Lactoferrin and SIgA Concentrations in Human Milk of SARS-CoV–Infected Mothers—Polish Cohort Study
Highlights
- SARS-CoV-2 infected mothers show reduced lactoferrin and SIgA in colostrum.
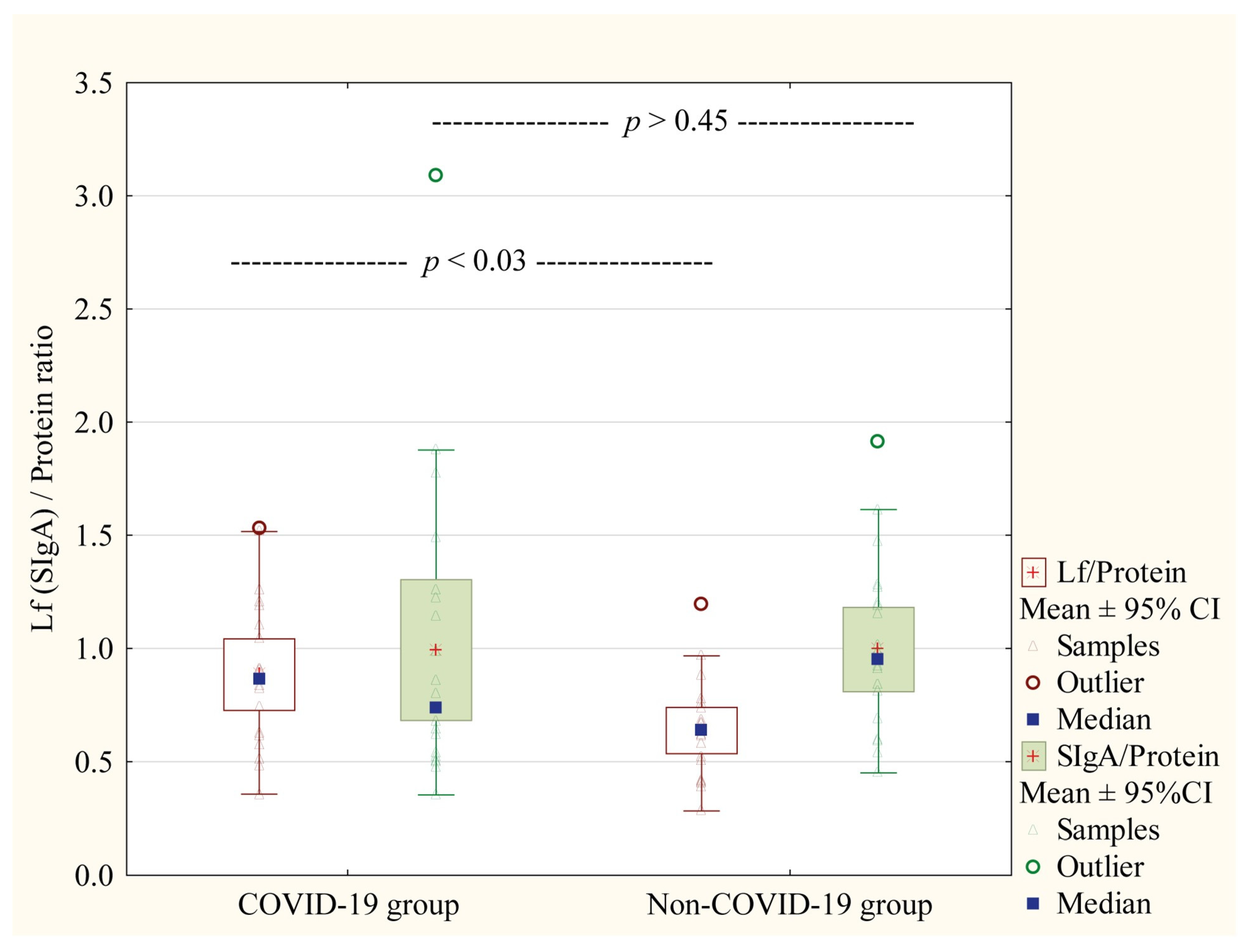
- They exhibit a compensatory increase in the Lf/protein ratio.
