Vitamin D Supplementation in Heart Failure—Confusion Without a Cause?
Highlights
- Vitamin D supplementation in patients with heart failure but without vitamin D deficiency had no consistent effects on primary endpoints such as mortality or HF-related hospitalization.
- While vitamin D deficiency is commonly observed in patients with heart failure, it is still a matter of research whether vitamin D is a marker of general health and a cardiovascular risk factor or whether it has a direct impact on cardiovascular health.
- Vitamin D supplementation may positively influence surrogate markers in selected patient groups, particularly those with reduced ejection fraction or severe vitamin D deficiency.
Abstract
1. Introduction
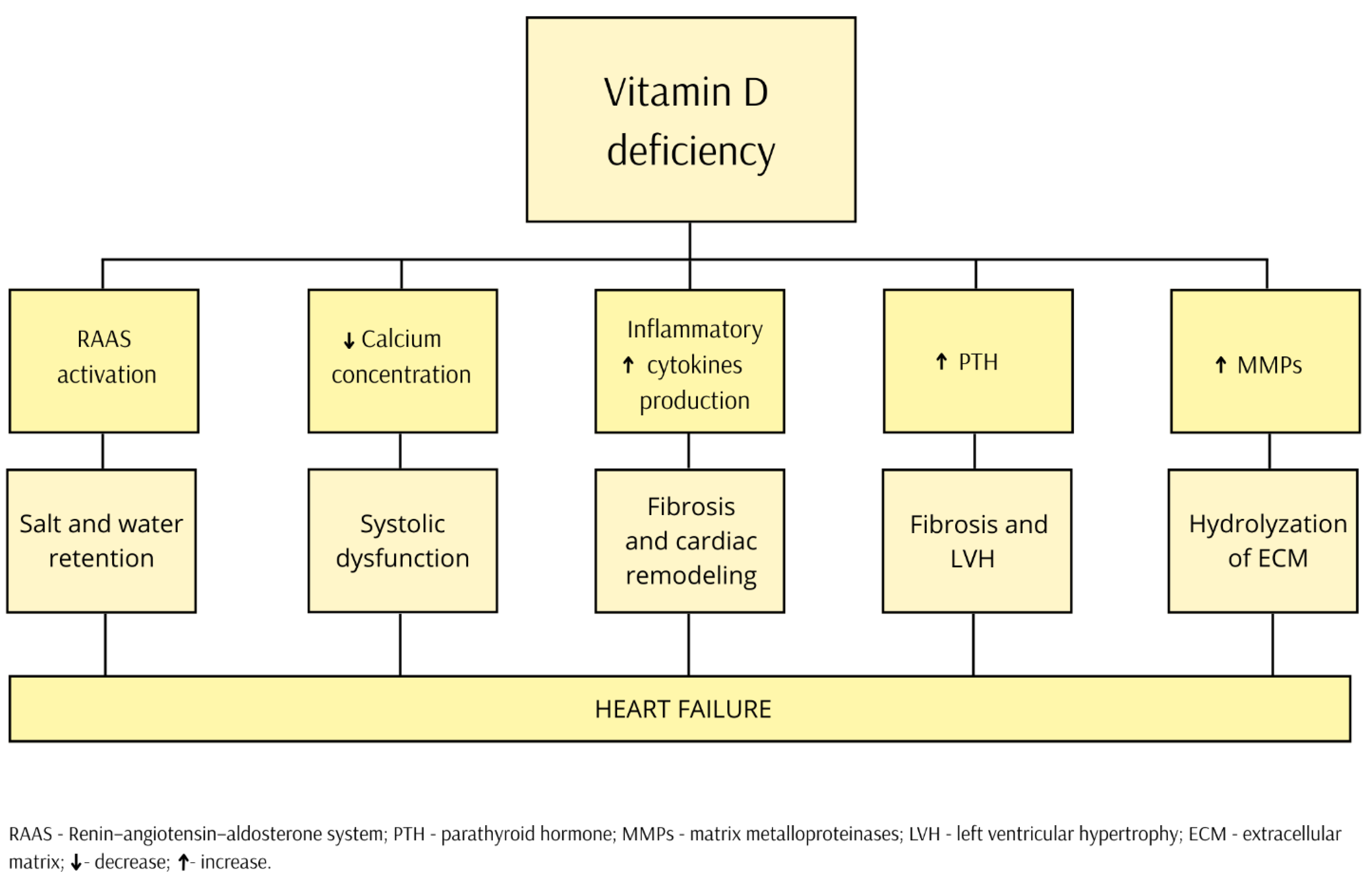
2. Pathophysiology
3. Vitamin D Deficiency
4. The Complicated Relationship
5. HFpEF
6. HFrEF
7. Chronic Kidney Disease
8. Metabolic Diseases
9. Sarcopenia
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D, calcitirol |
| 24,25(OH)2D | 24,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxyvitamin D, calcifediol |
| 6MWD | six-minute walking distance |
| BNP | B-type natriuretic peptide |
| CKD | chronic kidney disease |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| eGFR | estimated glomerular filtration rate |
| epi-25(OH)D | epimeric vitamin D3 |
| f25(OH)D | free fraction of 25-hydroxyvitamin D |
| HbA1c | glycated haemoglobin |
| HDL | high-density lipoprotein cholesterol |
| HF | heart failure |
| HFmrEF | heart failure with mildly reduced ejection fraction |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HT | hypertension |
| LDL | low-density lipoprotein cholesterol |
| LV | left ventricle |
| LVAD | left ventricular assist devices |
| LVEDD | left ventricular end-diastolic dimension |
| LVEF | left ventricular ejection fraction |
| LVESD | left ventricular end-systolic dimension |
| MACE | major adverse cardiovascular events |
| MMPs | metalloproteinases |
| MRAs | mineralocorticoid receptor antagonists |
| NO | nitric oxide |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| NYHA | New York Heart Association |
| PTH | parathyroid hormone |
| RAAs | renin-angiotensin-aldosterone system |
| SGLT2 | sodium/glucose cotransporter 2 |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| T25(OH)D | total of 25-hydroxyvitamin D metabolites |
