Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Experimental Animals and Model Induction
2.3. Liver Transcriptome Analysis
2.4. Gut Microbiota Composition Analysis
2.5. Serum Metabolites Analysis
2.6. Statistical Analysis
3. Results
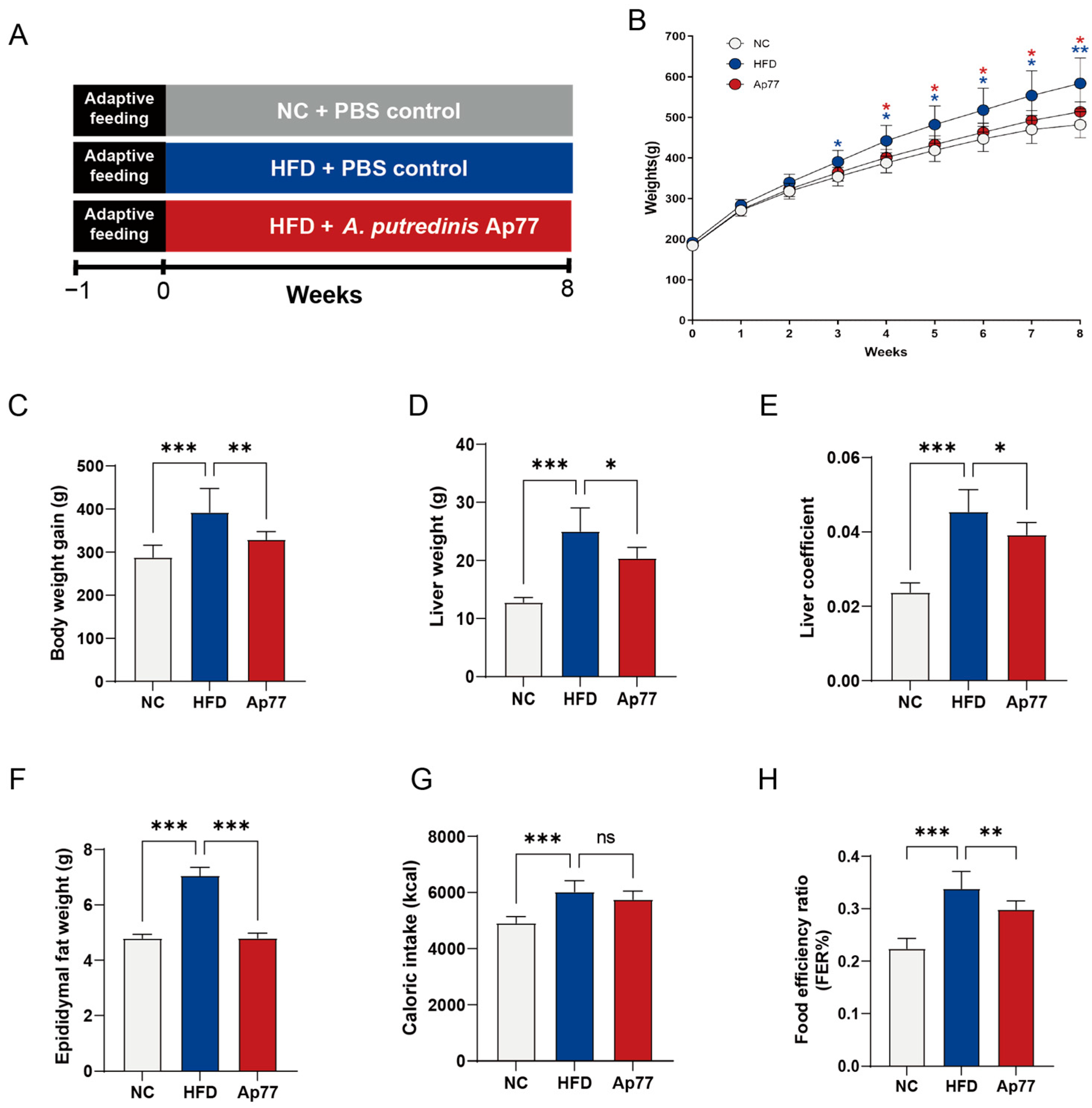
3.1. Ap77 Influences Physiological Parameters in MASLD Rats
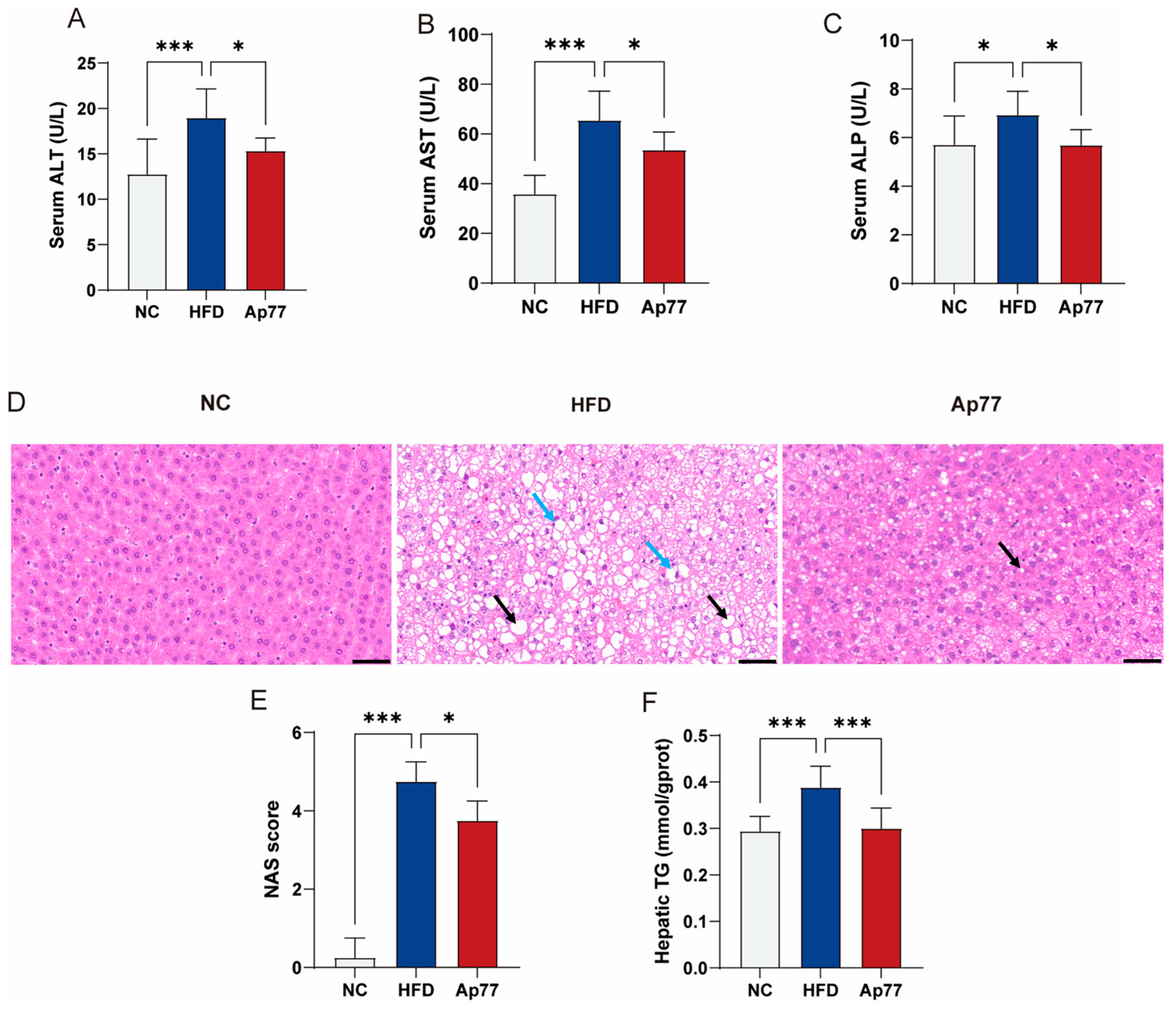
3.2. Ap77 Ameliorates Liver Function Impairment in MASLD Rats
3.3. Ap77 Modulates Lipid Profile and Adipose Tissue Morphology in MASLD Rats
3.4. Ap77 Suppresses Inflammation-Related Pathways
3.5. Ap77 Attenuates Inflammatory Responses in MASLD Rats
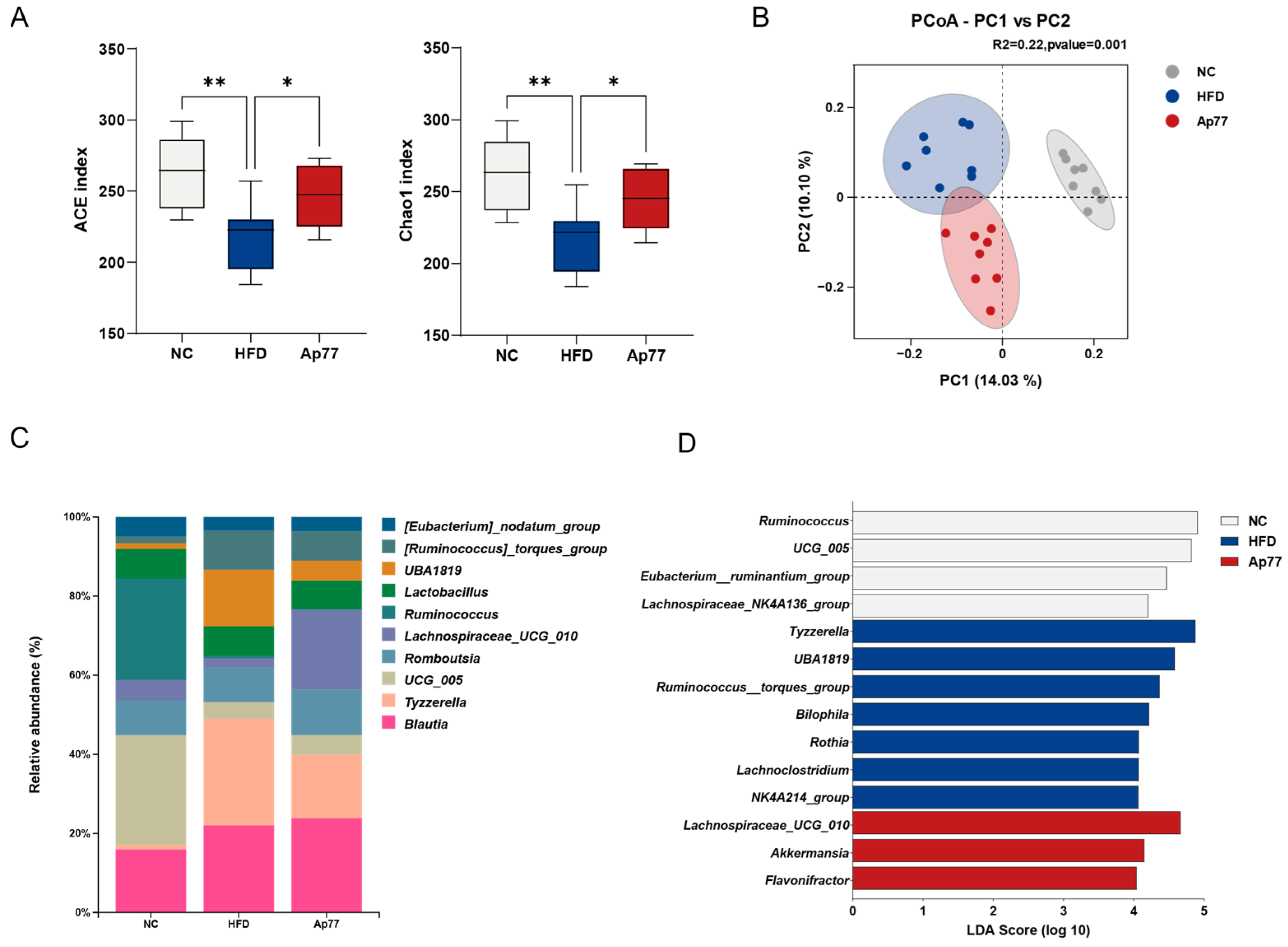
3.6. Gut Microbiota Remodeling by Ap77 in MASLD Rats
3.7. Modulation of Serum Metabolites by Ap77 in MASLD Rats
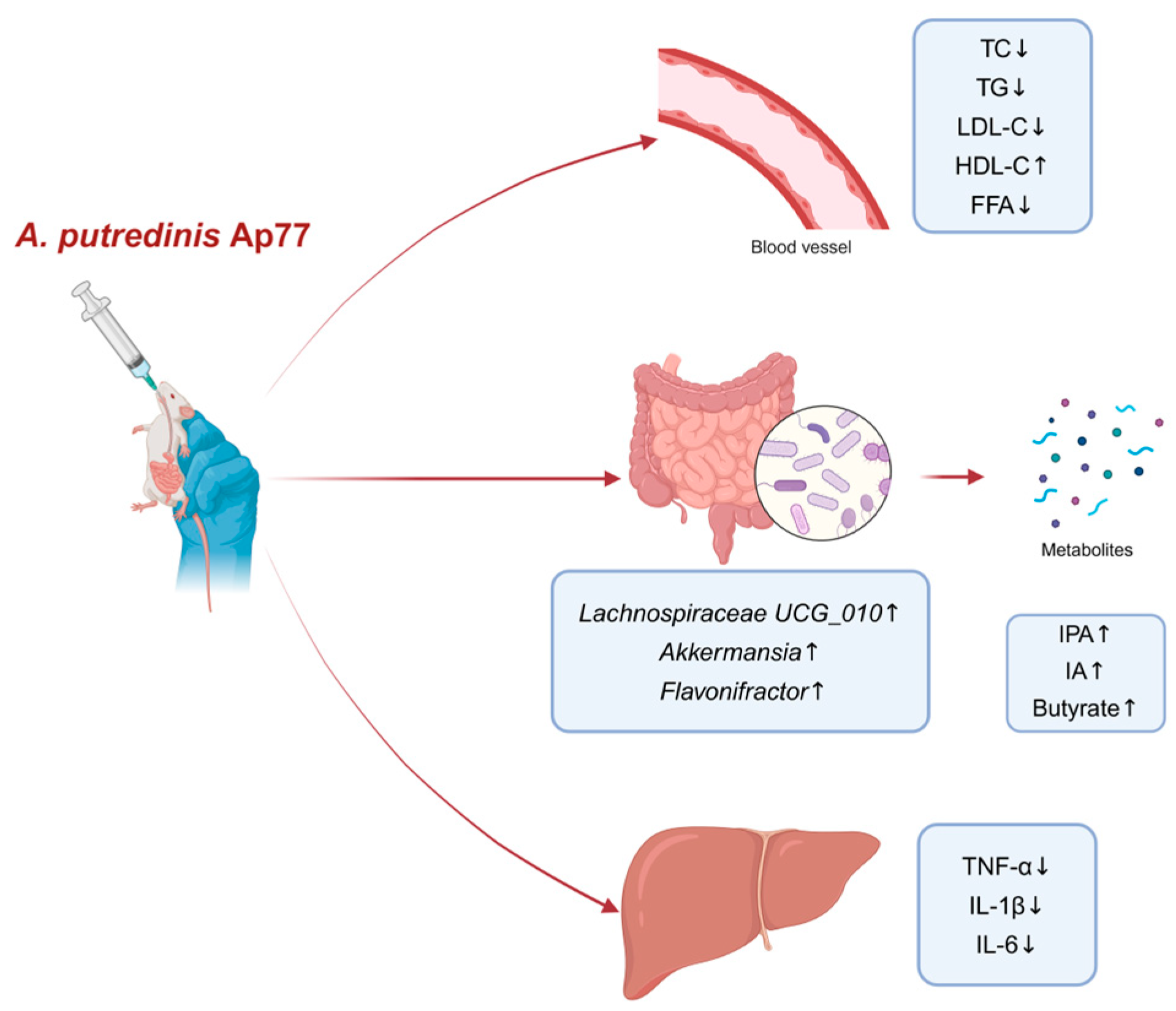
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic-dysfunction-associated steatotic liver disease |
| NAS | NAFLD Activity Score |
| NC | Normal chow diet |
| HFD | High-fat diet |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| TC | Total cholesterol |
| TG | Triglyceride |
| LDL-C | Low-density-lipoprotein cholesterol |
| HDL-C | High-density-lipoprotein cholesterol |
| FFA | Free fatty acid |
| DEGs | Differentially expressed genes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GSEA | Gene set enrichment analysis |
| TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| ASV | Amplicon Sequence Variant |
| PCoA | Principal Coordinate Analysis |
| LEfSe | Linear Discriminant Analysis Effect Size |
| SCFAs | Short-chain fatty acids |
| IPA | Indole-3-propionic acid |
| IA | Indoleacrylic acid |
References
