Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems
Abstract
1. Introduction
2. Caseins’ Structure
2.1. Casein Micelles
2.2. Functional Properties
3. Bioactive Peptides Derived from Caseins
3.1. Production of CDPs
3.2. Antibacterial Activities of CDPs
In Silico Prediction and Peptidomic Characterization of CDAMPs
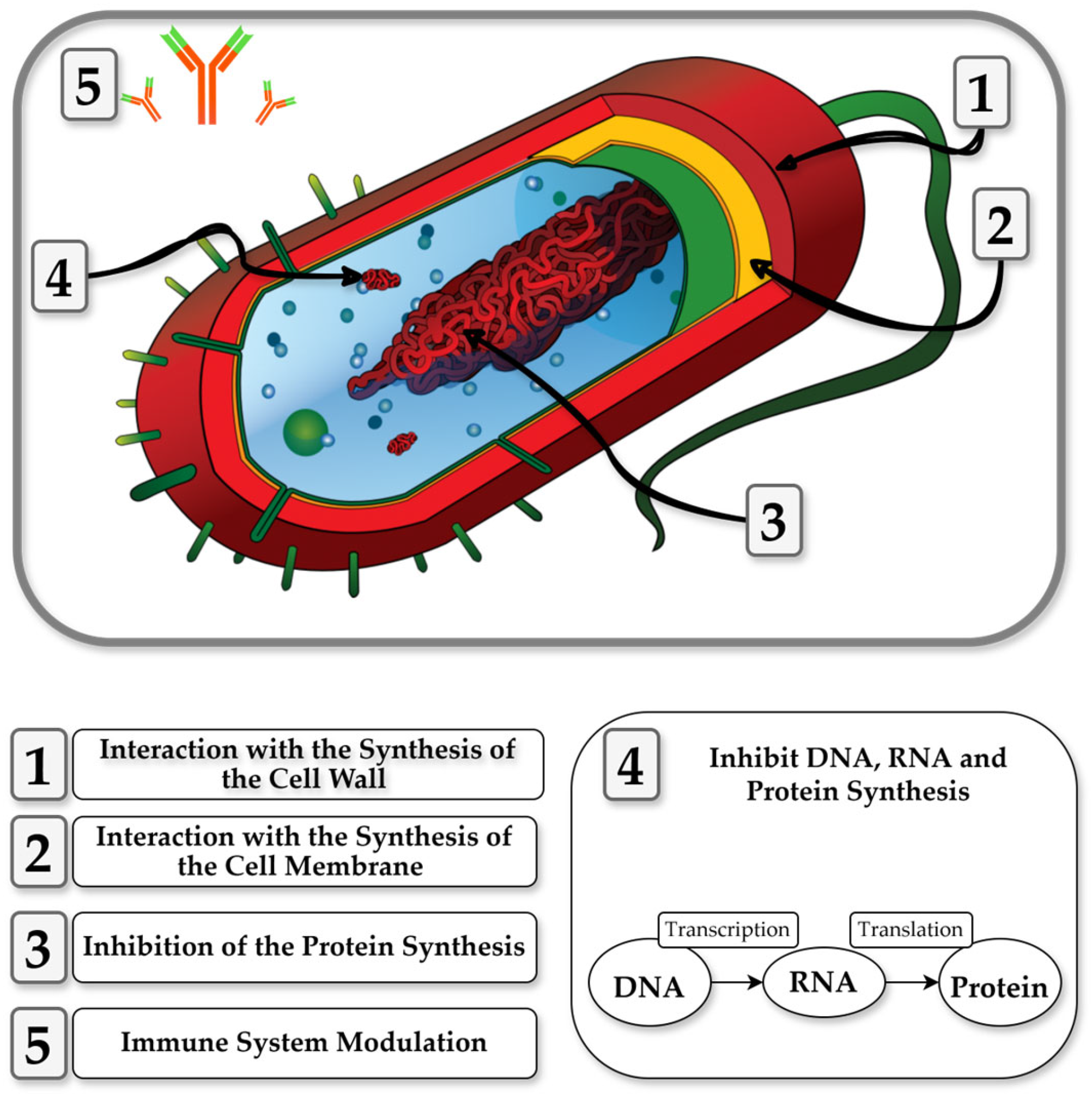
3.3. Mechanisms of Action of CDPs
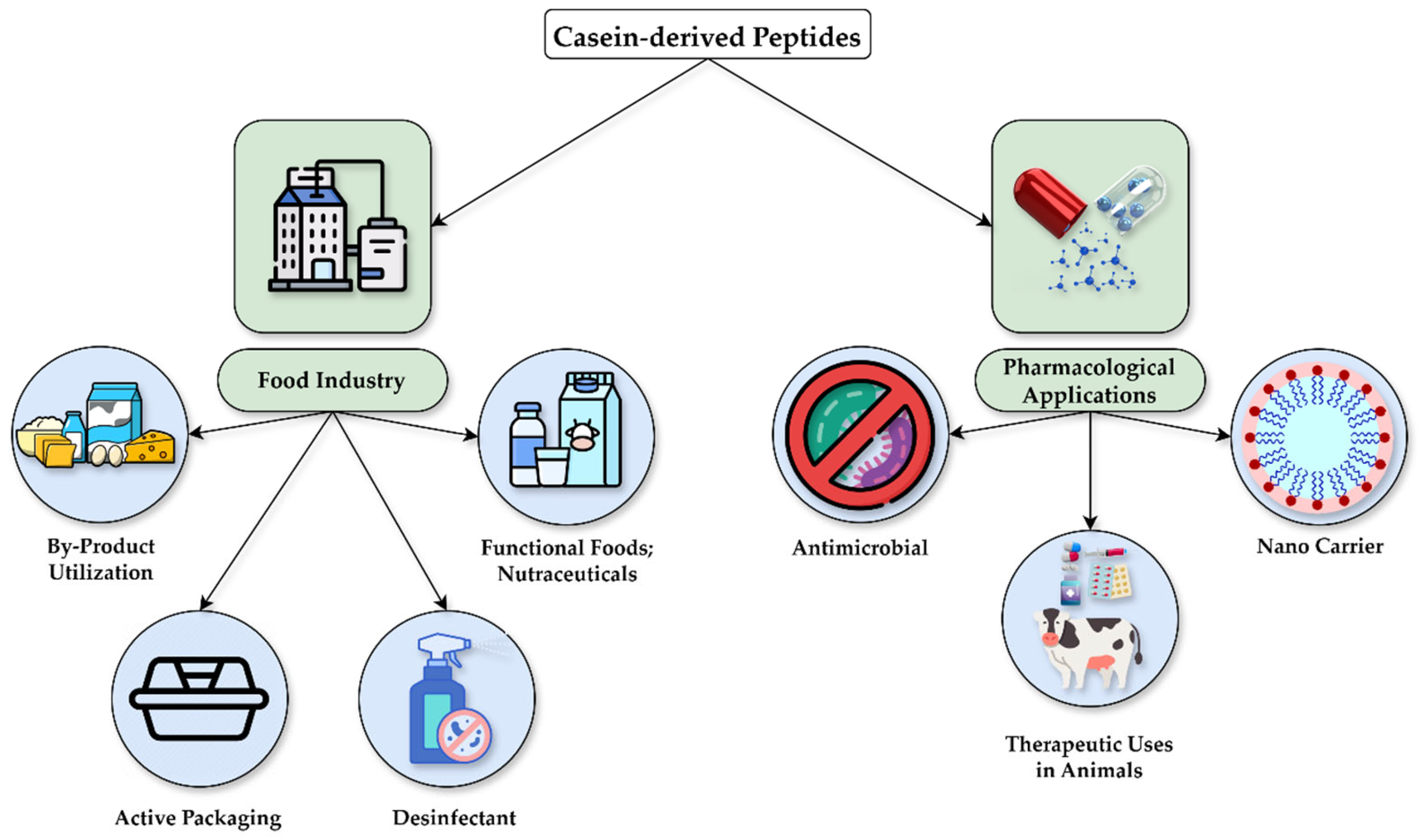
4. Practical Applications of CDPs
4.1. Application in the Food Industry
4.1.1. Food Preservation and Safety
4.1.2. Food Packaging
4.1.3. Functional Foods
4.2. Pharmacological Applications
4.3. Isolation, Stability, and Delivery Systems
4.4. Safety and Regulatory Aspects of Casein-Derived Bioactive Peptides
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Runthala, A.; Mbye, M.; Ayyash, M.; Xu, Y.; Kamal-Eldin, A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules 2023, 28, 2023. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Layman, D.K. Dairy bioactive proteins and peptides: A narrative review. Nutr. Rev. 2021, 79, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Pre-sleep casein protein ingestion: New paradigm in post-exercise recovery nutrition. Phys. Act. Nutr. 2020, 24, 6–10. [Google Scholar] [CrossRef]
- Leng, J.; Jiang, Y.; Zhou, T.; Zhang, S.; Zhu, C.; Wang, B.; Li, L.; Zhao, W. Unveiling the slow digestion and peptide profiles of polymerised whey gel via heat and TGase crosslinking: An in vitro/vivo perspective. Food Chem. 2025, 464, 141829. [Google Scholar] [CrossRef]
- Jiménez-Barrios, P.; Sánchez-Rivera, L.; Martínez-Maqueda, D.; Gouar, Y.L.; Dupont, D.; Miralles, B.; Recio, I. Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model. Nutrients 2023, 15, 1065. [Google Scholar] [CrossRef]
- Treweek, T. Alpha-Casein as a Molecular Chaperone. In Milk Protein; Walter, L., Hurley, W.L., Eds.; InTech: Rijeka, Croatia, 2012; pp. 85–118. [Google Scholar] [CrossRef][Green Version]
- Gebhardt, R.; Hohn, C.; Asaduzzaman, M. Stabilizing interactions of casein microparticles after a thermal post-treatment. Food Chem. 2024, 450, 139369. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Cao, S.; Wang, H.; Jiang, C.; Hussain, M.A.; Pang, S. Broad-Spectrum antimicrobial activity and low cytotoxicity against human cells of a peptide derived from bovine αS1-casein. Molecules 2018, 23, 1220. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, D.P.; Solanki, M.K.; Singh, P.; Srivastva, A.K.; Kumar, S.; Kashyap, P.L.; Saxena, A.K.; Singhal, P.K.; Arora, D.K. Optimization of media components for chitinase production by chickpea rhizosphere associated Lysinibacillus fusiformis B-CM18. J. Basic Microbiol. 2013, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Liyanaarachchi, W.S.; Chandrapala, J.; Dissanayake, M.; Vasiljevic, T. Utilizing unique properties of caseins and the casein micelle for delivery of sensitive food ingredients and bioactives. Trends Food Sci. Technol. 2016, 57, 178–187. [Google Scholar] [CrossRef]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; Mahoonak, A.S. Health Implications of Bioactive Peptides: A Review. Int. J. Vitam. Nutr. Res. 2018, 88, 319–340. [Google Scholar] [CrossRef]
- Petrova, S.Y.; Khlgatian, S.V.; Emelyanova, O.Y.; Pishulina, L.A.; Berzhets, V.M. Structure and biological functions of milk caseins. Russ. Open Med. J. 2022, 11, e0209. [Google Scholar] [CrossRef]
- Singh, A.; Duche, R.T.; Wandhare, A.G.; Sian, J.K.; Singh, B.P.; Sihag, M.K.; Singh, K.S.; Sangwan, V.; Talan, S.; Panwar, H. Milk-Derived Antimicrobial Peptides: Overview, Applications, and Future Perspectives. Probiotics Antimicrob. Proteins 2022, 15, 44–62. [Google Scholar] [CrossRef]
- Foroutan, A.; Guo, A.C.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Holt, C. Casein and casein micelle structures, functions and diversity in 20 species. Int. Dairy J. 2016, 60, 2–13. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. The caseins: Structure, stability, and functionality. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–92. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein Micelle Structure, Functions and Interactions. In Advanced Dairy Chemistry—1 Proteins, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer Science + Business Media: New York, NY, USA, 2003; pp. 233–276. [Google Scholar] [CrossRef]
- Kalyankar, S.D.; Khedkar, C.D.; Patil, A.M.; Deosarkar, S.S. Milk: Sources and Composition. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 741–747. [Google Scholar] [CrossRef]
- The UniProt Consortium, UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Moller, T.L.; Nielsen, S.B.; Corredig, M. Novel details on the dissociation of casein micelle suspensions as a function of pH and temperature. J. Dairy Sci. 2023, 106, 8368–8374. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-casein: Isolation, properties and functionality. A review. Int. Dairy J. 2016, 66, 115–125. [Google Scholar] [CrossRef]
