Nutritional Challenges Among Children Under Five in Limpopo Province, South Africa: Complementary Feeding Practices and Dietary Diversity Deficits
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Population
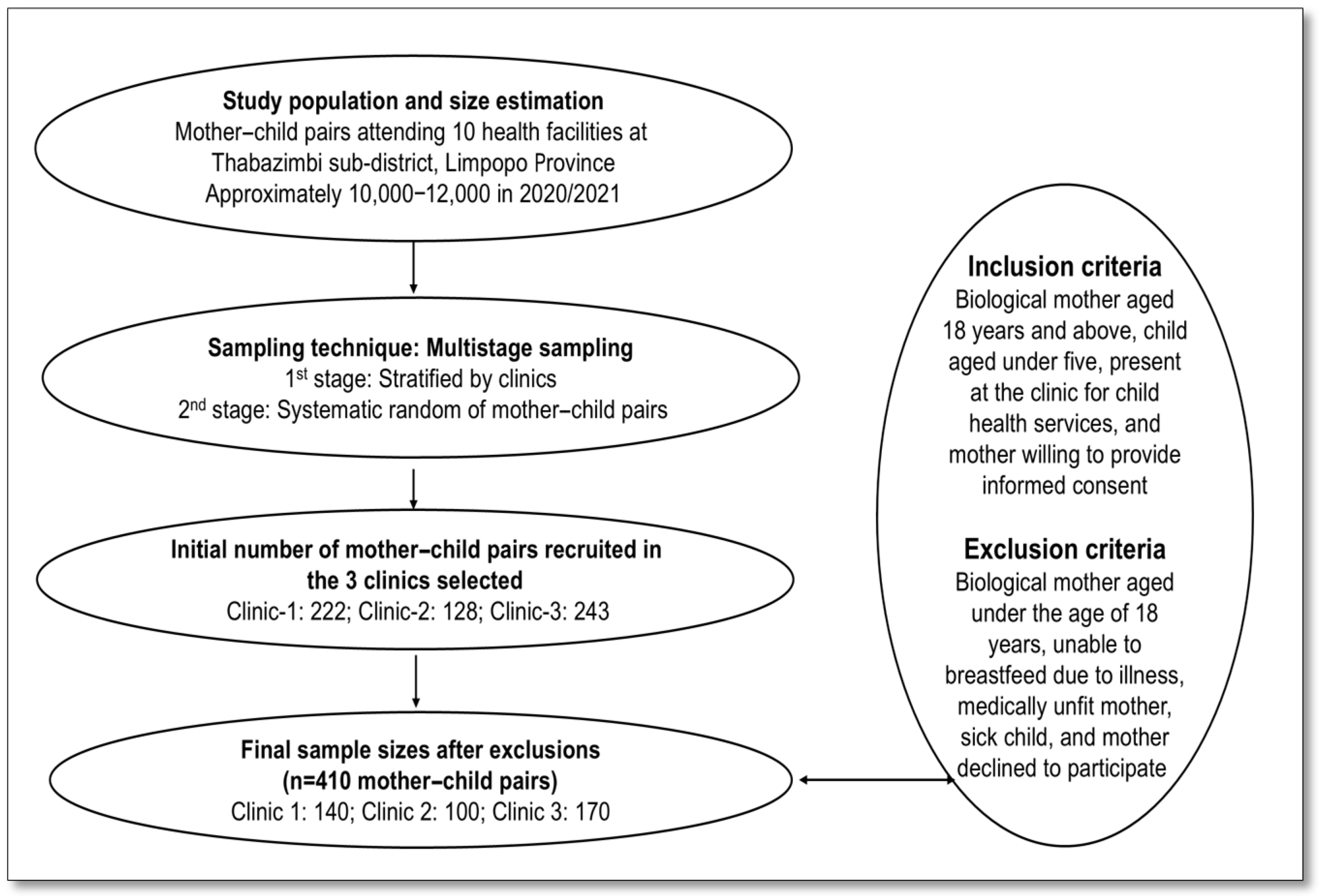
2.4. Sample Size and Sampling Procedure
2.5. Data Collection and Tools
2.5.1. Socio-Demographics of Study Participants
2.5.2. Complementary Feeding Practices and Dietary Diversity
2.5.3. Anthropometric Measurements and Nutritional Indicators of Children
2.5.4. Anthropometric Measurements of Mothers
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Mothers
3.2. Characteristics of Children
3.3. Comparison of the Nutritional Status of Children by Sex and Age
3.4. Infant and Complementary Feeding Practices
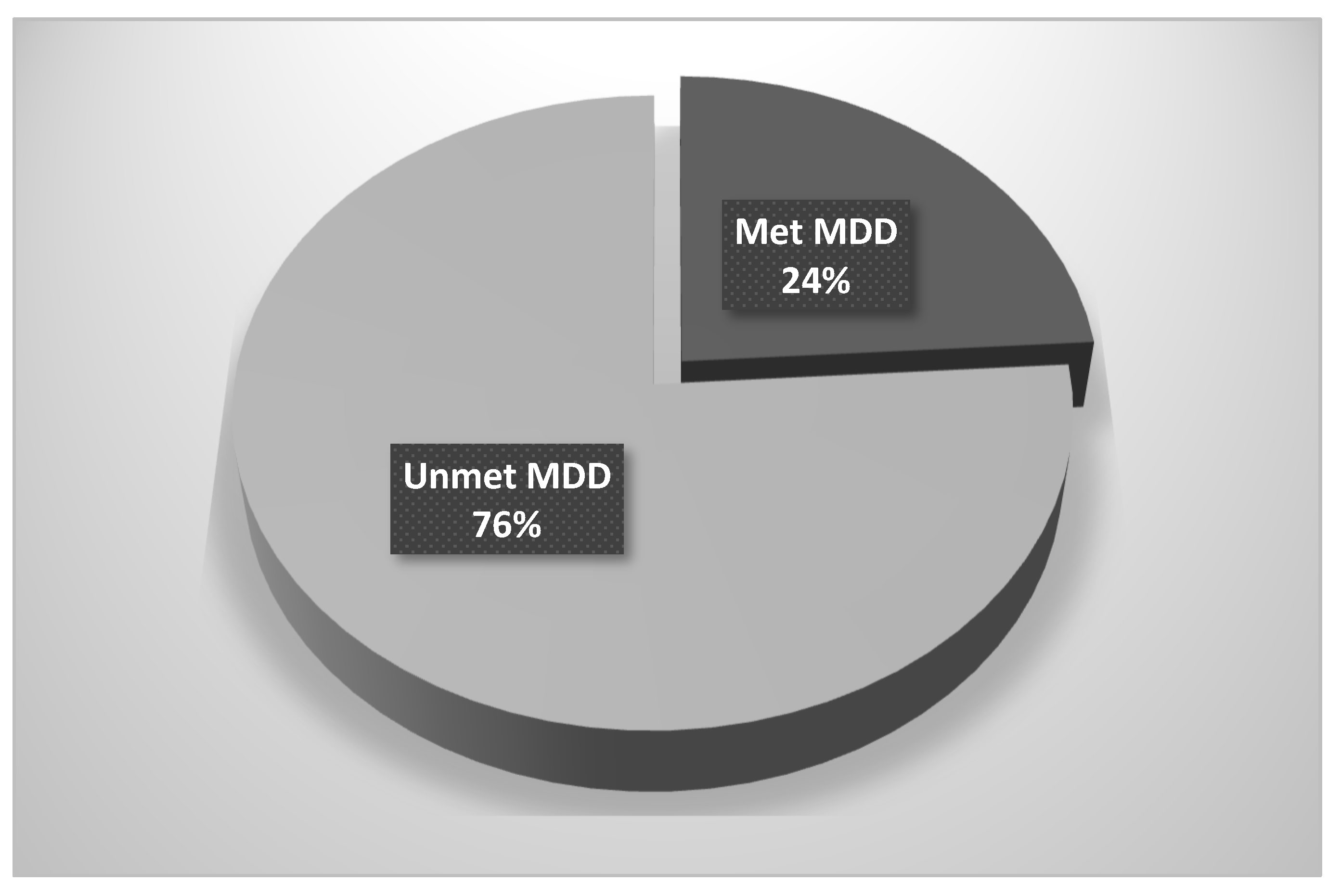
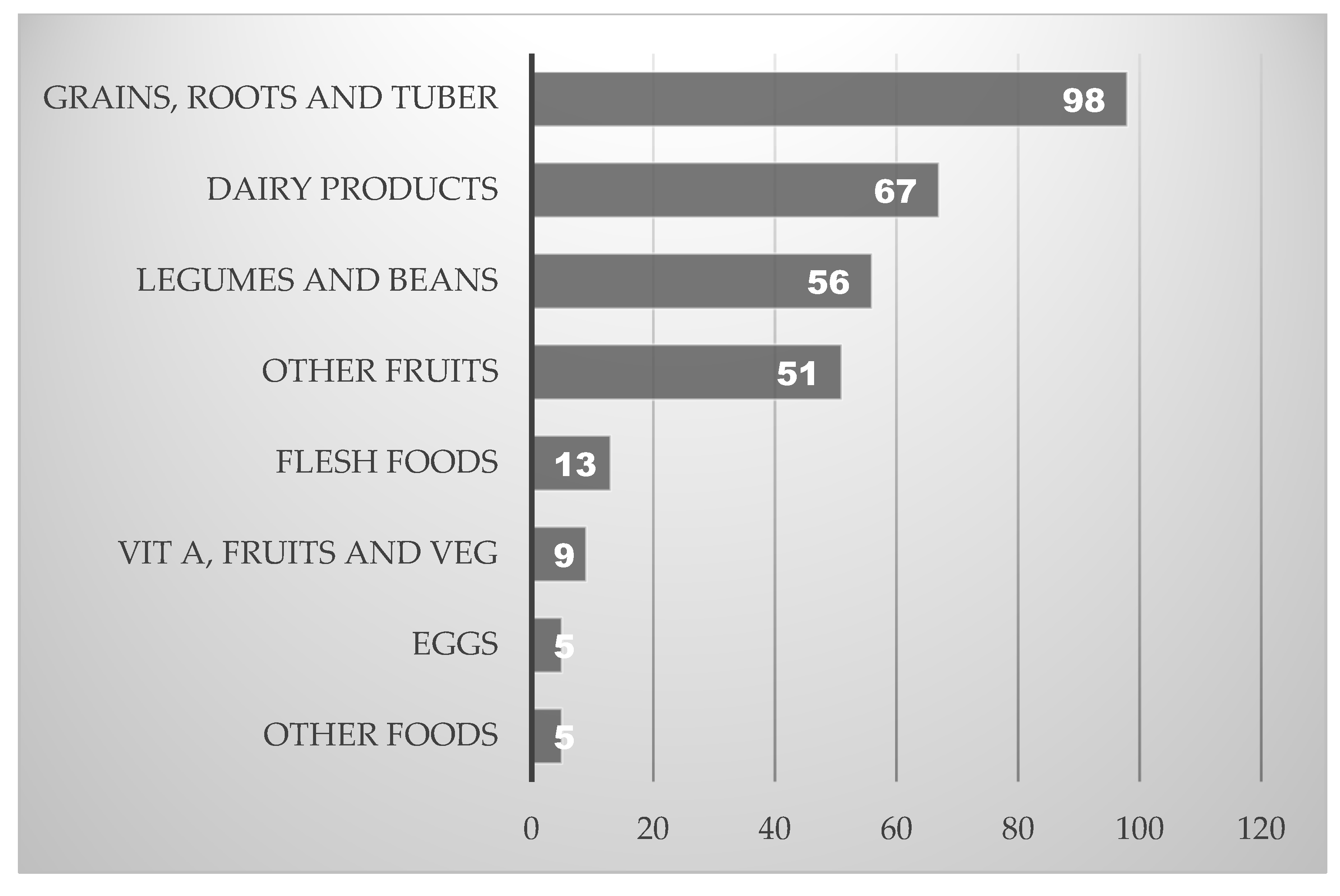
3.5. Dietary Diversity and Food Group Consumption Among Children
3.6. Multivariate Analysis of Factors Associated with Child Nutritional Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büttner, N.; Heemann, M.; De Neve, J.-W.; Verguet, S.; Vollmer, S.; Harttgen, K. Economic Growth and Childhood Malnutrition in Low- and Middle-Income Countries. JAMA Netw. Open 2023, 6, e2342654. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Wang, D.; Fawzi, W.W. School-based interventions targeting double burden of malnutrition and educational outcomes of adolescents in low- and middle-income countries: Protocol for a systematic review. Syst. Rev. 2021, 10, 204. [Google Scholar] [CrossRef]
- Mkhize, M.; Sibanda, M. A Review of Selected Studies on the Factors Associated with the Nutrition Status of Children Under the Age of Five Years in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 7973. [Google Scholar] [CrossRef] [PubMed]
- Malan, L.; Zandberg, L.; Visser, M.V.; Wicks, M.; Kruger, H.S.; Faber, M. Biochemical assessment of the nutritional status of infants, children and adolescents in South Africa (1997–2022): A systematic review. Public Health Nutr. 2024, 27, e210. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Mates, E.; Brennan, E.; Bijalwan, V.; Menezes, R.; Ray, S.; Ali, Z.; Yarparvar, A.; Sharma, D.; Lelijveld, N. Nutritional status of school-age children and adolescents in low- and middle-income countries across seven global regions: A synthesis of scoping reviews. Public Health Nutr. 2023, 26, 63–95. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods; World Health Organization and the United Nations Children’s Fund (UNICEF): Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240018389 (accessed on 10 October 2022).
- UNICEF. Child Malnutrition; World Health Organization: Geneva, Switzerland, 2023; Available online: https://data.unicef.org/topic/nutrition/malnutrition/ (accessed on 12 May 2025).