| TC | total cholesterol |
| TG | triglycerides |
| VD | vitamin D |
| VDD | vitamin D deficiency |
| VDR | vitamin D receptor |
References
- Li, H.; Hastings, M.H.; Rhee, J.; Trager, L.E.; Roh, J.D.; Rosenzweig, A. Targeting Age-Related Pathways in Heart Failure. Circ. Res. 2020, 126, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Nila, S.A.; Ahmed, M.; Okello, D.O.; Maqbool, M.; Dabas, M.M.; Nour, M.; Khan, S.M.; Ansari, F.; Anum, N.; et al. Comparative Efficacy and Long-Term Outcomes of Beta-Blockers Alone or in Combination with Angiotensin-Converting Enzyme (ACE) Inhibitors in Chronic Heart Failure: A Systematic Review. Cureus 2024, 16, e74329. [Google Scholar] [CrossRef] [PubMed]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. Int. J. Heart Fail. 2023, 5, 82–90. [Google Scholar] [CrossRef]
- Golla, M.S.G.; Hajouli, S.; Ludhwani, D. Heart Failure and Ejection Fraction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553115/ (accessed on 22 April 2025).
- Iyngkaran, P.; Thomas, M.; Horowitz, J.D.; Komesaroff, P.; Jelinek, M.; Hare, D.L. Common Comorbidities that Alter Heart Failure Prognosis—Shaping New Thinking for Practice. Curr. Cardiol. Rev. 2021, 17, e160721187934. [Google Scholar] [CrossRef]
- Kommuri, N.V.; Koelling, T.M.; Hummel, S.L. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am. J. Med. 2012, 125, 209.e9–209.e15. [Google Scholar] [CrossRef]
- Crafa, A.; Cannarella, R.; Cannarella, V.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Retrospective real world study on vitamin D supplementation: Looking for the most effective molecule and its frequency of use. Clin. Nutr. 2025, 47, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Jin, Z.; Bertholf, R.L.; Yi, X. Advances and challenges in the measurement of 1,25-dihydroxyvitamin D: A comprehensive review. Crit. Rev. Clin. Lab. Sci. 2023, 60, 535–548. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schöttker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3894. [Google Scholar] [CrossRef]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Pérez-López, F.R.; López-Baena, M.T.; Pérez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24,25- and 25,26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry—A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2020, 1158, 122394. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 24 April 2025).