- Continued breastfeeding is crucial for infant immunity during maternal infection.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Characteristics of the Study Participants
2.3. Milk Collection and Sample Pre-Treatment for Analysis
2.4. Protein Concentration
2.5. Skim Milk Lactoferrin and SIgA Concentration
2.6. CRP Concentration
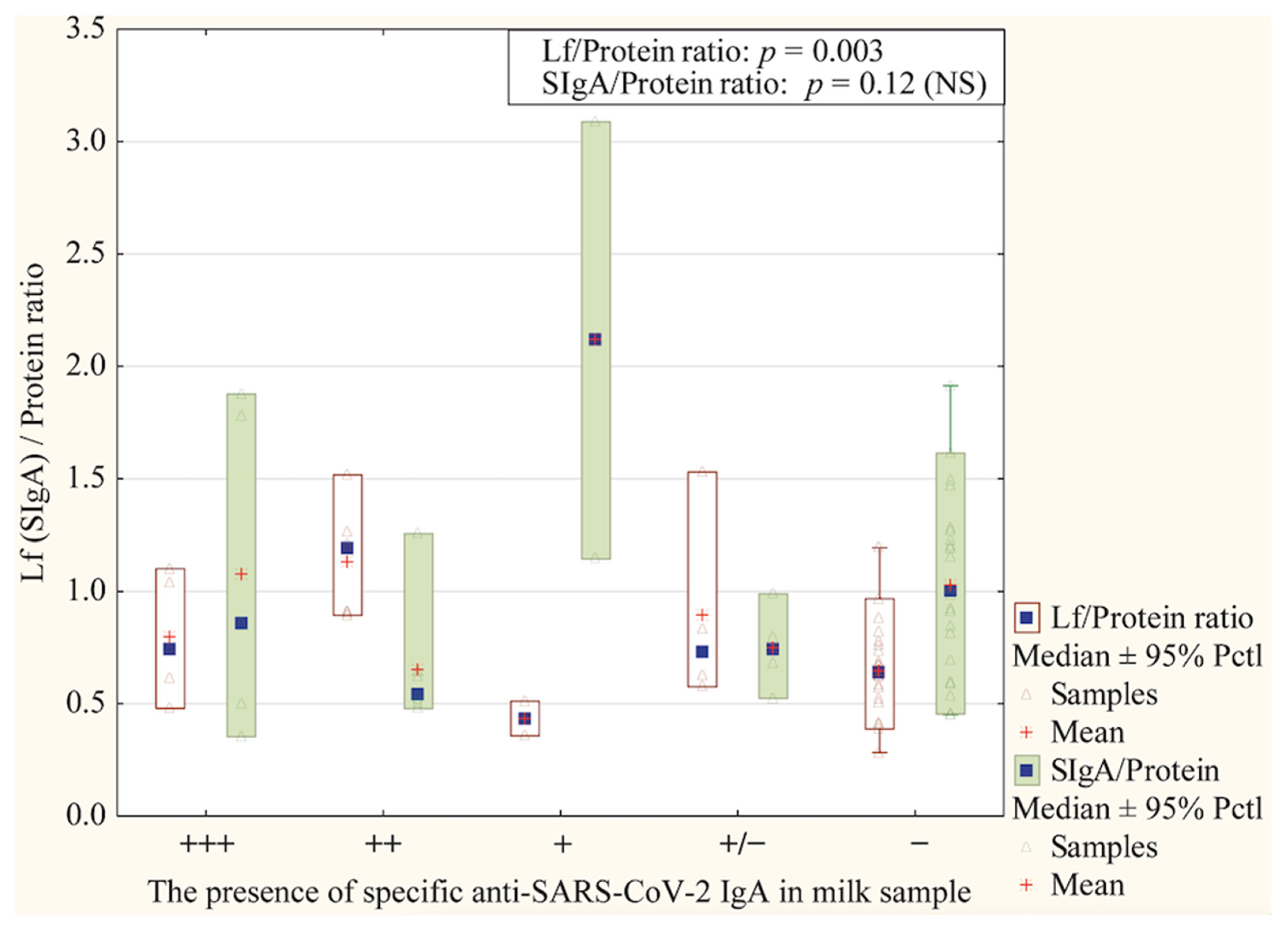
2.7. Anti-SARS-CoV-2 IgA Detection
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study and Control Group
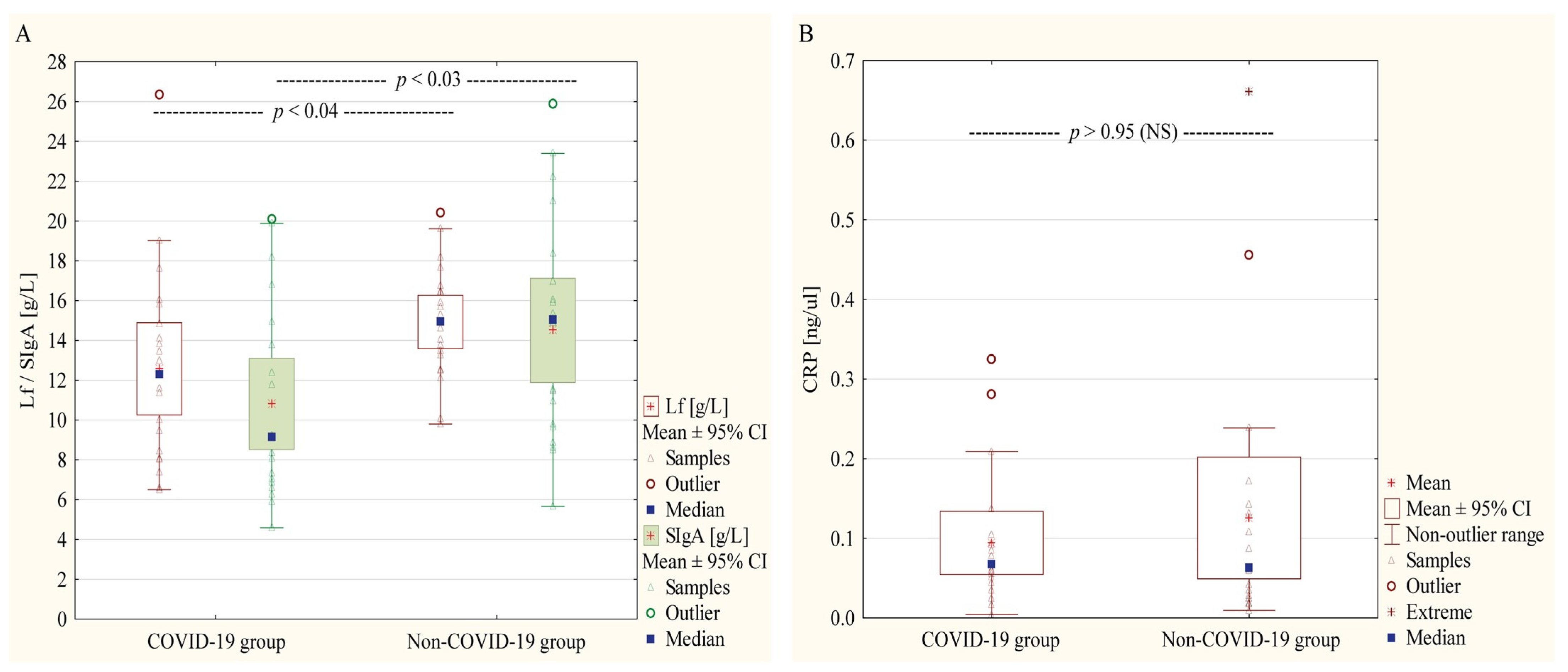
3.2. Proteins Concentrations in Skim Milk Samples
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quitadamo, P.A.; Comegna, L.; Cristalli, P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic from COVID-19. Front. Public Health 2020, 8, 589736. [Google Scholar] [CrossRef] [PubMed]
- Graciliano, N.G.; Goulart, M.O.F.; de Oliveira, A.C.M. Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk. Int. J. Mol. Sci. 2025, 26, 2600. [Google Scholar] [CrossRef] [PubMed]
- WHO. Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 18 April 2025).
- PITNUTS 2024—Kompleksowa Ocena Sposobu Żywienia Dzieci w Wieku od 5 do 72 Miesiąca Życia. Available online: https://imid.med.pl/pl/aktualnosci/nowe-wyniki-badania-pitnuts-2024-zdrowie-dzieci-w-polsce (accessed on 4 May 2025).
- Kumari, P.; Raval, A.; Rana, P.; Mahto, S.K. Regenerative Potential of Human Breast Milk: A Natural Reservoir of Nutrients, Bioactive Components and Stem cells. Stem Cell Rev. Rep. 2023, 19, 1307–1327. [Google Scholar] [CrossRef]
- Reniker, L.N.; Frazer, L.C.; Good, M. Key biologically active components of breast milk and their beneficial effects. Semin. Pediatr. Surg. 2023, 32, 151306. [Google Scholar] [CrossRef]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef]
- IFE Core Group. Infant and Young Child Feeding in Emergencies Operational Guidance. Available online: https://www.ennonline.net/operationalguidance-v3-2017 (accessed on 18 April 2025).