- UNICEF; WHO. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; UNICEF: New York, NY, USA; WHO: New York, NY, USA, 2023. [Google Scholar]
- Apostolopoulou, A.; Tranidou, A.; Tsakiridis, I.; Magriplis, E.; Dagklis, T.; Chourdakis, M. Effects of Nutrition on Maternal Health, Fetal Development, and Perinatal Outcomes. Nutrients 2024, 16, 375. [Google Scholar] [CrossRef]
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 2013, 9 (Suppl. S2), 27–45. [Google Scholar] [CrossRef]
- WHO. Stunting in a Nutshell. 2015. Available online: https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell (accessed on 12 February 2024).
- Du Plessis, L.M.; Kruger, H.; Sweet, L. Complementary feeding: A critical window of opportunity from six months onwards. S. Afr. J. Clin. Nutr. 2013, 26, S129–S140. [Google Scholar]
- Sayed, N.; Schonfeldt, H. A review of complementary feeding practices in South Africa. S. Afr. J. Clin. Nutr. 2018, 33, 36–43. [Google Scholar] [CrossRef]
- Mushaphi, L. Infant feeding practices of mothers and nutritional status of infant. S. Afr. J. Clin. Nutr. 2008, 21, 36–41. [Google Scholar]
- Faber, M.; Benade, A.J.S. Breastfeeding, complementary feeding and nutritional status of 6–12-month-old infants in rural KwaZulu-Natal. S. Afr. J. Clin. Nutr. 2007, 20, 16–24. [Google Scholar]
- Modjadji, P.; Seabela, E.S.; Ntuli, B.; Madiba, S. Beliefs and Norms Influencing Initiation and Sustenance of Exclusive Breastfeeding: Experiences of Mothers in Primary Health Care Facilities in Ermelo, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 1513. [Google Scholar] [CrossRef]
- Modjadji, P.; Mashishi, J. Persistent Malnutrition and Associated Factors among Children under Five Years Attending Primary Health Care Facilities in Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 7580. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.K.; Afful-Dadzie, E.; Marquis, G.S. Infant and young child feeding practices are associated with childhood anaemia and stunting in sub-Saharan Africa. BMC Nutr. 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Mekonen, E.G. Variation of continued breastfeeding by age of the child among children aged 12–23 months: Evidence from 21 sub-Saharan African countries. Nutr. Metab. 2025, 22, 31. [Google Scholar] [CrossRef]
- Mekonen, E.G.; Zegeye, A.F.; Workneh, B.S. Complementary feeding practices and associated factors among mothers of children aged 6 to 23 months in Sub-saharan African countries: A multilevel analysis of the recent demographic and health survey. BMC Public Health 2024, 24, 115. [Google Scholar] [CrossRef]
- Faber, M.; Laubscher, R.; Berti, C. Poor dietary diversity and low nutrient density of the complementary diet for 6-to 24-month-old children in urban and rural KwaZulu-Natal, South Africa. Matern. Child Nutr. 2016, 12, 528–545. [Google Scholar] [CrossRef]