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Ng, C.Y.; Wang, D.; Wang, J.; Li, G.; Liu, T. Meta-analysis of Vitamin D Deficiency and Risk of Atrial Fibrillation. Clin. Cardiol. 2016, 39, 537–543. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Agrawal, D.K. Vitamin D deficiency and essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 885–901. [Google Scholar] [CrossRef]
- Pilz, S.; März, W.; Wellnitz, B.; Seelhorst, U.; Fahrleitner-Pammer, A.; Dimai, H.P.; Boehm, B.O.; Dobnig, H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J. Clin. Endocrinol. Metab. 2008, 93, 3927–3935. [Google Scholar] [CrossRef]
- Gunta, S.S.; Thadhani, R.I.; Mak, R.H. The effect of vitamin D status on risk factors for cardiovascular disease. Nat. Rev. Nephrol. 2013, 9, 337–347. [Google Scholar] [CrossRef]
- O’Riordan, J.L.; Bijvoet, O.L. Rickets before the discovery of vitamin D. Bonekey Rep. 2014, 3, 478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pike, J.W.; Meyer, M.B.; Lee, S.M. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef]
- Gardner, D.G.; Chen, S.; Glenn, D.J. Vitamin D and the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R969–R977. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Glenn, D.J.; Ni, W.; Grigsby, C.L.; Olsen, K.; Nishimoto, M.; Law, C.S.; Gardner, D.G. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension 2008, 52, 1106–1112. [Google Scholar] [CrossRef]
- Merke, J.; Milde, P.; Lewicka, S.; Hügel, U.; Klaus, G.; Mangelsdorf, D.J.; Haussler, M.R.; Rauterberg, E.W.; Ritz, E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J. Clin. Investig. 1989, 83, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Deng, C.; Wu, Y. Vitamin D-Parathyroid Hormone-Fibroblast Growth Factor 23 Axis and Cardiac Remodeling. Am. J. Cardiovasc. Drugs 2025, 25, 25–36. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Dermato-Endocrinology 2015, 6, e983401. [Google Scholar] [CrossRef]
- Hagău, A.C.; Pușcaș, A.; Togănel, R.; Muntean, I. Is Hypovitaminosis D a Risk Factor for Heart Failure? Life 2023, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Busa, V.; Dardeir, A.; Marudhai, S.; Patel, M.; Subas, S.V.; Ghani, M.R.; Cancarevic, I. Role of Vitamin D Supplementation in Heart Failure Patients with Vitamin D Deficiency and Its Effects on Clinical Outcomes: A Literature Review. Cureus 2020, 12, e10840. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef]
- Mohanty, V.; Pathania, M.; Bhasi, A. Effect of vitamin supplementation in patients of congestive heart failure deficient in vitamin D: A study at a tertiary care center of North India. Ann. Afr. Med. 2022, 21, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.A.T.; Dams, O.C.; Gorter, T.M.; van Veldhuisen, C.L.; Verdonk, R.C.; van Veldhuisen, D.J. Exocrine Pancreatic Insufficiency in Heart Failure: Clinical Features and Association with Cardiac Cachexia. JCSM Commun. 2024, 7, 117–128. [Google Scholar] [CrossRef]
- Vijver, M.A.T.; Bomer, N.; Verdonk, R.C.; van der Meer, P.; van Veldhuisen, D.J.; Dams, O.C. Micronutrient Deficiencies in Heart Failure and Relationship with Exocrine Pancreatic Insufficiency. Nutrients 2024, 17, 56. [Google Scholar] [CrossRef]
- Dantas-Komatsu, R.C.S.; Freire, F.L.A.; de Lira, N.R.D.; Diniz, R.V.Z.; Lima, S.C.V.C.; Pedrosa, L.F.C. Vitamin D status and predictors of 25-hydroxyvitamin D levels in patients with heart failure living in a sunny region. Nutr. Hosp. 2021, 38, 349–357. [Google Scholar] [CrossRef]
- de Oliveira, L.B.; de Figueiredo Martins Siqueira, M.A.; de Macedo Gadêlha, R.B.; Garcia, J.; Bandeira, F. Vitamin D Deficiency in Patients Hospitalized for Heart Failure Living in the Tropics. Int. J. Heart Fail. 2023, 6, 84–90. [Google Scholar] [CrossRef]