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic Dysfunction–Associated Steatotic Liver Disease: Update and Impact of New Nomenclature on the American Association for the Study of Liver Diseases Practice Guidance on Nonalcoholic Fatty Liver Disease. Hepatology 2024, 79, 1212. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing Epidemiology, Global Trends and Implications for Outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The Gut–Liver Axis and Gut Microbiota in Health and Liver Disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the Barriers: The Role of Gut Homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting Gut Microbiota and Metabolism as the Major Probiotic Mechanism—An Evidence-Based Review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Mijangos-Trejo, A.; Nuño-Lambarri, N.; Barbero-Becerra, V.; Uribe-Esquivel, M.; Vidal-Cevallos, P.; Chávez-Tapia, N. Prebiotics and Probiotics: Therapeutic Tools for Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 14918. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.a.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental Factors Shaping the Gut Microbiome in a Dutch Population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Rautio, M.; Eerola, E.; Väisänen-Tunkelrott, M.-L.; Molitoris, D.; Lawson, P.; Collins, M.D.; Jousimies-Somer, H. Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a New Genus Alistipes Gen. Nov., as Alistipes putredinis Comb. Nov., and Description of Alistipes finegoldii Sp. Nov., from Human Sources. Syst. Appl. Microbiol. 2003, 26, 182–188. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut Microbial Carbohydrate Metabolism Contributes to Insulin Resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Wang, K.; Mehta, R.S.; Ma, W.; Nguyen, L.H.; Wang, D.D.; Ghazi, A.R.; Yan, Y.; Al-Shaar, L.; Wang, Y.; Hang, D.; et al. The Gut Microbiome Modifies the Associations of Short- and Long-Term Physical Activity with Body Weight Changes. Microbiome 2023, 11, 121. [Google Scholar] [CrossRef]