- Modjadji, P.; Molokwane, D.; Ukegbu, P.O. Dietary Diversity and Nutritional Status of Preschool Children in North West Province, South Africa: A Cross Sectional Study. Children 2020, 7, 174. [Google Scholar] [CrossRef]
- Motadi, S.A.; Zuma, M.K.; Freeland-Graves, J.H.; Gertrude Mbhenyane, X. Dietary diversity and nutritional status of children attending early childhood development centres in Vhembe District, Limpopo province, South Africa. J. Nutr. Sci. 2023, 12, e92. [Google Scholar] [CrossRef]
- Steyn, N.P.; Nel, J.H.; Nantel, G.; Kennedy, G.; Labadarios, D. Food variety and dietary diversity scores in children: Are they good indicators of dietary adequacy? Public Health Nutr. 2006, 9, 644–650. [Google Scholar] [CrossRef]
- Molani-Gol, R.; Kheirouri, S.; Alizadeh, M. Does the high dietary diversity score predict dietary micronutrients adequacy in children under 5 years old? A systematic review. J. Health Popul. Nutr. 2023, 42, 2. [Google Scholar] [CrossRef] [PubMed]
- Ty, H.; Krawinkel, M. Dietary Diversity Score: A Measure of Nutritional Adequacy or an Indicator of Healthy Diet? J. Nutr. Health Sci. 2016, 3, 303. [Google Scholar] [CrossRef]
- Dello Russo, M.; Formisano, A.; Lauria, F.; Ahrens, W.; Bogl, L.H.; Eiben, G.; De Henauw, S.; Hebestreit, A.; Intemann, T.; Hunsberger, M.; et al. Dietary Diversity and Its Association with Diet Quality and Health Status of European Children, Adolescents, and Adults: Results from the I. Family Study. Foods 2023, 12, 4458. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.M.; Ssentongo, P.; Gao, X.; Chinchilli, V.M.; Richie, J.P.; Jr Maiga, M.; Muscat, J.E. Prevalence and determinants of meeting minimum dietary diversity among children aged 6–23 months in three sub-Saharan African Countries: The Demographic and Health Surveys, 2019–2020. Front. Public Health 2022, 10, 846049. [Google Scholar] [CrossRef]
- Harper, A.; Goudge, J.; Chirwa, E.; Rothberg, A.; Sambu, W.; Mall, S. Dietary diversity, food insecurity and the double burden of malnutrition among children, adolescents and adults in South Africa: Findings from a national survey. Front. Public Health 2022, 10, 948090. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, J.V.; Quisumbing, A. Diet quality and micronutrient intakes in nutritional value chains: A synthesis and suggestions for further research. Food Policy 2025, 130, 102789. [Google Scholar] [CrossRef]
- Modjadji, P.; Madiba, S. The double burden of malnutrition in a rural health and demographic surveillance system site in South Africa: A study of primary schoolchildren and their mothers. BMC Public Health 2019, 19, 1087. [Google Scholar] [CrossRef]