- Polish Ministry of Health. Zalecenia Dla Kobiet W Okresie Okołoporodowym W Związku Z Ogłoszonym Na Obszarze Rzeczypospolitej Polskiej Stanem Epidemii W Związku Z Zakażeniami Wirusem SARS-CoV-2. Available online: https://www.gov.pl/web/zdrowie/zalecenia-dla-kobiet-w-okresie-okoloporodowym-w-zwiazku-z-zakazeniami-sars-cov-2 (accessed on 18 April 2025).
- Centeno-Tablante, E.; Medina-Rivera, M.; Finkelstein, J.L.; Rayco-Solon, P.; Garcia-Casal, M.N.; Rogers, L.; Ghezzi-Kopel, K.; Ridwan, P.; Pena-Rosas, J.P.; Mehta, S. Transmission of SARS-CoV-2 through breast milk and breastfeeding: A living systematic review. Ann. N. Y. Acad. Sci. 2021, 1484, 32–54. [Google Scholar] [CrossRef]
- Dawod, B.; Marshall, J.S.; Azad, M.B. Breastfeeding and the developmental origins of mucosal immunity: How human milk shapes the innate and adaptive mucosal immune systems. Curr. Opin. Gastroenterol. 2021, 37, 547–556. [Google Scholar] [CrossRef]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodriguez-Lagunas, M.J.; Perez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef]
- Low, J.M.; Low, Y.W.; Zhong, Y.; Lee, C.Y.C.; Chan, M.; Ng, N.B.H.; Amin, Z.; Ng, Y.P.M. Titres and neutralising capacity of SARS-CoV-2-specific antibodies in human milk: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 174–180. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef]
- Czosnykowska-Lukacka, M.; Orczyk-Pawilowicz, M.; Broers, B.; Krolak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Apanasewicz, A.; Danel, D.P.; Babiszewska, M.; Piosek, M.; Orczyk-Pawilowicz, M. Maternal Distress and Social Support Are Linked to Human Milk Immune Properties. Nutrients 2021, 13, 1857. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef]
- Aparicio, M.; Browne, P.D.; Hechler, C.; Beijers, R.; Rodriguez, J.M.; de Weerth, C.; Fernandez, L. Human milk cortisol and immune factors over the first three postnatal months: Relations to maternal psychosocial distress. PLoS ONE 2020, 15, e0233554. [Google Scholar] [CrossRef]
- Juncker, H.G.; Naninck, E.F.G.; van Keulen, B.J.; Harinck, J.E.; Schipper, L.; Lucassen, P.J.; van Goudoever, J.B.; de Rooij, S.R.; Korosi, A. Maternal stress is associated with higher protein-bound amino acid concentrations in human milk. Front. Nutr. 2023, 10, 1165764. [Google Scholar] [CrossRef]
- Moirasgenti, M.; Doulougeri, K.; Panagopoulou, E.; Theodoridis, T. Psychological Stress Reduces the Immunological Benefits of Breast Milk. Stress Health 2019, 35, 681–685. [Google Scholar] [CrossRef]
- Polish Ministry of Health. Zalecenia Dotyczące Sposobu Postępowania w Przypadku Noworodków Matek Zakażonych lub z Podejrzeniem COVID-19. Available online: https://www.gov.pl/web/zdrowie/wytyczne-dla-poszczegolnych-zakresow-i-rodzajow-swiadczen (accessed on 18 April 2025).