- Fitzgerald, R.J.; Murray, B.A. Bioactive Peptides and Lactic Fermentations. Int. J. Dairy Technol. 2006, 59, 118–125. [Google Scholar] [CrossRef]
- McCarthy, R.; Mills, S.; Ross, R.; Fitzgerald, G.; Stanton, C. Bioactive Peptides from Casein and Whey Proteins. In Milk and Dairy Products as Functional Foods; Kanekanian, A., Ed.; Wiley Blackwell: Oxford, UK, 2014; pp. 23–54. [Google Scholar] [CrossRef]
- Nielsen, S.D.H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.J.; Dallas, D.C. Bioactive milk peptides: An updated comprehensive overview and database. Crit. Rev. Food Sci. Nutr. 2023, 64, 11510–11529. [Google Scholar] [CrossRef] [PubMed]
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, T.; Zhang, X.; Luo, J.; An, P.; Luo, Y. Effect of casein hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4207. [Google Scholar] [CrossRef]
- De Vasconcelos, M.L.; Oliveira, L.M.F.S.; Hill, J.P.; Vidal, A.M.C. Difficulties in Establishing the Adverse Effects of β-Casomorphin-7 Released from β-Casein Variants—A Review. Foods 2023, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Yu, J.; Shao, S. Advances in the application and mechanism of bioactive peptides in the treatment of inflammation. Front. Immunol. 2024, 15, 1413179. [Google Scholar] [CrossRef]
- Meisel, H. Biochemical properties of peptides encrypted in bovine milk proteins. Curr. Med. Chem. 2005, 12, 1905–1919. [Google Scholar] [CrossRef]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takano, T. Antihypertensive peptides derived from milk proteins. Food/Nahrung 1999, 43, 159–164. [Google Scholar] [CrossRef]
- Pellegrini, A. Antimicrobial Peptides from Food Proteins. Curr. Pharm. Des. 2003, 9, 1225–1238. [Google Scholar] [CrossRef]
- McCann, K.; Shiell, B.; Michalski, W.; Lee, A.; Wan, J.; Roginski, H.; Coventry, M. Isolation and characterisation of antibacterial peptides derived from the f(164-207) region of bovine αS2-casein. Int. Dairy J. 2005, 15, 133–143. [Google Scholar] [CrossRef]
- Benoit, S.; Chaumontet, C.; Violle, N.; Boulier, A.; Hafeez, Z.; Cakir-Kiefer, C.; Tomé, D.; Schwarz, J.; Miclo, L. The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve. Nutrients 2022, 14, 2212. [Google Scholar] [CrossRef] [PubMed]
- Freret, T.; Largilliere, S.; Née, G.; Coolzaet, M.; Corvaisier, S.; Boulouard, M. Fast Anxiolytic-Like Effect Observed in the Rat Conditioned Defensive Burying Test, after a Single Oral Dose of Natural Protein Extract Products. Nutrients 2021, 13, 2445. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Antioxidative and antibacterial peptides derived from bovine milk proteins. Crit. Rev. Food Sci. Nutr. 2016, 58, 726–740. [Google Scholar] [CrossRef]
- Abril, A.G.; Pazos, M.; Villa, T.G.; Calo-Mata, P.; Barros-Velázquez, J.; Carrera, M. Proteomics Characterization of Food-Derived Bioactive Peptides with Anti-Allergic and Anti-Inflammatory Properties. Nutrients 2022, 14, 4400. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.W.; Sunwoo, H. Anti-inflammatory and antioxidant properties of peptides released from β-lactoglobulin by high hydrostatic pressure-assisted enzymatic hydrolysis. Molecules 2017, 22, 949. [Google Scholar] [CrossRef]
- Liu, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of peptides exhibiting biological activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef]
- Santos, I.; Silva, M.; Grácio, M.; Pedroso, L.; Lima, A. Milk Antiviral Proteins and Derived Peptides against Zoonoses. Int. J. Mol. Sci. 2024, 25, 1842. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Buradaleng, S.; Wattanachant, S.; Benjakul, S.; Tani, A.; Maneerat, S. Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control 2011, 22, 401–407. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini–review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2020, 108, 27–39. [Google Scholar] [CrossRef]
- Dong, X.; Li, J.; Jiang, G.; Li, H.; Zhao, M.; Jiang, Y. Effects of combined high pressure and enzymatic treatments on physicochemical and antioxidant properties of peanut proteins. Food Sci. Nutr. 2019, 7, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2015, 197, 273–284. [Google Scholar] [CrossRef]
- Miclo, L.; Roux, É.; Genay, M.; Brusseaux, É.; Poirson, C.; Jameh, N.; Perrin, C.; Dary, A. Variability of Hydrolysis of β-, αs1- and αs2-Caseins by 10 Strains of Streptococcus thermophilus and Resulting Bioactive Peptides. J. Agric. Food Chem. 2011, 60, 554–565. [Google Scholar] [CrossRef]
- Sforza, S.; Cavatorta, V.; Lambertini, F.; Galaverna, G.; Dossena, A.; Marchelli, R. Cheese peptidomics: A detailed study on the evolution of the oligopeptide fraction in Parmigiano-Reggiano cheese from curd to 24 months of aging. J. Dairy Sci. 2012, 95, 3514–3526. [Google Scholar] [CrossRef]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Identification of antioxidant peptides in cheddar cheese made with adjunct culture Lactobacillus casei ssp. casei 300. Milchwissenschaft 2010, 65, 396–399. Available online: https://www.cabdirect.org/cabdirect/abstract/20103340704 (accessed on 18 February 2025).