- Modjadji, P.; Masilela, L.N.; Cele, L.; Mathibe, M.; Mphekgwana, P.M. Evidence of Concurrent Stunting and Obesity among Children under 2 Years from Socio-Economically Disadvantaged Backgrounds in the Era of the Integrated Nutrition Programme in South Africa. Int. J. Environ. Res. Public Health 2022, 19, 12501. [Google Scholar] [CrossRef]
- Mamabolo, R.L.; Alberts, M.; Steyn, N.P.; Delemarre-van de Waal, H.A.; Levitt, N.S. Prevalence and determinants of stunting and overweight in 3-year-old black South African children residing in the Central Region of Limpopo Province, South Africa. Public Health Nutr. 2005, 8, 501–508. [Google Scholar] [CrossRef]
- Gallo, V.; Egger, M.; McCormack, V.; Farmer, P.B.; Ioannidis, J.P.A.; Kirsch-Volders, M.; Matullo, G.; Phillips, D.H.; Schoket, B.; Stromberg, U.; et al. STrengthening the reporting of Observational studies in Epidemiology—Molecular Epidemiology (STROBE-ME): An extension of the STROBE statement. Eur. J. Epidemiol. 2011, 26, 797–810. [Google Scholar] [CrossRef]
- Kieser, M. Methods and Applications of Sample Size Calculation and Recalculation in Clinical Trials; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Masilela, L.N.; Modjadji, P. Child Nutrition Outcomes and Maternal Nutrition-Related Knowledge in Rural Localities of Mbombela, South Africa. Children 2023, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P.; Madiba, S. Childhood Undernutrition and Its Predictors in a Rural Health and Demographic Surveillance System Site in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 3021. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices (Part 1); World Health Organization: Geneva, Switzerland, 2008; Available online: https://iris.who.int/handle/10665/43895 (accessed on 13 January 2024).
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- WHO. WHO Guideline for Complementary Feeding of Infants and Young Children 6–23 Months of Age; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/bitstream/handle/10665/373358/9789240081864-eng.pdf (accessed on 12 December 2022).
- FAO. Guidelines for Measuring Household and Individual Dietary Diversity. 2011. Available online: https://www.fao.org/fileadmin/user_upload/wa_workshop/docs/FAO-guidelines-dietary-diversity2011.pdf (accessed on 13 February 2024).
- Kennedy, G.L.; Pedro, M.R.; Seghieri, C.; Nantel, G.; Brouwer, I. Dietary Diversity Score Is a Useful Indicator of Micronutrient Intake in Non-Breast-Feeding Filipino Children2. J. Nutr. 2007, 137, 472–477. [Google Scholar] [CrossRef]
- Norton, K. Standards for Anthropometry Assessment; International Society for the Advancement of Kinanthropometry (ISAK): Glasgow, UK, 2018; pp. 68–137. [Google Scholar]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee 1995; WHO Technical report Series No. 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Weir, C.; Jan, A. Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 25 May 2025).