- Lis-Kuberka, J.; Berghausen-Mazur, M.; Orczyk-Pawilowicz, M. Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers. Nutrients 2021, 13, 818. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Katnik-Prastowska, I.; Berghausen-Mazur, M.; Orczyk-Pawilowicz, M. Lectin-based analysis of fucosylated glycoproteins of human skim milk during 47 days of lactation. Glycoconj. J. 2015, 32, 665–674. [Google Scholar] [CrossRef]
- Czosnykowska-Lukacka, M.; Lis-Kuberka, J.; Krolak-Olejnik, B.; Orczyk-Pawilowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Ma, H.; Ding, C.; Yang, Y.; Sun, Y.; Huang, X.; He, W.; Xiang, Y.; Gao, Y.; Jin, T. Characterization of SARS-CoV-2-specific antibodies in COVID-19 patients reveals highly potent neutralizing IgA. Signal Transduct. Target. Ther. 2021, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef]
- Lackey, K.A.; Pace, R.M.; Williams, J.E.; Bode, L.; Donovan, S.M.; Jarvinen, K.M.; Seppo, A.E.; Raiten, D.J.; Meehan, C.L.; McGuire, M.A.; et al. SARS-CoV-2 and human milk: What is the evidence? Matern Child Nutr. 2020, 16, e13032. [Google Scholar] [CrossRef]
- Wesołowska, A.; Mołas, A.; Socha, M.W. Karmienie piersią i mlekiem matki a ryzyko infekcji wirusowej SARS-CoV-2 u dziecka w obliczu pandemii COVID-19. Postępy Neonatol. 2020, 1, 9–15. [Google Scholar]
- Giuliani, F.; Oros, D.; Gunier, R.B.; Deantoni, S.; Rauch, S.; Casale, R.; Nieto, R.; Bertino, E.; Rego, A.; Menis, C.; et al. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: Results from the INTERCOVID Multinational Cohort Study. Am. J. Obstet. Gynecol. 2022, 227, 488.e1–488.e17. [Google Scholar] [CrossRef]
- Mickleson, K.N.; Moriarty, K.M. Immunoglobulin levels in human colostrum and milk. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 381–384. [Google Scholar] [CrossRef]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lonnerdal, B.; et al. Concentration of Lactoferrin in Human Milk and Its Variation during Lactation in Different Chinese Populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef]
- Kulesza-Bronczyk, B.; Bien, A.; Sobieraj, P.; Orczyk-Pawilowicz, M.; Lis-Kuberka, J.; Czosnykowska-Lukacka, M.; Bzikowska-Jura, A. Factors affecting total protein and lactoferrin in human milk. Sci. Rep. 2023, 13, 22434. [Google Scholar] [CrossRef]
- Gawel, P.; Lukianowski, B.; Koscielska-Kasprzak, K.; Bartoszek, D.; Krajewska, M.; Krolak-Olejnik, B. Colostrum Lactoferrin Following Active and Recovered SARS-CoV-2 Infections during Pregnancy. Biomedicines 2024, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Espinosa-Martos, I.; Garcia-Carral, C.; Manzano, S.; McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; et al. What’s Normal? Immune Profiling of Human Milk from Healthy Women Living in Different Geographical and Socioeconomic Settings. Front. Immunol. 2017, 8, 696. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.; Davis, M.; Steele, K. Associations between human milk SIgA and maternal immune, infectious, endocrine, and stress variables. J. Hum. Lact. 2004, 20, 153–158; quiz 159–163. [Google Scholar] [CrossRef]