- Toelstede, S.; Hofmann, T. Sensomics mapping and identification of the key bitter metabolites in gouda cheese. J. Agric. Food Chem. 2008, 56, 2795–2804. [Google Scholar] [CrossRef]
- Combes, C.; Paterson, E.; Amadò, R. Isolation and Identification of Low-Molecular-Weight Peptides from Emmentaler Cheese. J. Food Sci. 2002, 67, 553–559. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Toldrá, F. New approaches based on comparative proteomics for the assessment of food quality. Curr. Opin. Food Sci. 2018, 22, 22–27. [Google Scholar] [CrossRef]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; Van Berkel, L.A.; Van Berkel, P.H.C.; Pauwels, E.K.J.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef]
- Brück, W.M.; Kelleher, L.; Gibson, G.R.; Nielsen, E.K.; Chatterton, E.W.D.; Lönnerdal, B. rRNA Probes Used to Quantify the Effects of Glycomacropeptide and-Lactalbumin Supplementation on the Predominant Groups of Intestinal Bacteria of Infant Rhesus Monkeys Challenged with Enteropathogenic Escherichia coli. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 273–280. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wang, F.; You, L.; Cao, Y.; Tang, R.; Wen, J.; Cui, X. A novel endogenous antimicrobial peptide CAMP211-225 derived from casein in human milk. Food Funct. 2020, 11, 2291–2298. [Google Scholar] [CrossRef]
- Liu, Y.; Eichler, J.; Pischetsrieder, M. Virtual screening of a milk peptide database for the identification of food-derived antimicrobial peptides. Mol. Nutr. Food Res. 2015, 59, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-Derived Antimicrobial Peptides Generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.; Guinane, C.; O’Connor, P.; Fitzgerald, G.; Hill, C.; Stanton, C.; Ross, R. Production of the antimicrobial peptides Caseicin A and B by Bacillus isolates growing on sodium caseinate. Lett. Appl. Microbiol. 2012, 55, 141–148. [Google Scholar] [CrossRef]
- Norberg, S.; O’Connor, P.M.; Stanton, C.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Cotter, P.D. Extensive manipulation of caseicins A and B highlights the tolerance of these antimicrobial peptides to change. Appl. Environ. Microbiol. 2012, 78, 2353–2358. [Google Scholar] [CrossRef]
- McClean, S.; Beggs, L.B.; Welch, R.W. Antimicrobial activity of antihypertensive food-derived peptides and selected alanine analogues. Food Chem. 2013, 146, 443–447. [Google Scholar] [CrossRef]
- Lahov, E.; Regelson, W. Antibacterial and immunostimulating casein-derived substances from milk: Casecidin, isracidin peptides. Food Chem. Toxicol. 1996, 34, 131–145. [Google Scholar] [CrossRef]
- Birkemo, G.; O’Sullivan, O.; Ross, R.; Hill, C. Antimicrobial activity of two peptides casecidin 15 and 17, found naturally in bovine colostrum. J. Appl. Microbiol. 2008, 106, 233–240. [Google Scholar] [CrossRef]
- Rizzello, C.; Losito, I.; Gobbetti, M.; Carbonara, T.; De Bari, M.; Zambonin, P. Antibacterial Activities of Peptides from the Water-Soluble Extracts of Italian Cheese Varieties. J. Dairy Sci. 2005, 88, 2348–2360. [Google Scholar] [CrossRef]
- McCann, K.; Shiell, B.; Michalski, W.; Lee, A.; Wan, J.; Roginski, H.; Coventry, M. Isolation and characterisation of a novel antibacterial peptide from bovine αS1-casein. Int. Dairy J. 2005, 16, 316–323. [Google Scholar] [CrossRef]
- Tang, W.; Yuan, H.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. An antimicrobial peptide screened from casein hydrolyzate by Saccharomyces cerevisiae cell membrane affinity method. Food Control 2014, 50, 413–422. [Google Scholar] [CrossRef]
- Elbarbary, H.A.; Abdou, A.M.; Nakamura, Y.; Park, E.Y.; Mohamed, H.A.; Sato, K. Identification of novel antibacterial peptides isolated from a commercially available casein hydrolysate by autofocusing technique. BioFactors 2012, 38, 309–315. [Google Scholar] [CrossRef]
- López-Expósito, I.; Gómez-Ruiz, J.Á.; Amigo, L.; Recio, I. Identification of antibacterial peptides from ovine αs2-casein. Int. Dairy J. 2006, 16, 1072–1080. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Begley, M.; Clifford, T.; Deasy, T.; Considine, K.; Hill, C. Structure-Activity Relationship of Synthetic Variants of the Milk-Derived Antimicrobial Peptide α s2 -Casein f(183–207). Appl. Environ. Microbiol. 2013, 79, 5179–5185. [Google Scholar] [CrossRef] [PubMed]
- Recio, I.; Visser, S. Identification of two distinct antibacterial domains within the sequence of bovine αs2-casein. Biochim. Biophys. Acta Gen. Subj. 1999, 1428, 314–326. [Google Scholar] [CrossRef]
- López-Expósito, I.; Amigo, L.; Recio, I. Identification of the initial binding sites of αs2-casein f(183–207) and effect on bacterial membranes and cell morphology. Biochim. Biophys. Acta Biomembr. 2008, 1778, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Pellegrini, A.; Amigo, L.; Recio, I. Synergistic effect between different Milk-Derived peptides and proteins. Int. Dairy J. 2008, 91, 2184–2189. [Google Scholar] [CrossRef]
- Sistla, S. Structure–activity relationships of αs-casein peptides with multifunctional biological activities. Mol. Cell. Biochem. 2013, 384, 29–38. [Google Scholar] [CrossRef]
- Zucht, H.; Raida, M.; Adermann, K.; Mägert, H.; Forssmann, W. Casocidin-I: A casein-αs2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995, 372, 185–188. [Google Scholar] [CrossRef]
- Bougherra, F.; Dilmi-Bouras, A.; Balti, R.; Przybylski, R.; Adoui, F.; Elhameur, H.; Chevalier, M.; Flahaut, C.; Dhulster, P.; Naima, N. Antibacterial activity of new peptide from bovine casein hydrolyzed by a serine metalloprotease of Lactococcus lactis subsp lactis BR16. J. Funct. Foods 2017, 32, 112–122. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Hou, C.; Song, S. Inhibitory effect of milk-derived peptide αS2-casein151-181 against spore-forming bacteria. Int. Dairy J. 2020, 104, 104651. [Google Scholar] [CrossRef]
- Ouertani, A.; Chaabouni, I.; Mosbah, A.; Long, J.; Barakat, M.; Mansuelle, P.; Mghirbi, O.; Najjari, A.; Ouzari, H.; Masmoudi, A.S.; et al. Two New Secreted Proteases Generate a Casein-Derived Antimicrobial Peptide in Bacillus cereus Food Born Isolate Leading to Bacterial Competition in Milk. Front. Microbiol. 2018, 9, 1148. [Google Scholar] [CrossRef]
- Sedaghati, M.; Ezzatpanah, H.; Boojar, M.M.A.; Ebrahimi, M.T.; Kobarfard, F. Isolation and identification of some antibacterial peptides in the plasmin-digest of β-casein. LWT 2015, 68, 217–225. [Google Scholar] [CrossRef]
- López-Expósito, I.; Minervini, F.; Amigo, L.; Recio, I. Identification of Antibacterial Peptides from Bovine κ-Casein. J. Food Prot. 2006, 69, 2992–2997. [Google Scholar] [CrossRef]
- Pellegrini, A.; Dettling, C.; Thomas, U.; Hunziker, P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta Gen. Subj. 2001, 1526, 131–140. [Google Scholar] [CrossRef]