- Yang, C.; Xu, J.; Xu, X.; Xu, W.; Tong, B.; Wang, S.; Ji, R.; Tan, Y.; Zhu, Y. Characteristics of Gut Microbiota in Patients with Metabolic Associated Fatty Liver Disease. Sci. Rep. 2023, 13, 9988. [Google Scholar] [CrossRef]
- Shao, L.; Ling, Z.; Chen, D.; Liu, Y.; Yang, F.; Li, L. Disorganized Gut Microbiome Contributed to Liver Cirrhosis Progression: A Meta-Omics-Based Study. Front. Microbiol. 2018, 9, 3166. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Wang, Y.; Tan, X.; Zou, H.; Feng, S.; Zhang, H.; Zhang, Z.; He, J.; Cui, B.; et al. Human Fecal Microbiota Transplantation Reduces the Susceptibility to Dextran Sulfate Sodium-Induced Germ-Free Mouse Colitis. Front. Immunol. 2022, 13, 836542. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Ishikawa, D.; Zhang, X.; Nomura, K.; Shibuya, T.; Hojo, M.; Yamashita, M.; Koizumi, S.; Yamazaki, F.; Iwamoto, S.; Saito, M.; et al. Anti-Inflammatory Effects of Bacteroidota Strains Derived from Outstanding Donors of Fecal Microbiota Transplantation for the Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2024, 30, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Yang, Y.; Li, J.; Sun, X.; Zhang, Y.; Liu, R.; Chen, F.; Li, X. Special Correlation between Diet and MASLD: Positive or Negative? Cell Biosci. 2025, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pu, J.; Lu, S.; Bai, X.; Wu, Y.; Jin, D.; Cheng, Y.; Zhang, G.; Zhu, W.; Luo, X.; et al. Species-Level Analysis of Human Gut Microbiota With Metataxonomics. Front. Microbiol. 2020, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, R.; Wang, R.; Lu, Y.; Xu, M.; Lin, X.; Lan, R.; Zhang, S.; Tang, H.; Fan, Q.; et al. Weissella Viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD. Nutrients 2025, 17, 1585. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; for the NASH Clinical Research Network (CRN). Nonalcoholic Fatty Liver Disease (NAFLD) Activity Score and the Histopathologic Diagnosis in NAFLD: Distinct Clinicopathologic Meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Brown, R.A.M.; Epis, M.R.; Horsham, J.L.; Kabir, T.D.; Richardson, K.L.; Leedman, P.J. Total RNA Extraction from Tissues for microRNA and Target Gene Expression Analysis: Not All Kits Are Created Equal. BMC Biotechnol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Guo, D.; Deng, Y.; Yang, Q.; Li, M.; Wang, X.; Wan, X.; He, J.; Xu, Y.; Huang, W.; Lin, G.; et al. Symbiotic Probiotic Communities with Multiple Targets Successfully Combat Obesity in High-Fat-Diet-Fed Mice. Gut Microbes 2024, 16, 2420771. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The Intestinal Microbiota Fuelling Metabolic Inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Charles-Messance, H.; Mitchelson, K.A.J.; De Marco Castro, E.; Sheedy, F.J.; Roche, H.M. Regulating Metabolic Inflammation by Nutritional Modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.; Gall, J.; Byrne, R.; Yvan-Charvet, L.; McGillicuddy, F.C. Metabolic Inflammation in Obesity-At the Crossroads between Fatty Acid and Cholesterol Metabolism. Mol. Nutr. Food Res. 2021, 65, e1900482. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An Integrated View of Anti-Inflammatory and Antifibrotic Targets for the Treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The Bidirectional Immune Crosstalk in Metabolic Dysfunction-Associated Steatotic Liver Disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.-F.; Wu, Y.; Wang, Y.; et al. Rutin Ameliorated Lipid Metabolism Dysfunction of Diabetic NAFLD via AMPK/SREBP1 Pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef]