- Kawano, A.; Emori, Y. The relationship between maternal postpartum psychological state and breast milk secretory immunoglobulin A level. J. Am. Psychiatr. Nurses Assoc. 2015, 21, 23–30. [Google Scholar] [CrossRef]
- Skowron, K.; Lichocki, I.; Godziszewski, F.; Orczyk-Pawiłowicz, M. From Mind to Milk: The Influence of Psychological Factors on the Composition of Human Breast Milk. Nutrients 2025, 17, 1093. [Google Scholar] [CrossRef]
- Wesolowska, A.; Orczyk-Pawilowicz, M.; Bzikowska-Jura, A.; Gawronska, M.; Walczak, B. Protecting Breastfeeding during the COVID-19 Pandemic: A Scoping Review of Perinatal Care Recommendations in the Context of Maternal and Child Well-Being. Int. J. Environ. Res. Public Health 2022, 19, 3347. [Google Scholar] [CrossRef]
- Samuel, T.M.; Thielecke, F.; Lavalle, L.; Chen, C.; Fogel, P.; Giuffrida, F.; Dubascoux, S.; Martinez-Costa, C.; Haaland, K.; Marchini, G.; et al. Mode of Neonatal Delivery Influences the Nutrient Composition of Human Milk: Results From a Multicenter European Cohort of Lactating Women. Front. Nutr. 2022, 9, 834394. [Google Scholar] [CrossRef]
- Dizdar, E.A.; Sari, F.N.; Degirmencioglu, H.; Canpolat, F.E.; Oguz, S.S.; Uras, N.; Dilmen, U. Effect of mode of delivery on macronutrient content of breast milk. J. Matern Fetal Neonatal Med. 2014, 27, 1099–1102. [Google Scholar] [CrossRef]
- Villavicencio, A.; Rueda, M.S.; Turin, C.G.; Ochoa, T.J. Factors affecting lactoferrin concentration in human milk: How much do we know? Biochem. Cell Biol. 2017, 95, 12–21. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Mathijssen, G.; Dapra, C.; Do, D.M.; Medo, E. Active free secretory component and secretory IgA in human milk: Do maternal vaccination, allergy, infection, mode of delivery, nutrition and active lifestyle change their concentrations? Pediatr. Res. 2021, 89, 795–802. [Google Scholar] [CrossRef]
- Trofin, F.; Cianga, P.; Constantinescu, D.; Iancu, L.S.; Iancu, R.I.; Paduraru, D.; Nastase, E.V.; Buzila, E.R.; Lunca, C.; Cianga, C.M.; et al. The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection. Curr. Issues Mol. Biol. 2025, 47, 182. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Papadopoulou, A.; Syridou, G.; Marchisio, E.; Kapsabeli, E.; Daskalaki, A.; Papaevangelou, V. Early human milk lactoferrin during SARS-CoV-2 infection. J. Matern Fetal Neonatal Med. 2022, 35, 6704–6707. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.J.; Hartmann, P.E. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Peroni, D.G.; Fanos, V. Lactoferrin is an important factor when breastfeeding and COVID-19 are considered. Acta Paediatr. 2020, 109, 2139–2140. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Elavarasi, A.; Prasad, M.; Seth, T.; Sahoo, R.K.; Madan, K.; Nischal, N.; Soneja, M.; Sharma, A.; Maulik, S.K.; Shalimar; et al. Chloroquine and Hydroxychloroquine for the Treatment of COVID-19: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2020, 35, 3308–3314. [Google Scholar] [CrossRef]