- Mthethwa, S.; Wale, E. The impact of the social grants programme on household vulnerability to food insecurity in South Africa: Application of a two-stage least squares and implications. Afr. J. Dev. Stud. (Former. AFFRIKA J. Politics Econ. Soc.) 2023, 13, 241–263. [Google Scholar] [CrossRef]
- Ralston, M.; Schatz, E.; Menken, J.; Gómez-Olivé, F.X.; Tollman, S. Who Benefits-Or Does not-From South Africa’s Old Age Pension? Evidence from Characteristics of Rural Pensioners and Non-Pensioners. Int. J. Environ. Res. Public Health 2015, 13, 85. [Google Scholar] [CrossRef]
- Cavazos-Rehg, P.A.; Krauss, M.J.; Spitznagel, E.L.; Bommarito, K.; Madden, T.; Olsen, M.A.; Subramaniam, H.; Peipert, J.F.; Bierut, L.J. Maternal age and risk of labor and delivery complications. Matern. Child Health J. 2015, 19, 1202–1211. [Google Scholar] [CrossRef]
- Karlsson, O.; Subramanian, S.V. Refrigerator ownership and child health and nutrition in low- and middle-income countries. Glob. Food Secur. 2023, 37, 100698. [Google Scholar] [CrossRef]
- Swope, C.B.; Hernández, D. Housing as a determinant of health equity: A conceptual model. Soc. Sci. Med. (1982) 2019, 243, 112571. [Google Scholar] [CrossRef]
- Akombi, B.J.; Agho, K.E.; Merom, D.; Renzaho, A.M.; Hall, J.J. Child malnutrition in sub-Saharan Africa: A meta-analysis of demographic and health surveys (2006–2016). PLoS ONE 2017, 12, e0177338. [Google Scholar] [CrossRef]
- Wand, H.; Naidoo, S.; Govender, V.; Reddy, T.; Moodley, J. Preventing Stunting in South African Children Under 5: Evaluating the Combined Impacts of Maternal Characteristics and Low Socioeconomic Conditions. J. Prev. 2024, 45, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Akombi, B.J.; Agho, K.E.; Hall, J.J.; Wali, N.; Renzaho, A.M.N.; Merom, D. Stunting, Wasting and Underweight in Sub-Saharan Africa: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, H.; Grant, C.; Viljoen, M. Overweight and obesity in children and adolescents: The South African problem. S. Afr. J. Sci. 2012, 108, 907. [Google Scholar] [CrossRef]
- Kruger, H.S.; Visser, M.; Malan, L.; Zandberg, L.; Wicks, M.; Ricci, C.; Faber, M. Anthropometric nutritional status of children (0–18 years) in South Africa 1997–2022: A systematic review and meta-analysis. Public Health Nutr. 2023, 26, 2226–2242. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, G.; Gerard Bryan, G.; Mubarek, A.; Natasha, L.; Debbie, T.; Melkamu, B.; Alemseged, A.; Tsinuel, G.; Marko, K. Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: A systematic review. BMJ Glob. Health 2021, 6, e003161. [Google Scholar]
- Makwela, M.S.; Mashaba, R.G.; Ntimana, C.B.; Seakamela, K.P.; Maimela, E. Barriers and enablers to exclusive breastfeeding by mothers in Polokwane, South Africa. Front. Glob. Women’s Health 2024, 5, 1209784. [Google Scholar] [CrossRef]
- Vitalis, D.; Vilar-Compte, M.; Nyhan, K.; Pérez-Escamilla, R. Breastfeeding inequities in South Africa: Can enforcement of the WHO Code help address them?—A systematic scoping review. Int. J. Equity Health 2021, 20, 114. [Google Scholar] [CrossRef]
- Trafford, Z.; Jewett, S.; Swartz, A.; LeFevre, A.E.; Winch, P.J.; Colvin, C.J.; Barron, P.; Bamford, L. Reported infant feeding practices and contextual influences on breastfeeding: Qualitative interviews with women registered to MomConnect in three South African provinces. Int. Breastfeed. J. 2020, 15, 81. [Google Scholar] [CrossRef]
- Maonga, A.R.; Mahande, M.J.; Damian, D.J.; Msuya, S.E. Factors Affecting Exclusive Breastfeeding among Women in Muheza District Tanga Northeastern Tanzania: A Mixed Method Community Based Study. Matern. Child Health J. 2016, 20, 77–87. [Google Scholar] [CrossRef]
- Ogbo, F.A.; Page, A.; Idoko, J.; Claudio, F.; Agho, K.E. Trends in complementary feeding indicators in Nigeria, 2003–2013. BMJ Open 2015, 5, e008467. [Google Scholar] [CrossRef]
- Seabela, E.S.; Modjadji, P.; Mokwena, K.E. Facilitators and barriers associated with breastfeeding among mothers attending primary healthcare facilities in Mpumalanga, South Africa. Front. Nutr. 2023, 10, 1062817. [Google Scholar] [CrossRef] [PubMed]