- Sedaghati, M.; Ezzatpanah, H.; Boojar, M.M.; Ebrahimi, M.T.; Aminafshar, M. Plasmin-digest of β-lactoglobulin with antibacterial properties. Food Agric. Immunol. 2014, 26, 218–230. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Gauthier, S.F.; Britten, M.; Fliss, I.; Robitaille, G.; Jean, J. Antibacterial activity of peptides extracted from tryptic hydrolyzate of whey protein by nanofiltration. Int. Dairy J. 2012, 28, 94–101. [Google Scholar] [CrossRef]
- Théolier, J.; Hammami, R.; Labelle, P.; Fliss, I.; Jean, J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods 2013, 5, 706–714. [Google Scholar] [CrossRef]
- Liu, V.; Dashper, S.; Parashos, P.; Liu, S.; Stanton, D.; Shen, P.; Chivatxaranukul, P.; Reynolds, E. Antibacterial efficacy of casein-derived peptides against Enterococcus faecalis. Aust. Dent. J. 2012, 57, 339–343. [Google Scholar] [CrossRef]
- Malkoski, M.; Dashper, S.G.; O’Brien-Simpson, N.M.; Talbo, G.H.; Macris, M.; Cross, K.J.; Reynolds, E.C. Kappacin, a Novel Antibacterial Peptide from Bovine Milk. Antimicrob. Agents Chemother. 2001, 45, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, M.; Ezzatpanah, H.; Boojar, M.M.A.; Ebrahimi, M.T.; Aminafshar, M. Plasmin digest of κ-casein as a source of antibacterial peptides. J. Dairy Res. 2014, 81, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, G.; Lapointe, C.; Leclerc, D.; Britten, M. Effect of pepsin-treated bovine and goat caseinomacropeptide on Escherichia coli and Lactobacillus rhamnosus in acidic conditions. J. Dairy Sci. 2011, 95, 1–8. [Google Scholar] [CrossRef]
- Popa, G.L.; Papa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 15, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Gambushe, S.M.; Zishiri, O.T.; Zowalaty, M.E. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef]
- Romano, A.; Carrella, S.; Rezza, S.; Nia, Y.; Hennekinne, J.; Bianchi, D.; Martucci, F.; Zuccon, F.; Gulino, M.; Mari, C.; et al. First report of food poisoning due to staphylococcal enterotoxin type B in döner kebab (Italy). Pathogens 2023, 12, 1139. [Google Scholar] [CrossRef]
- Yang, K.; Shi, Y.; Li, Y.; Wei, G.; Zhao, Q.; Huang, A. iTRAQ-based quantitative proteomic analysis of antibacterial mechanism of milk-derived peptide BCp12 against Escherichia coli. Foods 2022, 11, 672. [Google Scholar] [CrossRef]
- Qi, S.; Zhao, S.; Zhang, H.; Liu, S.; Liu, J.; Yang, J.; Qi, Y.; Zhao, Q.; Jin, Y.; Wang, F. Novel casein antimicrobial peptides for the inhibition of oral pathogenic bacteria. Food Chem. 2023, 425, 136454. [Google Scholar] [CrossRef]
- Sansi, M.S.; Iram, D.; Zanab, S.; Vij, S.; Puniya, A.K.; Singh, A.; Ashutosh; Meena, S. Antimicrobial bioactive peptides from goat Milk proteins: In silico prediction and analysis. J. Food Biochem. 2022, 46, 14311. [Google Scholar] [CrossRef]
- Tomazou, M.; Oulas, A.; Anagnostopoulos, A.K.; Tsangaris, G.T.; Spyrou, G.M. In Silico Identification of Antimicrobial Peptides in the Proteomes of Goat and Sheep Milk and Feta Cheese. Proteomes 2019, 7, 32. [Google Scholar] [CrossRef]
- Tu, M.; Liu, H.; Zhang, R.; Chen, H.; Fan, F.; Shi, P.; Xu, X.; Lu, W.; Du, M. Bioactive hydrolysates from casein: Generation, identification, and in silico toxicity and allergenicity prediction of peptides. J. Sci. Food Agric. 2017, 98, 3416–3426. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.A.F.; Evangelista, A.G.; Nazareth, T.M.; Luciano, F.B. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Acta Mater. 2019, 8, 100494. [Google Scholar] [CrossRef]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive peptides derived from whey proteins for health and functional beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Łojewska, E.; Sakowicz, T. An alternative to antibiotics: Selected methods to combat zoonotic foodborne bacterial infections. Curr. Microbiol. 2021, 78, 4037–4049. [Google Scholar] [CrossRef]
- Liu, Z.; Brady, A.; Young, A.; Rasimick, B.; Chen, K.; Zhou, C.; Kallenbach, N. Length effects in antimicrobial peptides of the (RW)n series. Antimicrob. Agents Chemother. 2007, 51, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Liu, X.; Zhang, F.; Yan, L.; Chen, L.; Wang, X.; Ruan, H.; Ji, C.; Cui, X.; et al. Antimicrobial activity and mechanism of PDC213, an endogenous peptide from human milk. Biochem. Biophys. Res. Commun. 2017, 484, 132–137. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Suo, H.; Tan, J.; Zhang, Y.; Song, J. Identification and molecular mechanism of action of antibacterial peptides from Flavourzyme-hydrolyzed yak casein against Staphylococcus aureus. J. Dairy Sci. 2023, 106, 3779–3790. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial peptides—Mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Xu, Y.; Li, Y.; Yang, T.; Yu, X.; Yang, X.; Huang, A.; Yu, Y.; Shi, Y. Milk-derived antimicrobial peptide GMp7: Disrupting protein networks for multi-target antibacterial inhibition and enhanced dairy preservation. J. Dairy Sci. 2025, 108, 3428–3443. [Google Scholar] [CrossRef]
- Amiri, E.O.; Farmani, J.; Amiri, Z.R.; Dehestani, A.; Mohseni, M. Antimicrobial activity, environmental sensitivity, mechanism of action, and food application of αs165-181 peptide. Int. J. Food Microbiol. 2021, 358, 109403. [Google Scholar] [CrossRef]
- Mudgil, P.; AlMazroui, M.; Redha, A.A.; Kilari, B.P.; Srikumar, S.; Maqsood, S. Cow and camel milk-derived whey and casein protein hydrolysates demonstrated effective antifungal properties against selected Candida species. J. Dairy Sci. 2021, 105, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Ningsih, D.R.; Raharjo, T.J.; Haryadi, W.; Wikandari, R. Antifungal activity and identification of bioactive peptide from Etawa crossbreed goat (Capra hircus) milk protein hydrolyzed using trypsin enzyme. Arab. J. Chem. 2023, 16, 105249. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Y.; Kang, J.; Yao, Q.; Dong, A.; Liu, Y. Reuse of waste casein peptides to capture Cu (II) for long-term antibacterial reutilization. Colloid Interface Sci. Commun. 2024, 60, 100781. [Google Scholar] [CrossRef]
- Blondelle, S.E.; Lohner, K. Combinatorial libraries: A tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 2000, 55, 74–87. [Google Scholar] [CrossRef]
- Meisel, H. Bioactive peptides from milk proteins: A perspective for consumers and producers. Aust. J. Dairy Technol. 2001, 56, 83–92. [Google Scholar]
- Milk Protein Market Size, Share & Trends Analysis Report by Product (Concentrates, Isolates, Hydrolyzed), by Form (Powder, Liquid), by Application (Food & Beverages, Dietary Supplements), by Region, and Segment Forecasts, 2025–2030. 2025. Available online: https://www.grandviewresearch.com/industry-analysis/milk-protein-market-report (accessed on 20 February 2025).