- Mirabelli, C.; Wotring, J.W.; Zhang, C.J.; McCarty, S.M.; Fursmidt, R.; Pretto, C.D.; Qiao, Y.; Zhang, Y.; Frum, T.; Kadambi, N.S.; et al. Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105815118. [Google Scholar] [CrossRef]
- Brink, L.R.; Chichlowski, M.; Pastor, N.; Thimmasandra Narayanappa, A.; Shah, N. In the Age of Viral Pandemic, Can Ingredients Inspired by Human Milk and Infant Nutrition Be Repurposed to Support the Immune System? Nutrients 2021, 13, 870. [Google Scholar] [CrossRef]
- Bode, L.; Bertrand, K.; Najera, J.A.; Furst, A.; Honerkamp-Smith, G.; Shandling, A.D.; Chambers, C.D.; Camerini, D.; Campo, J.J. Characterization of SARS-CoV-2 antibodies in human milk from 21 women with confirmed COVID-19 infection. Pediatr. Res. 2023, 93, 1626–1633. [Google Scholar] [CrossRef]
- Szczygiol, P.; Lukianowski, B.; Koscielska-Kasprzak, K.; Jakuszko, K.; Bartoszek, D.; Krajewska, M.; Krolak-Olejnik, B. Antibodies in the breastmilk of COVID-19 recovered women. BMC Pregnancy Childbirth 2022, 22, 635. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Buhigas, I.; Rayo, N.; Silos, J.C.; Serrano, B.; Ocon-Hernandez, O.; Leung, B.W.; Delgado, J.L.; Fernandez, D.S.; Valle, S.; De Miguel, L.; et al. Anti-SARS-CoV-2-specific antibodies in human breast milk following SARS-CoV-2 infection during pregnancy: A prospective cohort study. Int. Breastfeed. J. 2024, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Pullen, K.M.; Atyeo, C.; Collier, A.Y.; Gray, K.J.; Belfort, M.B.; Lauffenburger, D.A.; Edlow, A.G.; Alter, G. Selective functional antibody transfer into the breastmilk after SARS-CoV-2 infection. Cell Rep. 2021, 37, 109959. [Google Scholar] [CrossRef]
- Sheehan, J.; Andres, A.; Yeruva, L.; Ramsay, A.J. SARS-CoV-2 infection induces human milk antibodies capable of mediating multiple functional activities. Clin. Nutr. Open Sci. 2024, 58, 215–226. [Google Scholar] [CrossRef]
- Hochmayr, C.; Winkler, I.; Hammerl, M.; Höller, A.; Huber, E.; Urbanek, M.; Kiechl-Kohlendorfer, U.; Griesmaier, E.; Posod, A.J.N. Factors Influencing Breast Milk Antibody Titers during the Coronavirus Disease 2019 Pandemic: An Observational Study. Nutrients 2024, 16, 2320. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, K.I.; Liu, S.; Yan, Z.; Xu, C.; Qiao, Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 18. [Google Scholar] [CrossRef]
- Abdullah, A.J.; Arif, A.T.; Rahman, H.A.; Sofihussein, K.Q.; Hadi, J.M.; Aziz, J.M.A.; Tofiq, S.S.; Mustafa, A.M. Assessing serum C-reactive protein as a predictor of COVID-19 outcomes: A retrospective cross-sectional study. Ann. Med. Surg. 2023, 85, 3359–3363. [Google Scholar] [CrossRef]