- Kamimura, D.; Yimer, W.K.; Shah, A.M.; Mentz, R.J.; Oshunbade, A.; Hamid, A.; Suzuki, T.; Clark, D.; Waller, J.; Fox, E.R.; et al. Vitamin D Levels in Black Americans and the Association with Left Ventricular Remodeling and Incident Heart Failure with Preserved Ejectin Fraction: The Jackson Heart Study. J. Card. Fail. 2023, 29, 150–157. [Google Scholar] [CrossRef]
- Khanolkar, S.; Hirani, S.; Mishra, A.; Vardhan, S.; Hirani, S.; Prasad, R.; Wanjari, M. Exploring the Role of Vitamin D in Atherosclerosis and Its Impact on Cardiovascular Events: A Comprehensive Review. Cureus 2023, 15, e42470. [Google Scholar] [CrossRef]
- Chunbin, W.; Han, W.; Lin, C. Efficacy of Vitamin D on Chronic Heart Failure Among Adults. Int. J. Vitam. Nutr. Res. 2020, 90, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Li, X.; Kong, M.; Ni, M.; Wei, D.; Zhu, X.; Wang, Y.; Hong, Z.; Dong, A. Associations Between Vitamin D Levels and Risk of Heart Failure: A Bidirectional Mendelian Randomization Study. Front. Nutr. 2022, 9, 910949. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; MacLennan, G.S.; Avenell, A.; Bolland, M.; Grey, A.; Witham, M. Cardiovascular disease and vitamin D supplementation: Trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 746–755. [Google Scholar] [CrossRef]
- Zhao, J.D.; Jia, J.J.; Dong, P.S.; Zhao, D.; Yang, X.-M.; Li, D.-L.; Zhang, H.-F. Effect of vitamin D on ventricular remodelling in heart failure: A meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef]
- Szabo, T.M.; Nagy, E.E.; Kirchmaier, Á.; Heidenhoffer, E.; Gábor-Kelemen, H.-L.; Frăsineanu, M.; Cseke, J.; Germán-Salló, M.; Frigy, A. Total 25-Hydroxyvitamin D Is an Independent Marker of Left Ventricular Ejection Fraction in Heart Failure with Reduced and Mildly Reduced Ejection Fraction. Biomolecules 2023, 13, 1578. [Google Scholar] [CrossRef]
- Sun, L.; Du, J. Magnesium status, serum vitamin D concentration and mortality among congestive heart failure patients: A cohort study from NHANES 2007–2018. Magnes. Res. 2024, 37, 61–75. [Google Scholar] [CrossRef]
- Herrmann, M.; Keppel, M.H.; Zelzer, S.; Alonso, N.; Cavalier, E.; Kleber, M.; Enko, D.; Scharnagl, H.; Pilz, S.; März, W. The role of functional vitamin D deficiency and low vitamin D reservoirs in relation to cardiovascular health and mortality. Clin. Chem. Lab. Med. 2024, 63, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Ojaroodi, A.F.; Jafarnezhad, F.; Eskandari, Z.; Keramat, S.; Stanek, A. Recent Updates and Advances in the Association Between Vitamin D Deficiency and Risk of Thrombotic Disease. Nutrients 2025, 17, 90. [Google Scholar] [CrossRef]
- Pirrotta, F.; Cavati, G.; Mingiano, C.; Merlotti, D.; Nuti, R.; Gennari, L.; Palazzuoli, A. Vitamin D Deficiency and Cardiovascular Mortality: Retrospective Analysis “Siena Osteoporosis” Cohort. Nutrients 2023, 15, 3303. [Google Scholar] [CrossRef]
- Long, B.; Robertson, J.; Koyfman, A.; Brady, W. Left ventricular assist devices and their complications: A review for emergency clinicians. Am. J. Emerg. Med. 2019, 37, 1562–1570. [Google Scholar] [CrossRef]
- Zittermann, A.; Pilz, S.; Morshuis, M.; Gummert, J.F.; Milting, H. Vitamin D deficiency and driveline infection in patients with a left ventricular assist device implant. Int. J. Artif. Organs 2023, 46, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Obeid, F.A.; Yost, G.; Bhat, G.; Drever, E.; Tatooles, A. Effect of Vitamin D Level on Clinical Outcomes in Patients Undergoing Left Ventricular Assist Device Implantation. Nutr. Clin. Pract. 2018, 33, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Djoussé, L.; Cook, N.R.; Kim, E.; Bodar, V.; Walter, J.; Bubes, V.; Luttmann-Gibson, H.; Mora, S.; Joseph, J.; Lee, I.-M.; et al. Supplementation with Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation 2020, 141, 784–786. [Google Scholar] [CrossRef]
- Golla, M.S.G.; Shams, P. Heart Failure with Preserved Ejection Fraction (HFpEF). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599960/ (accessed on 22 April 2025).