- Yu, J.S.; Youn, G.S.; Choi, J.; Kim, C.; Kim, B.Y.; Yang, S.; Lee, J.H.; Park, T.; Kim, B.K.; Kim, Y.B.; et al. Lactobacillus lactis and Pediococcus pentosaceus-driven Reprogramming of Gut Microbiome and Metabolome Ameliorates the Progression of Non-alcoholic Fatty Liver Disease. Clin. Transl. Med. 2021, 11, e634. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, W.; Shan, M.; Lu, Z.; Lu, Y. Yogurt-Derived Lactobacillus Plantarum Q16 Alleviated High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Food Sci. Hum. Wellness 2022, 11, 1428–1439. [Google Scholar] [CrossRef]
- Pabst, O.; Hornef, M.W.; Schaap, F.G.; Cerovic, V.; Clavel, T.; Bruns, T. Gut–Liver Axis: Barriers and Functional Circuits. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, Z.; Zhong, H.; Luo, R.; Liu, W.; Xiong, S.; Liu, Q.; Liu, M. Gut Microbial Metabolites in MASLD: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Hepatol. Commun. 2024, 8, e0484. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gao, W.; Liu, Z.; Fang, Z.; Wang, H.; Zhao, J.; Zhang, H.; Lu, W.; Chen, W. Specific Strains of Faecalibacterium Prausnitzii Ameliorate Nonalcoholic Fatty Liver Disease in Mice in Association with Gut Microbiota Regulation. Nutrients 2022, 14, 2945. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Luo, G.; Wang, Y.; Du, K.; Gao, X. Metabolome Combined with Gut Microbiome Revealed the Lipid-Lowering Mechanism of Xuezhiping Capsule on Hyperlipidemic Hamster Induced by High Fat Diet. Front. Mol. Biosci. 2023, 10, 1147910. [Google Scholar] [CrossRef]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia Muciniphila: Is It the Holy Grail for Ameliorating Metabolic Diseases? Gut Microbes 2021, 13, 1984104. [Google Scholar] [CrossRef]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia Muciniphila Alleviates High-Fat-Diet-Related Metabolic-Associated Fatty Liver Disease by Modulating Gut Microbiota and Bile Acids. Microb. Biotechnol. 