- Fetherston, C.M.; Wells, J.I.; Hartmann, P.E. Severity of mastitis symptoms as a predictor of C-reactive protein in milk and blood during lactation. Breastfeed. Med. 2006, 1, 127–135. [Google Scholar] [CrossRef]
- Landau-Crangle, E.; O’Connor, D.; Unger, S.; Hopperton, K.; Somerset, E.; Nir, H.; Hoban, R. Associations of maternal inflammatory states with human milk composition in mothers of preterm infants. Front. Nutr. 2023, 10, 1290690. [Google Scholar] [CrossRef]
- Whitaker, K.M.; Marino, R.C.; Haapala, J.L.; Foster, L.; Smith, K.D.; Teague, A.M.; Jacobs, D.R.; Fontaine, P.L.; McGovern, P.M.; Schoenfuss, T.C. Associations of maternal weight status before, during, and after pregnancy with inflammatory markers in breast milk. Obesity 2017, 25, 2092–2099. [Google Scholar] [CrossRef]
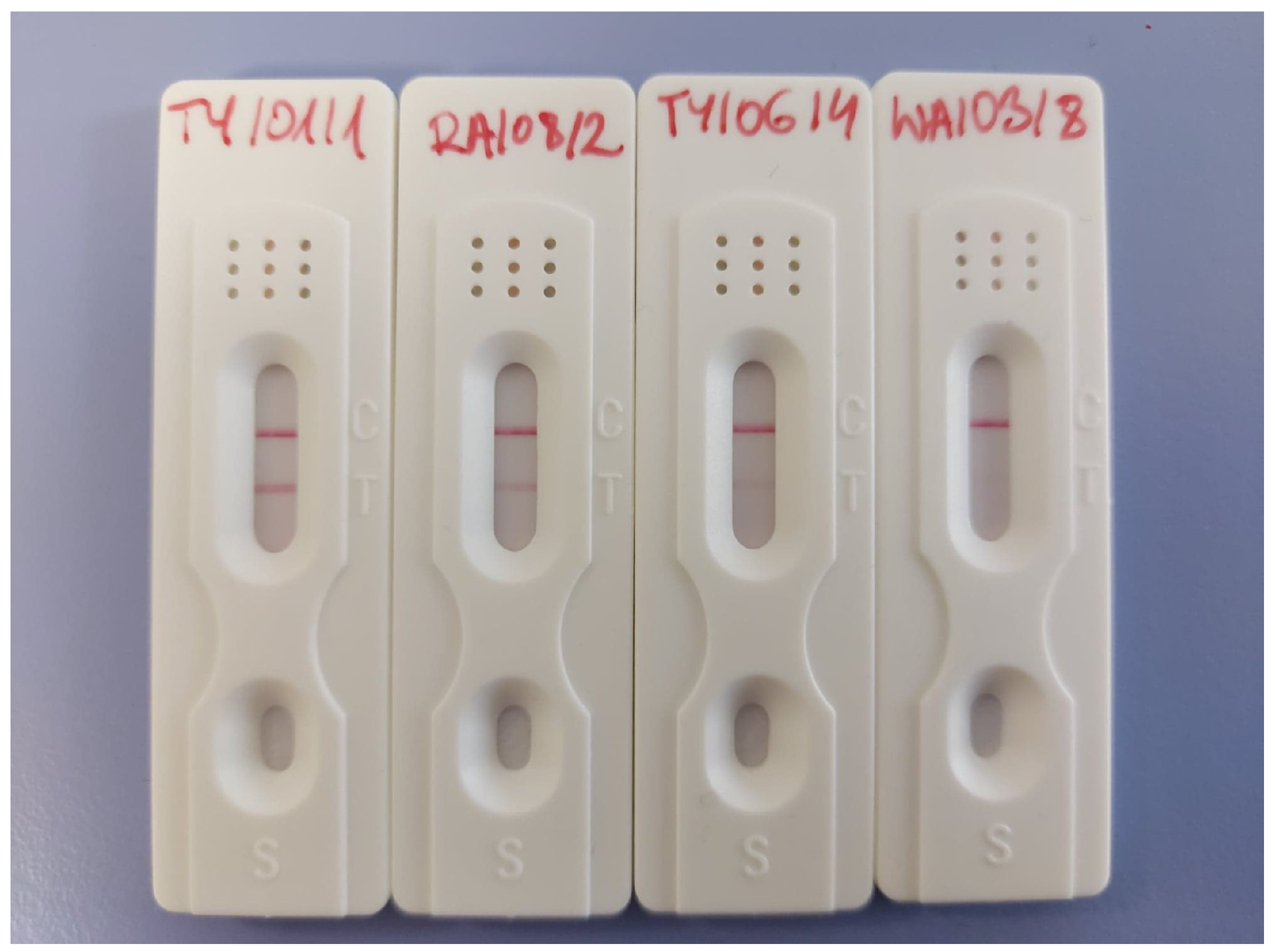


| (−) | No detection |
| (+/−) | Unknown |
| (+) | Low level |
| (++)/(+++) | Very noticeable |
| COVID-19-Positive Mothers N = 20 (% (n/N)) | COVID-19-Negative Mothers N = 20 (% (n/N)) | p-Value | |
|---|---|---|---|
Race/ethnicity
| 100% (20/20) | 100% (20/20) | NA |
| Maternal age (mean ± SD) | 30.95 ± 5.52 (median: 31) | 30.90 ± 3.75 (median: 31) | 0.75 (NS) |
| 35.0% (7/20) | 45.0% (9/20) | |
| 60.0% (12/20) | 55.0% (11/20) | |
| 5.0% (1/20) | 0.0% (0/20) | |
| Gestational age (mean ± SD) | 38.56 ± 1.46 (median: 39) | 40.35 ± 1.27 (median: 41) | 0.41 (NS) |
| 10.0% (2/20) | 5.0% (1/20) | |
| 80.0% (16/20) | 95.0% (19/20) | |
| 10.0% (2/20) | 0.0% (0/20) | |
| Delivery mode | 0.047 | ||
| 15.0% (3/20) | 0.0% (0/20) | |
| 75.0% (15/20) | 100.0% (20/20) | |
| 10.0% (2/20) | 0.0% (0/20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mołas, A.; Lis-Kuberka, J.; Bzikowska-Jura, A.; Wesołowska, A.; Jin, T.; Socha, M.W.; Orczyk-Pawiłowicz, M. Lactoferrin and SIgA Concentrations in Human Milk of SARS-CoV–Infected Mothers—Polish Cohort Study. Nutrients 2025, 17, 1840. https://doi.org/10.3390/nu17111840
Mołas A, Lis-Kuberka J, Bzikowska-Jura A, Wesołowska A, Jin T, Socha MW, Orczyk-Pawiłowicz M. Lactoferrin and SIgA Concentrations in Human Milk of SARS-CoV–Infected Mothers—Polish Cohort Study. Nutrients. 2025; 17(11):1840. https://doi.org/10.3390/nu17111840
Chicago/Turabian StyleMołas, Aleksandra, Jolanta Lis-Kuberka, Agnieszka Bzikowska-Jura, Aleksandra Wesołowska, Tengchuan Jin, Maciej W. Socha, and Magdalena Orczyk-Pawiłowicz. 2025. "Lactoferrin and SIgA Concentrations in Human Milk of SARS-CoV–Infected Mothers—Polish Cohort Study" Nutrients 17, no. 11: 1840. https://doi.org/10.3390/nu17111840
APA StyleMołas, A., Lis-Kuberka, J., Bzikowska-Jura, A., Wesołowska, A., Jin, T., Socha, M. W., & Orczyk-Pawiłowicz, M. (2025). Lactoferrin and SIgA Concentrations in Human Milk of SARS-CoV–Infected Mothers—Polish Cohort Study. Nutrients, 17(11), 1840. https://doi.org/10.3390/nu17111840