- Kubeka, Z.; Modjadji, P. Association of Stunting with Socio-Demographic Factors and Feeding Practices among Children under Two Years in Informal Settlements in Gauteng, South Africa. Children 2023, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- Oyelana, O.; Kamanzi, J.; Richter, S. A critical look at exclusive breastfeeding in Africa: Through the lens of diffusion of innovation theory. Int. J. Afr. Nurs. Sci. 2021, 14, 100267. [Google Scholar] [CrossRef]
- Semenekane, N.M.; Witten, C.B.; Swanepoel, E.; Kruger, H.S. Sociodemographic factors associated with mixed-feeding practices among a cohort of mothers with infants aged 4–14 weeks in Tlokwe subdistrict, North West Province, South Africa. S. Afr. J. Child Health 2022, 16, 192–197. [Google Scholar] [CrossRef]
- Martin, M. Mixed-feeding in humans: Evolution and current implications. In Breastfeeding: New Anthropological Approaches; Routeledge: London, UK, 2017; pp. 140–154. [Google Scholar]
- Faber, M.; Wenhold, F. Nutrition in contemporary South Africa. Water SA 2007, 33, 407–412. [Google Scholar] [CrossRef]
- Kruger, S.H.; Labadarios, D.; Swart, R.; Maunder, E.; Gericke, G.J.; Kuzwayo, P.M.; Ntsie, P.R.; Steyn, N.P.; Schloss, I.; Dhansay, M.A.; et al. National Food Consumption Survey-Fortification Baselin (NFCS-FB-I); Nutrition, Department of Health: Pretoria, South Africa, 2008. [Google Scholar]
- Kasimba, S.; Covic, N.; Motswagole, B.; Laubscher, R.; Claasen, N. Consumption of Traditional and Indigenous Foods and Their Contribution to Nutrient Intake among Children and Women in Botswana. Ecol. Food Nutr. 2019, 58, 281–298. [Google Scholar] [CrossRef]
- Ekesa, B.; Walingo mk Abukutsa-Onyango, M. Dietary diversity, nutrition status and morbidity of pre-school children in Matungu division, Western Kenya. Int. J. Food Saf. Nutr. Public Health 2009, 2, 131–144. [Google Scholar] [CrossRef]
- Keyata, E.O.; Daselegn, A.; Oljira, A. Dietary diversity and associated factors among preschool children in selected kindergarten school of Horo Guduru Wollega Zone, Oromia Region, Ethiopia. BMC Nutr. 2022, 8, 71. [Google Scholar] [CrossRef]
- Khamis, A.G.; Mwanri, A.W.; Ntwenya, J.E.; Kreppel, K. The influence of dietary diversity on the nutritional status of children between 6 and 23 months of age in Tanzania. BMC Pediatr. 2019, 19, 518. [Google Scholar] [CrossRef]
- Sealey-Potts, C.; Potts, A.; Citation: Sealey-Potts, C.; Potts, A.C. An Assessment of Dietary Diversity and Nutritional Status Austin Journal of Nutrition and Food Sciences. Austin J. Nutr. Food Sci. 2014, 2, 7–2014. [Google Scholar]
- Asmare, A.A.; Agmas, Y.A. Determinants of coexistence of stunting, wasting, and underweight among children under five years in the Gambia; evidence from 2019/20 Gambian demographic health survey: Application of multivariate binary logistic regression model. BMC Public Health 2022, 22, 1621. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Stewart, K. Does Household Income Affect children’s Outcomes? A Systematic Review of the Evidence. Child Indic. Res. 2021, 14, 981–1005. [Google Scholar] [CrossRef]
- Vazquez, C.E.; Cubbin, C. Socioeconomic Status and Childhood Obesity: A Review of Literature from the Past Decade to Inform Intervention Research. Curr. Obes. Rep. 2020, 9, 562–570. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
| Variables | All | <25 Years (G1) | 25–34 Years (G2) | ≥35 Years (G3) | p | |
|---|---|---|---|---|---|---|
| n (%) | (n = 282); n (%) | (n = 71); n (%) | (n = 56); n (%) | |||
| BMI (Kg/m2) | <18.5 (underweight) | 10 (2) | 7 (3) | 1 (1) | 2 (4) | P0 = 0.090 |
| >18.5–24.99 (normal) | 289 (71) | 194 (69) | 55 (78) | 40 (71) | P1 = 0.130 | |
| 25–29.9 (overweight) | 87 (21) | 66 (23) | 14 (20) | 7 (13) | P2 = 0.901 | |
| ≥30 (obesity) | 23 (6) | 15 (5) | 1 (1) | 7 (12) | P3 = 0.319 | |
| Marital status | Single | 333 (81) | 226 (80) | 61 (86) | 46 (82) | P1 = 0.529; P1 ≤ 0.0001 * |
| Ever married | 76 (19) | 56 (20) | 10 (14) | 10 (18) | P2 ≤ 0.0001 *; P3 ≤ 0.0001 * | |
| Education | No school/primary | 23 (6) | 19 (7) | 4 (6) | 0 (0) | |
| Secondary/grade 12 | 284 (69) | 231 (82) | 64 (90) | 52 (93) | P0 = 0.072; P1 = 0.130 | |