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef]
- Hammam, A.R.A.; Martínez-Monteagudo, S.I.; Metzger, L.E. Progress in micellar casein concentrate: Production and applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4426–4449. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, H.; Tian, J.; Shu, C.; Sun, R.; Li, B.; Meng, X. Protective effects of α-casein or β-casein on the stability and antioxidant capacity of blueberry anthocyanins and their interaction mechanism. Lebensm. Wiss. Technol. 2019, 115, 108434. [Google Scholar] [CrossRef]
- El-Salam, M.H.A.; El-Shibiny, S. Preparation and potential applications of casein–polysaccharide conjugates: A review. J. Sci. Food Agric. 2019, 100, 1852–1859. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. Antimicrobial Peptides of Dairy Proteins: From Fundamental to Applications. Food Rev. Int. 2014, 30, 134–154. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Paul, M. Enzymatic fragmentation of the antimicrobial peptides casocidin and isracidin by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. Appl. Microbiol. Biotechnol. 2010, 87, 235–242. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Wang, X.; Huang, A. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. J. Dairy Sci. 2020, 103, 11116–11128. [Google Scholar] [CrossRef]
- Ribeiro, J.; Santos, M.; Silva, L.; Pereira, L.; Santos, I.; Da Silva Lannes, S.; Da Silva, M. Antioxidant and antimicrobial peptides derived from casein: Current trends in food preservation. Food Res. Int. 2019, 121, 204–211. [Google Scholar]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Keyata, E.O. Potentials of Natural preservatives to Enhance food Safety and shelf life: A review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; Da Silva Lannes, S.C.; Da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2018, 148, 181–188. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gómez Oliván, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive peptides: A promising alternative to chemical preservatives for food preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef]
- Murray, N.M.; Jacquier, J.C.; O’Sullivan, M.; Hallihan, A.; Murphy, E.; Feeney, E.L.; O´Riordan, D. Using rejection thresholds to determine acceptability of novel bioactive compounds added to milk-based beverages. Food Qual. Prefer. 2019, 73, 276–283. [Google Scholar] [CrossRef]
- Guinane, C.M.; Kent, R.M.; Norberg, S.; O’Connor, P.M.; Cotter, P.D.; Hill, C.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Generation of the antimicrobial peptide caseicin A from casein by hydrolysis with thermolysin enzymes. Int. Dairy J. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Zhang, F.; Zheng, K.; Liu, Y. Milk-derived antimicrobial peptides incorporated whey protein film as active coating to improve microbial stability of refrigerated soft cheese. Int. J. Food Microbiol. 2024, 419, 110751. [Google Scholar] [CrossRef] [PubMed]
- Rydlo, T.; Miltz, J.; Mor, A. Eukaryotic antimicrobial peptides: Promises and premises in food safety. J. Food Sci. 2006, 71, R125–R135. [Google Scholar] [CrossRef]
- Gogliettino, M.; Balestrieri, M.; Ambrosio, R.L.; Anastasio, A.; Smaldone, G.; Proroga, Y.T.R.; Moretta, R.; Rea, I.; De Stefano, L.; Agrillo, B.; et al. Extending the Shelf-Life of meat and dairy products via PET-Modified packaging activated with the antimicrobial peptide MTP1. Front. Microbiol. 2020, 10, 2963. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings—A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Santos, J.C.; Sousa, R.C.; Otoni, C.G.; Moraes, A.R.; Souza, V.G.; Medeiros, E.A.; Espitia, P.J.; Pires, A.C.; Coimbra, J.S.; Soares, N.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Khan, M.R.; Volpe, S.; Valentino, M.; Miele, N.A.; Cavella, S.; Torrieri, E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings 2021, 11, 899. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Takano, T. Antihypertensive Effect of Sour Milk and Peptides Isolated from It That are Inhibitors to Angiotensin I-Converting Enzyme. J. Dairy Sci. 1995, 78, 1253–1257. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J. Dairy Sci. 2000, 83, 1434–4140. [Google Scholar] [CrossRef]
- Gagnaire, V.; Mollé, D.; Herrouin, M.; Léonil, J. Peptides identified during Emmental cheese ripening: Origin and proteolytic systems involved. J. Agric. Food Chem. 2001, 49, 4402–4413. [Google Scholar] [CrossRef]
- Gobbetti, M.; Morea, M.; Baruzzi, F.; Corbo, M.R.; Matarante, A.; Considine, T.; Di Cagno, R.; Guineee, T.; Fox, P.F. 23 Microbiological, compositional, biochemical and textural characterisation of Caciocavallo Pugliese cheese during ripening. Int. Dairy J. 2002, 12, 511–523. [Google Scholar] [CrossRef]
- Meisel, H.; Bockelmann, W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropo-functional properties. Antonie Van Leeuwenhoek 1999, 76, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.; Goepfert, A.; Gunther, S. ACE inhibitory activities in milk products. Milchwissenschaft 1997, 52, 307–311. [Google Scholar]
- Rajanna, D.; Pushpadass, H.A.; Emerald, F.M.E.; Padaki, N.V.; Nath, B.S. Nanoencapsulation of CDP within electrospun nanofibres. J. Sci. Food Agric. 2022, 102, 1684–1698. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Silva, S.V.; Malcata, F.X. Partial Identification of Water-Soluble Peptides Released at Early Stages of Proteolysis in Sterilized Ovine Cheese-Like Systems: Influence of Type of Coagulant and Starter. J. Dairy Sci. 2005, 88, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Guo, T.; Li, W.; Chen, J.; Li, F.; Wang, C.; Shi, Y.; Li, D.X.; Zhang, S. Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Sci. Hum. Wellness 2019, 8, 156–176. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Batista, P.; Gomes, J.E.G.; da Silva, R.; Pintado, M.M. Screening of Novel Bioactive Peptides from Goat Casein: In Silico to In Vitro Validation. Int. J. Mol. Sci. 2022, 23, 2439. [Google Scholar] [CrossRef]
- Tu, M.; Qiao, X.; Wang, C.; Liu, H.; Cheng, S.; Xu, Z.; Du, M. In vitro and in silico analysis of dual-function peptides derived from casein hydrolysate. Food Sci. Hum. Wellness 2020, 10, 32–37. [Google Scholar] [CrossRef]
- Nascimento, L.G.L.; Casanova, F.; Silva, N.F.N.; De Carvalho Teixeira, A.V.N.; De Carvalho, A.F. Casein-based hydrogels: A mini–review. Food Chem. 2019, 314, 126063. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
- Balandrán-Quintana, R.R.; Mendoza-Wilson, A.M.; Montfort, G.R.; Huerta-Ocampo, J.Á.; Mazorra-Manzano, M.A. Peptides and proteins. Bioact. Compd. Health Dis. 2021, 2021, 79–117. [Google Scholar] [CrossRef]
- Dashper, S.G.; Liu, S.W.; Reynolds, E.C. Antimicrobial peptides and their potential as oral therapeutic agents. Int. J. Pept. Res. Ther. 2007, 13, 505–516. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Dashper, S.G.; O’Brien-Simpson, N.M.; Talbo, G.H.; Malkoski, M. Antimicrobial Peptides. European Patent EP1032592B1, 25 July 2007. Available online: https://patents.google.com/patent/EP1032592B1/en (accessed on 15 February 2025).
- Reynolds, E.C.; Dashper, S.G.; Paolini, R.A. Antimicrobial Composition. Australian Patent AU2010224414B2, 23 May 2013. Available online: https://patents.google.com/patent/AU2010224414B2/en (accessed on 15 February 2025).