- Nolte, K.; Herrmann-Lingen, C.; Platschek, L.; Holzendorf, V.; Pilz, S.; Tomaschitz, A.; Düngen, H.; Angermann, C.E.; Hasenfuß, G.; Pieske, B.; et al. Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. ESC Heart Fail. 2019, 6, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Shiue, I.; Bergeå af Geijerstam, P.; Sundström, J.; Ärnlöv, J.; Larsson, A.; Melhus, H.; Lind, L.; Ingelsson, E. Relations of circulating vitamin D concentrations with left ventricular geometry and function. Eur. J. Heart Fail. 2012, 14, 985–991. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Snijder, M.B.; Visser, M.; Hurk, K.v.D.; Kamp, O.; Dekker, J.; Nijpels, G.; Stehouwer, C.; Henry, R.; Paulus, W.; et al. Vitamin D in relation to myocardial structure and function after eight years of follow-up: The Hoorn study. Ann. Nutr. Metab. 2012, 60, 69–77. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Wu, M.; Xu, K.; Wu, Y.; Lin, L. Role of Vitamin D in Patients with Heart Failure with Reduced Ejection Fraction. Am. J. Cardiovasc. Drugs 2019, 19, 541–552. [Google Scholar] [CrossRef]
- Vervloet, M.G.; Hsu, S.; de Boer, I.H. Vitamin D supplementation in people with chronic kidney disease. Kidney Int. 2023, 104, 698–706. [Google Scholar] [CrossRef]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; Defilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Zannad, F.; Butler, J.; Filippatos, G.; Pocock, S.J.; Brueckmann, M.; Steubl, D.; Schueler, E.; Anker, S.D.; Packer, M. Association of Empagliflozin Treatment with Albuminuria Levels in Patients with Heart Failure: A Secondary Analysis of EMPEROR-Pooled. JAMA Cardiol. 2022, 7, 1148–1159. [Google Scholar] [CrossRef]
- Reimer, K.C.; Nadal, J.; Meiselbach, H.; Schmid, M.; Schultheiss, U.T.; Kotsis, F.; Stockmann, H.; Friedrich, N.; Nauck, M.; Krane, V.; et al. Association of mineral and bone biomarkers with adverse cardiovascular outcomes and mortality in the German Chronic Kidney Disease (GCKD) cohort. Bone Res. 2023, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Zelnick, L.R.; Bansal, N.; Brown, J.; Denburg, M.; Feldman, H.I.; Ginsberg, C.; Hoofnagle, A.N.; Isakova, T.; Leonard, M.B.; et al. Vitamin D Metabolites and Risk of Cardiovascular Disease in Chronic Kidney Disease: The CRIC Study. J. Am. Heart Assoc. 2023, 12, e028561. [Google Scholar] [CrossRef]
- Arroyo, E.; Leber, C.A.; Burney, H.N.; Li, Y.; Li, X.; Lu, T.-S.; Jones, G.; Kaufmann, M.; Ting, S.M.S.; Hiemstra, T.F.; et al. Epimeric vitamin D and cardiovascular structure and function in advanced CKD and after kidney transplantation. Nephrol. Dial. Transplant. 2024, 39, 264–276. [Google Scholar] [CrossRef]
- Cheng, J.H.; Hoofnagle, A.N.; Katz, R.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H.; Ginsberg, C. Serum free 25(OH)D concentrations and cardiovascular disease, heart failure, kidney function decline, and fracture: The health, aging, and body composition study. JBMR Plus 2025, 9, ziaf001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhen, Y.; Wang, Z.; Qi, L.; Li, Y.; Ren, L.; Chen, S. The Relationship Between Vitamin D Deficiency and Glycated Hemoglobin Levels in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 3899–3907. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Wan, Z.; Geng, T.; Zhu, K.; Li, R.; Lu, Q.; Lin, X.; Liu, S.; Ou, Y.; et al. Vitamin D and heart failure risk among individuals with type 2 diabetes: Observational and Mendelian randomization studies. Am. J. Clin. Nutr. 2024, 120, 491–498. [Google Scholar] [CrossRef]