| Post-grade 12 | 39 (10) | 32 (11) | 3 (4) | 4 (7) | P2 = 0.705; P3 = 0.107 | |
| Employed | No | 382 (93) | 265 (94) | 64 (90) | 53 (95) | P0 = 0.500; P1 = 0.252 |
| Yes | 27 (7) | 17 (6) | 7 (10) | 3 (5) | P2 = 0.846; P3 = 0.352 | |
| Child grant | No | 69 (17) | 58 (21) | 8 (11) | 3 (5) | P0 = 0.006 *; P1 = 0.073 |
| Yes | 340 (83) | 224 (79) | 63 (89) | 53 (94) | P2 = 0.007 *; P3 = 0.242 | |
| Household head | Self-headed | 27 (7) | 22 (8) | 4 (6) | 1 (2) | |
| Husband/partner | 183 (45) | 121 (43) | 32 (45) | 30 (54) | P0 = 0.432; P1 = 0.872 | |
| Parents/relatives | 199 (49) | 139 (49) | 35 (49) | 25 (45) | P2 = 0.870; P3 = 0.790 | |
| Household size | <5 | 182 (44) | 130 (46) | 24 (34) | 28 (50) | P0 = 0.118; P1 = 0.062; P2 = 0.594; P3 = 0.067 |
| ≥5 | 227 (56) | 152 (54) | 47 (66) | 28 (50) | ||
| Household income | <ZAR 5000 | 140 (34) | 96 (34) | 28 (39) | 17 (30) | P0 = 0.330 |
| ZAR 5000–ZAR 10,000 | 187 (46) | 129 (46) | 31 (44) | 29 (52) | P1 = 0.368 | |
| ZAR 10,001–ZAR 15,000 | 57 (14) | 44 (15) | 9 (13) | 4 (7) | P2 = 0.728 | |
| >ZAR 15,000 | 25 (6) | 13 (5) | 3 (4) | 6 (11) | P3 = 0.067 | |
| Dwelling place | Non-brick | 236 (58) | 175 (62) | 36 (51) | 25 (45) | P0 = 0.023 *; P1 = 0.082 P2 = 0.723; P3 = 0.336 |
| Brick house | 173 (42) | 107 (38) | 35 (49) | 31 (55) | ||
| Access to electricity | No | 9 (2) | 7 (3) | 2 (3) | 0 (0) | P0 = 0.668; P1 = 0.873 P2 = 0.234; P3 = 0.207 |
| Yes | 400 (98) | 275 (97 | 69 (97) | 56 (100) | ||
| Refrigerator use | No | 28 (7) | 22 (8) | 6 (8) | 0 (0) | P0 = 0.050 *; P1 = 0.857 P2 = 0.234; P3 = 0.207 |
| Yes | 381 (93) | 260 (92) | 65 (92) | 56 (100) | ||
| Access to water | No | 301 (73) | 74 (26) | 23 (32) | 11 (20) | P0 = 0.268; P1 = 0.082 P2 = 0.723; P3 = 0.336 |
| Yes | 108 (27) | 208 (74) | 48 (68) | 45 (80) | ||
| Mother’s age at 1st birth | <30 | 266 (65) | 181 (64) | 45 (63) | 40 (71) | P0 = 0.554; P1 = 0.900 P2 = 0.299; P3 = 0.340 |
| ≥30 | 143 (35) | 101 (36) | 26 (37) | 16 (29) | ||
| Parity | 1–2 | 258 (63) | 184 (65) | 43 (61) | 31 (55) | P0 = 0.333; P1 = 0.462 P2 = 0.161; P3 = 0.556 |
| ≥3 | 151 (37) | 98 (35) | 28 (39) | 25 (45) | ||
| Pregnancy full term | No | 14 (3) | 13 (5) | 0 (0) | 1 (2) | P0 = 0.106; P1 = 0.066 P2 = 0.333; P3 = 0.260 |
| Yes | 395 (97) | 269 (95) | 71 (100) | 55 (98) | ||
| Obstetric complications | No | 337 (82) | 223 (79) | 60 (85) | 54 (96) | P0 = 0.003 *; P1 = 0.301 P2 = 0.002 *; P3 = 0.336 |
| Yes | 72 (18) | 59 (21) | 11 (915) | 2 (4) | ||
| Variables | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|
| Age (months) | 12 | 12;24 | 12 | 60 |
| Weight (kg) | 9.9 | 8.2; 11.5 | 6 | 21 |
| Length (cm) | 76.1 | 69; 80 | 60 | 111 |
| LAZ/HAZ | −1.37 | −2.72; 0.02 | −6.2 | 6 |
| WAZ | −0.46 | −1.26; −0.33 | −4.14 | 4.22 |
| BAZ | 0.51 | −0.42; 1.44 | −4.49 | −6.31 |
| Variables | All (n = 409) | Boys (n = 202) | Girls (n = 207) | p |
|---|---|---|---|---|
| LAZ/HAZ | 0.034 * | |||
| Normal Stunted Tallness | 236 (58) 157 (38) 16 (4) | 106 (52) 90 (45) 6 (3) | 130 (63) 67 (32) 10 (5) | 0.011 * 0.446 |
| WAZ | 0.004 * | |||
| Normal Underweight Growth problem | 333 (81) 53 (13) 23 (6) | 152 (75) 37 (18) 13 (7) | 181 (87) 16 (8) 10 (5) | 0.001 * 0.481 |
| BAZ | 0.506 | |||
| Normal Thinness Overweight risk Overweight/obesity | 247 (60) 19 (5) 79 (19) 64 (17) | 116 (57) 11 (5) 39 (19) 36 (18) | 131 (63) 8 (4) 40 (19) 28 (14) | 0.489 0.997 0.232 |
| Variables | ≤12 Months (n = 282) | 13–24 Months (n = 71) | >24 Months (n = 56) | p |
| LAZ/HAZ | 0.001 * | |||
| Normal Stunted Tallness | 155 (55) 117 (41) 10 (4) | 35 (49) 33 (47) 3 (4) | 46 (82) 7 (13) 3 (5) | ≤0.0001 * 0.677 |
| WAZ | 0.233 | |||
| Normal Underweight Growth problem | 222 (79) 40 (18) 20 (7) | 61 (86) 8 (11) 2 (3) | 50 (89) 5 (9) 1 (2) | 0.153 0.506 |
| BAZ | 0.061 | |||
| Normal Thinness Overweight risk Overweight/obesity | 178 (63) 9 (3) 55 (20) 40 (14) | 34 (48) 6 (8) 13 (18) 18 (25) | 35 (63) 4 (7) 11 (20) 6 (11) | 0.096 0.972 0.038 * |
| Variables | All n (%) | <25 Years (G1) n (%) | 25–34 Years (G2) n (%) | ≥35 Years (G3) n (%) | p |
|---|---|---|---|---|---|
| Advice on CF No Yes | 80 (20) 329 (80) | 21 (18) 93 (82) | 49 (12) 183 (79) | 10 (16) 53 (84) | P0 = 0.607; P1 = 0.167 P2 = 0.001 *; P3 = 0.149 |