- Wusigale, N.; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Sarode, A.; Sawale, P.; Khedkar, C.; Kalyankar, S.; Pawshe, R. Casein and Caseinate: Methods of Manufacture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 676–682. [Google Scholar] [CrossRef]
- Troch, T.; Lefébure, É.; Baeten, V.; Colinet, F.; Gengler, N.; Sindic, M. Cow milk coagulation: Process description, variation factors and evaluation methodologies. A review. BASE 2017, 21, 276–287. [Google Scholar] [CrossRef]
- Amaro-Hernández, J.; Olivas, G.; Acosta-Muñiz, C.; Gutiérrez-Méndez, N.; Rios-Velasco, C.; Sepulveda, D.R. Chemical interactions among caseins during rennet coagulation of milk. J. Dairy Sci. 2021, 105, 981–989. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Ramos, M.; Olano, A. Rennet coagulation of milk subjected to high pressures. J. Agric. Food Chem. 1997, 45, 3233–3237. [Google Scholar] [CrossRef]
- De Kort, E.; Minor, M.; Snoeren, T.; Van Hooijdonk, T.; Van Der Linden, E. Effect of calcium chelators on heat coagulation and heat-induced changes of concentrated micellar casein solutions: The role of calcium-ion activity and micellar integrity. Int. Dairy J. 2012, 26, 112–119. [Google Scholar] [CrossRef]
- O’Connell, J.; Fox, P. The Two-Stage coagulation of milk proteins in the minimum of the heat coagulation Time-PH profile of milk: Effect of Casein micelle size. J. Dairy Sci. 2000, 83, 378–386. [Google Scholar] [CrossRef]
- Carter, B.; Cheng, N.; Kapoor, R.; Meletharayil, G.; Drake, M. Invited review: Microfiltration-derived casein and whey proteins from milk. J. Dairy Sci. 2021, 104, 2465–2479. [Google Scholar] [CrossRef]
- Tomasula, P.M.; Craig, J.C.; Boswell, R. A continuous process for casein production using high-pressure carbon dioxide. J. Food Eng. 1997, 33, 405–419. [Google Scholar] [CrossRef]
- Nelson, B.; Lynch, J.; Barbano, D. Impact of Milk Preacidification with CO2 on the Aging and Proteolysis of Cheddar Cheese. J. Dairy Sci. 2004, 87, 3590–3600. [Google Scholar] [CrossRef]
- Pomastowski, P.; Walczak, J.; Gawin, M.; Bocian, S.; Piekoszewski, W.; Buszewski, B. HPLC separation of casein components on a diol-bonded silica column with MALDI TOF/TOF MS identification. Anal. Methods 2014, 6, 5236–5244. [Google Scholar] [CrossRef]
- Yaguchi, M.; Rose, D. Chromatographic Separation of milk Proteins: A review. J. Dairy Sci. 1971, 54, 1725–1743. [Google Scholar] [CrossRef]
- Gani, A.; Broadway, A.A.; Masoodi, F.A.; Wani, A.A.; Maqsood, S.; Ashwar, B.A.; Shah, A.; Rather, S.A.; Gani, A. Enzymatic hydrolysis of whey and casein protein- effect on functional, rheological, textural and sensory properties of breads. J. Food Sci. Technol. 2015, 52, 7697–7709. [Google Scholar] [CrossRef]
- Wang, L.; Shao, X.; Cheng, M.; Fan, X.; Wang, C.; Jiang, H.; Zhang, X. Mechanisms and applications of milk-derived bioactive peptides in Food for Special Medical Purposes. Int. J. Food Sci. Technol. 2022, 57, 2830–2839. [Google Scholar] [CrossRef]
- Ao, J.; Li, B. Stability and antioxidative activities of casein peptide fractions during simulated gastrointestinal digestion in vitro: Charge properties of peptides affect digestive stability. Food Res. Int. 2013, 52, 334–341. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.; Li, B. Bioavailability of peptides from casein hydrolysate in vitro: Amino acid compositions of peptides affect the antioxidant efficacy and resistance to intestinal peptidases. Food Res. Int. 2015, 81, 188–196. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Q.; Liang, Y. Recent advances in the application of novel carriers for peptide delivery. J. Agric. Food Res. 2025, 19, 101628. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L.; Castejon, A.M. Recent Advances in Peptide-Loaded PLGA Nanocarriers for Drug Delivery and Regenerative Medicine. Pharmaceuticals 2025, 18, 127. [Google Scholar] [CrossRef]
- Jiang, E.Y.; Desroches, S.T.; Mikos, A.G. Particle carriers for controlled release of peptides. J. Control. Release 2023, 360, 953–968. [Google Scholar] [CrossRef]
- Oliveira, C.B.P.; Gomes, V.; Ferreira, P.M.T.; Martins, J.A.; Jervis, P.J. Peptide-Based Supramolecular Hydrogels as Drug Delivery Agents: Recent Advances. Gels 2022, 8, 706. [Google Scholar] [CrossRef]
- Peng, T.; Fu, H.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nanotoday 2021, 39, 101229. [Google Scholar] [CrossRef]
- Wenck, C.; Meier, N.; Heinrich, E.; Grützner, V.; Wiekhorst, F.; Bleul, R. Design and characterisation of casein coated and drug loaded magnetic nanoparticles for theranostic applications. RSC Adv. 2024, 14, 26388–26399. [Google Scholar] [CrossRef]
- Lombardi, L.; Li, J.; Williams, D.R. Peptide-Based Biomaterials for Combatting Infections and Improving Drug Delivery. Pharmaceutics 2024, 16, 1468. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial Compounds in Food Packaging. Int. J. Mol. Sci. 2023, 24, 2457. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Razavi, S.H.; Alizadeh, H.; Ghorbanpour, M. Production of recombinant antimicrobial polymeric protein beta casein-E 50-52 and its antimicrobial synergistic effects assessment with thymol. Molecules 2017, 22, 822. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based hydrogels for wound tissue repair. Adv. Sci. 2022, 9, 2104165. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2019, 103, 52–67. [Google Scholar] [CrossRef]