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor Plautii Attenuates Inflammatory Responses in Obese Adipose Tissue. Mol Biol Rep 2020, 47, 6717–6725. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wu, X.; Cai, T.; Dong, L.; Liang, S.; Zhu, L.; Song, X.; Dong, Y.; Zheng, Y.; et al. Administration of Alistipes indistinctus Prevented the Progression from Nonalcoholic Fatty Liver Disease to Nonalcoholic Steatohepatitis by Enhancing the Gut Barrier and Increasing Lactobacillus Spp. Biochem. Biophys. Res. Commun. 2024, 741, 151033. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small Metabolites, Possible Big Changes: A Microbiota-Centered View of Non-Alcoholic Fatty Liver Disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.-J.; Sharma, S.P.; Won, S.-M.; Oh, K.-K.; Yoon, S.J.; Park, H.J.; et al. Gut Microbiota-Derived Indole Compounds Attenuate Metabolic Dysfunction-Associated Steatotic Liver Disease by Improving Fat Metabolism and Inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef]
- Sarkar, A.; Mitra, P.; Lahiri, A.; Das, T.; Sarkar, J.; Paul, S.; Chakrabarti, P. Butyrate Limits Inflammatory Macrophage Niche in NASH. Cell Death Dis. 2023, 14, 332. [Google Scholar] [CrossRef]
- Raso, G.M.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, M.C.; Canani, R.B.; Calignano, A.; Meli, R. Effects of Sodium Butyrate and Its Synthetic Amide Derivative on Liver Inflammation and Glucose Tolerance in an Animal Model of Steatosis Induced by High Fat Diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef]
- Molino, S.; Lerma-Aguilera, A.; Jiménez-Hernández, N.; Rufián Henares, J.Á.; Francino, M.P. Evaluation of the Effects of a Short Supplementation with Tannins on the Gut Microbiota of Healthy Subjects. Front. Microbiol. 2022, 13, 848611. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Liu, L.-D.; Zhu, J.-Y.; Zhu, S.-S.; Ye, B.-Q.; Yang, J.-L.; Huang, J.-Y.; Huang, Z.-H.; You, Y.; Li, W.-K.; et al. Alistipes indistinctus-Derived Hippuric Acid Promotes Intestinal Urate Excretion to Alleviate Hyperuricemia. Cell Host Microbe 2024, 32, 366–381.e9. [Google Scholar] [CrossRef]