| Introduction of CF (did not exclusively breastfed) <6 months ≥6 months | 194 (47) 213 (52) | 130 (46) 150 (54) | 34 (48) 37 (52) | 30 (54) 26 (46) | P0 = 0.620; P1 = 0.826 P2 = 0.329; P3 = 0.526 |
| Delayed CF No Yes | 403 (98) 6 (2) | 277 (98) 5 (2) | 70 (99) 1 (1) | 56 (100) 0(0) | P0 = 0.836; P1 = 0.832 P2 = 0.316; P3 = 0.375 |
| Ever breastfed No Yes | 6 (2) 403 (98) | 5 (2) 277 (98) | 1 (1) 70 (99) | 0 (0) 56 (100) | P0 = 0.836; P1 = 0.832 P2 = 0.316; P3 = 0.375 |
| Source of influence to breastfeed Self HCW Family | 72 (18) 266 (66) 64 (16) | 63 (23) 165 (60) 48 (17) | 8 (11) 52 (74) 10 (14) | 1 (2) 49 (89) 6 (11) | P0 ≤ 0.0001 *; P1 = 0.292 P2 = 0.089; P3 = 0.480 |
| Initiation of breastfeeding (colostrum) <1 h 2–3 h ≥4 h After 24 h | 287 (71) 79 (19) 28 (7) 12 (3) | 191 (68) 55 (20) 22 (8) 11 (4) | 50 (70) 16 (23) 5 (7) 0 (0) | 46 (82) 79 (19) 1 (2) 1 92) | P0 = 0.227; P1 = 0.292 P2 = 0.089; P3 = 0.480 |
| Mixed feeding No Yes | 142 (35) 261 (64) | 114 (41) 163 (59) | 15 (21) 55 (79) | 13 (23) 43 (77) | P0 = 0.001 *; P1 = 0.002 * P2 = 0.012 *; P3 = 0.811 |
| Stunting | Unadjusted PR (95% CI) | p | Adjusted PR (95% CI) | p |
|---|---|---|---|---|
| Child’s age (months) <12 12–24 >24 | 1 (ref) 1.20 (0.92–1.57) 0.32 (0.16–0.65) | 0.178 0.002 * | 1 (ref) 1.18 (0.90–1.55) 0.33 (0.16–0.65) | 0.239 0.001 * |
| Child’s sex Girls Boys | 1 (ref) 1.24 (0.97–1.58) | 0.083 | 1 (ref) 1.27 (1.00–1.61) | 0.049 * |
| DDS <4 ≥4 | 1 (ref) 0.71 (0.51–0.99) | 0.041 * | 1 (ref) 0.72 (0.52–0.99) | 0.041 * |
| House type Non-brick Brick | 1 (ref) 1.14 (0.90–1.45) | 0.227 | 1 (ref) 1.24 (0.98–1.57) | 0.069 |
| Underweight | Unadjusted PR (95% CI) | p | Adjusted (95%CI) | p |
| Child’s sex Girls Boys | 1 (ref) 1.96 (1.59–2.42) | ≤0.0001 * | 1 (ref) 2.40 (1.40–4.11) | 0.001 * |
| DDS <4 ≥4 | 1 (ref) 0.47 (0.22–1.00) | 0.050 * | 1 (ref) 0.43 (0.20- 0.92) | 0.029 * |
| Household head Self-headed Spouse Partner Parents Relatives | 1 (ref) 0.34 (0.15–0.79) 0.31 (0.12–0.81) 0.51 (0.26–1.00) 0.44 (0.13–1.47) | 0.012 * 0.017 * 0.051 0.182 | 1 (ref) 0.35 (0.15–0.78) 0.33 (0.13–0.87) 0.49 (0.24–0.99) 0.43 (0.13–1.71) | 0.011 * 0.024 * 0.046 * 0.254 |
| Thinness | Unadjusted PR (95% CI) | p | AOR (95%CI) | p |
| DDS <4 ≥4 | 1 (ref) 3.02 (1.29–7.12) | 0.011 * | 1 (ref) 2.70 (1.13–6.45) | 0.025 * |
| Child’s age <12 12–24 >24 | 1 (ref) 3.12 (1.17–8.28) 2.13 (0.69–6.59) | 0.023 * 0.189 | 1 (ref) 2.80 (1.02–7.64) 1.70 (0.56–5.18) | 0.045 * 0.352 |
| Overweight/obesity | Unadjusted PR (95% CI) | p | AOR (95%CI) | p |
| Child’s age <12 12–24 >24 | 1 (ref) 1.89 (1.18–3.01) 0.80 (0.36–1.76) | 0.008 * 0.576 | 1 (ref) 1.94 (1.25–3.03) 0.70 (0.32–1.70) | 0.003 * 0.479 |
| Household income <ZAR 5000 ZAR 5000–ZAR 10,000 ZAR 10,001–ZAR 15,000 >ZAR 15,000 | 1.24 (0.72–2.14) 1.02 (0.47–2.20) 3.24 (2.14–6.82) | 0.437 0.961 ≤0.0001 * | 1 (ref) 1.28 (0.74–2.20) 1.02 (0.47–2.21) 4.09 (2.33–7.17) | 0.378 0.958 ≤0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafhungo, T.; Cele, L.P.; Mathibe, M.; Modjadji, P. Nutritional Challenges Among Children Under Five in Limpopo Province, South Africa: Complementary Feeding Practices and Dietary Diversity Deficits. Nutrients 2025, 17, 1919. https://doi.org/10.3390/nu17111919
Mafhungo T, Cele LP, Mathibe M, Modjadji P. Nutritional Challenges Among Children Under Five in Limpopo Province, South Africa: Complementary Feeding Practices and Dietary Diversity Deficits. Nutrients. 2025; 17(11):1919. https://doi.org/10.3390/nu17111919
Chicago/Turabian StyleMafhungo, Tshilidzi, Lindiwe Priscilla Cele, Mmampedi Mathibe, and Perpetua Modjadji. 2025. "Nutritional Challenges Among Children Under Five in Limpopo Province, South Africa: Complementary Feeding Practices and Dietary Diversity Deficits" Nutrients 17, no. 11: 1919. https://doi.org/10.3390/nu17111919
APA StyleMafhungo, T., Cele, L. P., Mathibe, M., & Modjadji, P. (2025). Nutritional Challenges Among Children Under Five in Limpopo Province, South Africa: Complementary Feeding Practices and Dietary Diversity Deficits. Nutrients, 17(11), 1919. https://doi.org/10.3390/nu17111919