- Patel, H.R.; Patel, G.N.; Patel, R.K. Formulation and evaluation of oral mucosal casein salt film for the anti-diabetic activity. Polym. Sci. 2017, 03. [Google Scholar] [CrossRef]
- Tan, S.; Hadinoto, K.; Ebrahimi, A.; Langrish, T. Fabrication of novel casein gel with controlled release property via acidification, spray drying and tableting approach. Colloids Surf. B Biointerfaces 2019, 177, 329–337. [Google Scholar] [CrossRef]
- Simão, A.R.; Fragal, V.H.; Pellá, M.C.G.; Garcia, F.P.; Nakamura, C.V.; Silva, R.; Tambourgi, E.B.; Rubira, A.F. Drug polarity effect over the controlled release in casein and chondroitin sulfate-based hydrogels. Int. J. Biol. Macromol. 2020, 158, 116–126. [Google Scholar] [CrossRef]
- Simão, A.R.; Fragal, V.H.; De Oliveira Lima, A.M.; Pellá, M.C.G.; Garcia, F.P.; Nakamura, C.V.; Tambourgi, E.B.; Rubira, A.F. pH-responsive hybrid hydrogels: Chondroitin sulfate/casein trapped silica nanospheres for controlled drug release. Int. J. Biol. Macromol. 2020, 148, 302–315. [Google Scholar] [CrossRef]
- Li, N.; Fu, C.; Zhang, L. Using casein and oxidized hyaluronic acid to form biocompatible composite hydrogels for controlled drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Yang, R.; Borelli, L.P.P.; Alves, T.; Mehta, M.; Chaud, M.V.; Mazzola, P.G.; Kohane, D.S. Enhancement of the Mechanical and Drug-Releasing Properties of Poloxamer 407 Hydrogels with Casein. Pharm. Res. 2021, 38, 515–522. [Google Scholar] [CrossRef]
- Upputuri, R.T.P.; Mandal, A.K.A. Mathematical Modeling and Release Kinetics of Green Tea Polyphenols Released from Casein Nanoparticles. Iran. J. Pharm. Res. 2019, 18, 1137–1146. [Google Scholar] [CrossRef]
- Maeno, M.; Nakamura, Y.; Mennear, J.H.; Bernard, B.K. Studies of the Toxicological Potential of Tripeptides (L-Valyl-L-prolyl-L-proline and L-lsoleucyl-L-prolyl-L-proline): III. Single- and/or Repeated-Dose Toxicity of Tripeptides-Containing Lactobacillus helveticus-Fermented Milk Powder and Casein Hydrolysate in Rats. Int. J. Toxicol. 2005, 24, 13–23. [Google Scholar] [CrossRef]
- Mizuno, S.; Mennear, J.H.; Matsuura, K.; Bernard, B.K. Studies of the toxicological potential of tripeptides (L-Valyl-L-prolyl-L-proline and L-lsoleucyl-L-prolyl-L-proline): V. A 13-Week toxicity Study of Tripeptides-Containing Casein Hydrolysate in male and female rats. Int. J. Toxicol. 2005, 24, 41–59. [Google Scholar] [CrossRef]
- Kurosaki, T.; Maeno, M.; Mennear, J.H.; Bernard, B.K. Studies of the Toxicological Potential of Tripeptides (L-Valyl-L-prolyl-L-proline and L-lsoleucyl-L-prolyl-L-proline): VI. Effects of Lactobacillus helveticus-fermented Milk Powder on Fertility and Reproductive Performance of Rats. Int. J. Toxicol. 2005, 24, 61–89. [Google Scholar] [CrossRef]
- Doorten, A.P.; Wiel, J.V.; Jonker, D. Safety evaluation of an IPP tripeptide-containing milk protein hydrolysate. Food Chem. Toxicol. 2008, 47, 55–61. [Google Scholar] [CrossRef]
- Matsufuji, H.; Matsui, T.; Ohshige, S.; Kawasaki, T.; Osajima, K.; Osajima, Y. Antihypertensive effects of angiotensin fragments in SHR. Biosci. Biotechnol. Biochem. 1995, 59, 1398–1401. [Google Scholar] [CrossRef]
- Tsuchita, H.; Suzuki, T.; Kuwata, T. The effect of casein phosphopeptides on calcium absorption from calcium-fortified milk in growing rats. Br. J. Nutr. 2001, 85, 5–10. [Google Scholar] [CrossRef]
- Scientific Concepts of Functional Foods in Europe Consensus Document. Br. J. Nutr. 1999, 81, 1–27. [CrossRef]
- Hartmann, R.; Meisel, H. Caseinophosphopeptides and their cell modulating potential. BioFactors 2004, 21, 73–78. [Google Scholar] [CrossRef]
- Urista, C.M.; Fernández, R.Á.; Rodriguez, F.R.; Cuenca, A.A.; Jurado, A.T. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef]
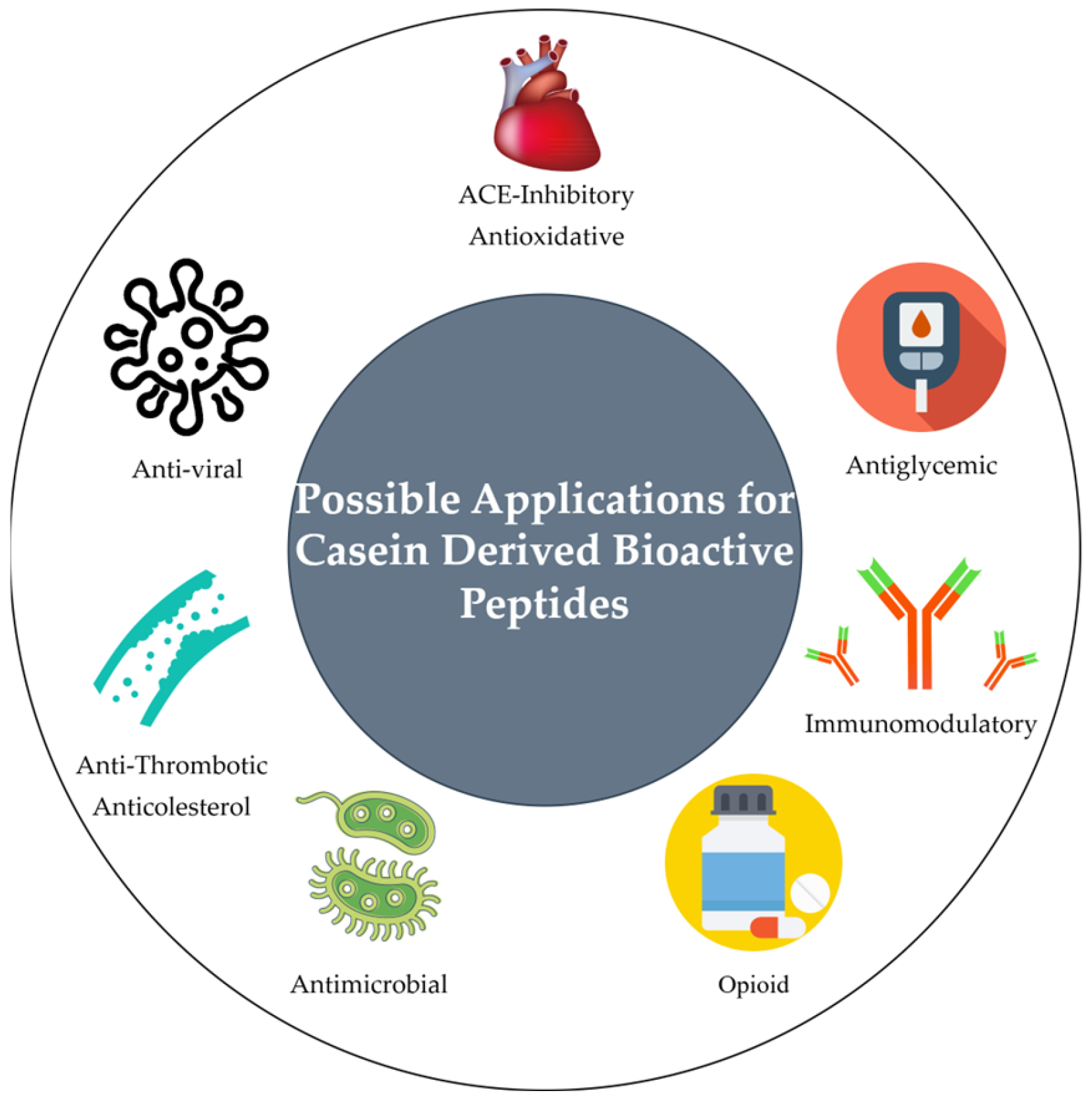
| Category | Peptide Name | Sequence | Bioactivity | Concentration | Applications | References |
|---|---|---|---|---|---|---|
| 1. Casomorphins | β-Casomorphin-5; β-Casomorphin-7 | YPFPGPIPNSL; YPFPGPI | Opioid, ACE-inhibitory, immunomodulatory (µM) | 10.00 | Potential influence on immune and neurological systems; excessive intake may lead to gastrointestinal discomfort or altered immune responses. | [41,42] |
| 2. Casokinins | Casokinin-10 | YQQPVLGPVR | ACE-inhibitory (µM) | 300.00 | Potential applications in functional foods to manage hypertension, as a natural alternative to ACE inhibitors. | [43,44] |
| 3. Lactoferricin-Like Peptides | Lactoferricin-like peptides derived from β-casein | FKCRRWQWRMKKLGAPSITCVRRAF | Antimicrobial (MIC) mg/mL | B. cereus—400; B. subtilis—6.3; L. innocua—6.3; L. monocytogenes—6.3; E. coli—12,5; S. entiritidis—25; S. thyphimurium—6.3 | Natural antimicrobials for food safety and potential therapeutic agents in animal health. | [45,46] |
| 4. αs1-Casein Hexapeptide | α-casozepine | YLGYLEQLLR | Anxiolytic | Used in stress relief products, studied for calming effects on stress-related behaviors. | [47,48] |
| Peptide | Name | Sequence | Type of Assay | Bacteria Inhibition | References |
|---|---|---|---|---|---|
| Alpha-S1-Casein | YLEQLLR | MIC (μg/mL) | B. subtilis—53.6; E. coli NEB 5α—241.0; E. coli ATCC 25922—40.2 | [75] | |
| Alpha-S1-Casein | Caseicin B | VLNENLLR | MIC (mM); well diffusion assay | (1) E. coli—0.22; C. sakazakii +++; L. innocua ++; L. bulgaricus ++; S. mutans ++, | [76] |
| MIC (mg/mL) | (2) C. sakazakii—0.97 | [77] | |||