- Sehgal, R.; de Mello, V.D.; Männistö, V.; Lindström, J.; Tuomilehto, J.; Pihlajamäki, J.; Uusitupa, M. Indolepropionic Acid, a Gut Bacteria-Produced Tryptophan Metabolite and the Risk of Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 4695. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Marascio, N.; Scarlata, G.G.M.; Romeo, F.; Cicino, C.; Trecarichi, E.M.; Quirino, A.; Torti, C.; Matera, G.; Russo, A. The Role of Gut Microbiota in the Clinical Outcome of Septic Patients: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 9307. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, R.; Zhao, R.; Lu, Y.; Xu, M.; Lin, X.; Lan, R.; Zhang, S.; Tang, H.; Fan, Q.; et al. Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression. Nutrients 2025, 17, 2013. https://doi.org/10.3390/nu17122013
Zhang S, Wang R, Zhao R, Lu Y, Xu M, Lin X, Lan R, Zhang S, Tang H, Fan Q, et al. Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression. Nutrients. 2025; 17(12):2013. https://doi.org/10.3390/nu17122013
Chicago/Turabian StyleZhang, Shuwei, Ruoshi Wang, Ruiqing Zhao, Yao Lu, Mingchao Xu, Xiaoying Lin, Ruiting Lan, Suping Zhang, Huijing Tang, Qianhua Fan, and et al. 2025. "Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression" Nutrients 17, no. 12: 2013. https://doi.org/10.3390/nu17122013
APA StyleZhang, S., Wang, R., Zhao, R., Lu, Y., Xu, M., Lin, X., Lan, R., Zhang, S., Tang, H., Fan, Q., Yang, J., Liu, L., & Xu, J. (2025). Alistipes putredinis Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease in Rats via Gut Microbiota Remodeling and Inflammatory Suppression. Nutrients, 17(12), 2013. https://doi.org/10.3390/nu17122013




