Turabian Stylede Souza, Leticia C., Jose M. Moris, Paul M. Gordon, Jeffery L. Heileson, and LesLee K. Funderburk. 2025. "From Fish Oil to Resolution: A Narrative Review on the Potential of SPM-Enriched Marine Oil for Exercise-Induced Muscle Damage Recovery" Nutrients 17, no. 12: 2014. https://doi.org/10.3390/nu17122014
APA Stylede Souza, L. C., Moris, J. M., Gordon, P. M., Heileson, J. L., & Funderburk, L. K. (2025). From Fish Oil to Resolution: A Narrative Review on the Potential of SPM-Enriched Marine Oil for Exercise-Induced Muscle Damage Recovery. Nutrients, 17(12), 2014. https://doi.org/10.3390/nu17122014