- Tougaard, N.H.; Hansen, T.W.; Rossing, P. Vitamin D deficiency and development of complications in individuals with type 1 and type 2 diabetes: A cohort study. J. Diabetes Complicat. 2023, 37, 108611. [Google Scholar] [CrossRef]
- Vasdeki, D.; Tsamos, G.; Dimakakos, E.; Patriarcheas, V.; Koufakis, T.; Kotsa, K.; Cholewka, A.; Stanek, A. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients 2024, 16, 3651. [Google Scholar] [CrossRef]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, F.; Fonarow, G.C.; Sreenivasan, J.; Greene, S.J.; Khan, S.U.; Usman, M.S.; Vaduganathan, M.; Fudim, M.; Anker, S.D.; et al. Dietary interventions and nutritional supplements for heart failure: A systematic appraisal and evidence map. Eur. J. Heart Fail. 2021, 23, 1468–1476. [Google Scholar] [CrossRef]
- Chandrashekhar Iyer, L.; Vaishali, K.; Babu, A.S. Prevalence of sarcopenia in heart failure: A systematic review. Indian Heart J. 2023, 75, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Duan, J.; Liu, W.; Huang, K.; Chen, Z.; Yang, C.; Yang, L. The Role of Sarcopenia in Heart Failure with Depression. Rev. Cardiovasc. Med. 2022, 23, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, M.; Lei, Y.; Tian, J.; Zhang, L.; Xu, D. Sarcopenia Predicts Adverse Prognosis in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2023, 24, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, W. Vitamin D and Sarcopenia in the Senior People: A Review of Mechanisms and Comprehensive Prevention and Treatment Strategies. Ther. Clin. Risk Manag. 2024, 20, 577–595. [Google Scholar] [CrossRef]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Collamati, A.; Marzetti, E.; Calvani, R.; Tosato, M.; D’Angelo, E.; Sisto, A.N.; Lando, F. Sarcopenia in heart failure: Mechanisms and therapeutic strategies. J. Geriatr. Cardiol. 2016, 13, 615–624. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study. Nutrients 2023, 15, 2703. [Google Scholar] [CrossRef]
- Nagaoka, R.; Katano, S.; Yano, T.; Numazawa, R.; Yamano, K.; Fujisawa, Y.; Honma, S.; Kamoda, T.; Sato, K.; Kouzu, H.; et al. Optimal serum 25-hydroxyvitamin D level to prevent sarcopenia in patients with heart failure: Insights from a dose-response relationship. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 606–617. [Google Scholar] [CrossRef]
- Hung, M.; Birmingham, W.C.; Ocampo, M.; Mohajeri, A. The Role of Vitamin D in Cardiovascular Diseases. Nutrients 2023, 15, 3547. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampka, Z.; Czapla, D.; Wojakowski, W.; Stanek, A. Vitamin D Supplementation in Heart Failure—Confusion Without a Cause? Nutrients 2025, 17, 1839. https://doi.org/10.3390/nu17111839
Kampka Z, Czapla D, Wojakowski W, Stanek A. Vitamin D Supplementation in Heart Failure—Confusion Without a Cause? Nutrients. 2025; 17(11):1839. https://doi.org/10.3390/nu17111839
Chicago/Turabian StyleKampka, Zofia, Dominika Czapla, Wojciech Wojakowski, and Agata Stanek. 2025. "Vitamin D Supplementation in Heart Failure—Confusion Without a Cause?" Nutrients 17, no. 11: 1839. https://doi.org/10.3390/nu17111839
APA StyleKampka, Z., Czapla, D., Wojakowski, W., & Stanek, A. (2025). Vitamin D Supplementation in Heart Failure—Confusion Without a Cause? Nutrients, 17(11), 1839. https://doi.org/10.3390/nu17111839