| MIC (mM) | (3) C. sakazakii—1.25; C. muytjensii—0.625 | [78] | |||
| Alpha-S1-Casein | TTMPLW | MIC (μM) | E. coli—193; S. aureus—193.0; M. luteus—64.0; C. albicans—144.0 | [79] | |
| Alpha-S1-Casein | Caseicin C | SDIPNPIGSENSEK | MIC (mM) | L. innocua—1.0 | [76] |
| Alpha-S1-Casein | RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKV | MIC (μg/mL) | B. subtilis—29.0; E. coli NEB 5α—58.0; E. coli ATCC 25922—58.0 | [75] | |
| Alpha-S1-Casein | RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEK | MIC (μg/mL) | B. subtilis—59.0; E. coli NEB 5α—59.0; E. coli ATCC 25922—59.0 | [75] | |
| Alpha-S1-Casein | RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGK | MIC (μg/mL) | B. subtilis—31.4; E. coli NEB 5α—31.4; E. coli ATCC 25922—31.4 | [75] | |
| Alpha-S1-Casein | RPKHPIKHQGLPQEVLNENLLRFF | MIC (μg/mL) | B. subtilis—23.6; E. coli NEB 5α—47.2; E. coli ATCC 25922—189.0 | [75] | |
| Alpha-S1-Casein | Isracidin | RPKHPIKHQGLPQEVLNENLLRF | MBC (mg/mL) | E. coli—0.1—1.0; | [80] |
| MIC (mg/mL) | C. sakazakii—0.5; E. coli—0.2 | [81] | |||
| Alpha-S1-Casein | RPKHPIK | MIC (μg/mL) | Lactobacillus sakei A15—200.0; Escherichia coli K12—400.0; Bacillus megaterium F6—400.0 | [82] | |
| Alpha-S1-Casein | CP1/Cpep11 | LRLKKYKVPQL | MIC (μM) | (1) E. coli ATCC 25922—64.0; E. coli UB1005—128.0; S. pullorum—256.0; Salmonella enterica subsp enterica CMCC 50071—256.0; S. aureus ATCC 29213—640.0, L. monocytogenes—65.0 | [9] |
| MIC (μg/mL) | (2) B. subtilis—125; L. innocua—125.0; L. monocytogenes FSAW 2310—125.0; L. monocytogenes NCTC 11994—125.0; C. freundii—500; E. aerogenes—>1000; E. coli—250.0; S. enteritidis—250.0; S. thyphimurium—125.0 | [83] | |||
| MIC (μg/mL) | (3) S. dysenteriae ATCC51302—300.0; E. coli ATCC 25922—250.0; Salmonella enterica serovar Typhimurium ATCC 14028—125.0; B. subtilis ATCC 9372—275.0; S.aureus ATCC25923—125.0; Streptococcus pneumoniae ATCC 49619—150.0 | [84] | |||
| Alpha-S1-Casein | LGYLEQLLRL | MIC (μg/mL) | B. subtilis—NA; E. coli NEB 5α—NA; E. coli ATCC 25922—1356.0 | [75] | |
| Alpha-S1-Casein | LEQLLRLKKY | MIC (μg/mL) | B. subtilis—1266.0; E. coli NEB 5α—633.0; E. coli ATCC 25922—NA | [75] | |
| Alpha-S1-Casein | Fragment of Isradicin | IKHQGLPQEV | MIC (μg/mL) and MBC (μg/mL) | E. coli—MIC 25.0–50.0, MBC 50.0; B. subtilis MIC 50.0, MBC 100.0 | [85] |
| Alpha-S1-Casein | Caseicin A | IKHQGLPQE | MIC (mg/mL) | (1) E. coli—2.0; L. monocytogenes—1.0; S. thyphimurium—2.0; P. putida—1.0; S. aureus—0.5 | [71] |
| MIC (mM) | (2) E. coli DPC6053—0.05 | [76] | |||
| MIC (mM) | (3) C. sakazakii—0.625; C. muytjensii—0.625; S. thyphimurium—1.25; E. coli—1.25; K. pneumoniae—1.25; P. fluorescens—1.25 | [78] | |||
| Alpha-S1-Casein | HIQKEDVPSERYLGYLEQLLRLKKYK | MIC (μg/mL) | B. subtilis—42.4; E. coli NEB 5α—169.0; E. coli ATCC 25922—NA | [75] | |
| Alpha-S1-Casein | HIQKEDVPSERYLGYLEQLLRLKK | MIC (μg/mL) | B. subtilis—186.0; E. coli NEB 5α—NA; E. coli ATCC 25922—NA | [75] | |
| Alpha-S1-Casein | HIQKEDVPSERYLGYLEQLLRLK | MIC (μg/mL) | B. subtilis—292.0; E. coli NEB 5α—292.0; E. coli ATCC 25922—584.0 | [75] | |
| Alpha-S2-Casein | PYVRYL | log(N0/Nf) | E. coli—0.27; S. carnosus—2.23; S. epidermis—2.06; L. innocua—1.13 | [86] | |
| Alpha-S2-Casein | WIQPKTKVIPYVRYL | MIC (μM) | C. sakazakii—78.125; L. monocytogenes—39.063 | [87] | |
| Alpha-S2-Casein | Casein F/CP2 | VYQHQKAMKPWIQPKTKVIPYVRYL | MIC (mM) | (1) B. subtilis—21; L. innocua—21; L. monocytogenes—21; C. freundii—664; E. coli—332; S. enteritidis—664; S. typhmirium—21, | [83] |
| MIC (μM) | (2) C. sakazakii—312.5 L. monocytogenes—78.125 | [87] | |||
| MIC (μM) | (3) E. coli—16.0; L. innocua—16.0; B. cereus—16.0; M. flavus—16.0; St. thermophilus—8.0 | [88] | |||
| MIC (μg/mL) | (4) E. coli—8.0–16.0; S. carnosus—8.0–16.0, | [89] | |||
| MIC (μM) | (5) E. coli—1.25; S. choleraesuis—0.5; S. Epidermidis—2.5; L. monocytogenes—0.05 | [90] | |||
| Alpha-S2-Casein | TVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—2.7; E. coli NEB 5α—21.4; E. coli ATCC 25922—172.0 | [75] | |
| Alpha-S2-Casein | TKVIPYVRYL | MIC (μM) | C. sakazakii—156.25 L. monocytogenes—78.125 | [87] | |
| Alpha-S2-Casein | TKLTEEEKNRLNFLKKISQRYQKFALPQYLK | MIC (μg/mL) | B. subtilis—4.0; E. coli NEB 5α—16.2; E. coli ATCC 25922—16.2 | [75] | |
| Alpha-S2-Casein | P14 | TKKTKLTEEEKNRL | MIC (μM) | B. cereus—2.99; S. aureus—2.3; L. monocytogenes—2.99 | [91] |
| Alpha-S2-Casein | CR7 | QKFALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—312.5; L. innocua—625.0 | [46] |
| Alpha-S2-Casein | P10 | QKALNEINQF | MIC (μM) | B. cereus 0.87; S. aureus—1.75; L. monocytogenes 1.75; H. pylori—0.083 | [91] |
| Alpha-S2-Casein | CR3 | LKTVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—312.5; L. innocua—625.0 | [46] |
| Alpha-S2-Casein | CR5/CR6 | LKKISQRYQKFALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—4.8; L. innocua—4.8; L. monocytogenes 2310—4.8 | [46] |
| Alpha-S2-Casein | LKKISQRYQKFALPQY | MIC (μM) | E. coli—25.0; L. innocua—50.0; B. cereus—75.0; M. flavus—75.0; St. thermophilus—50.0 | [88] | |
| Alpha-S2-Casein | CR1 | KTVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—21.0; L. innocua—21.0; L. monocytogenes 2310—21.0; S. typhimirium—21.0 | [46] |
| Alpha-S2-Casein | KTKLTEEEKNRLNFLKKISQRYQKFALPQYLKTVYQHQK | Diffusion Assay | E. coli—NA; S. carnosus—NA | [92] | |
| Alpha-S2-Casein | SR4 | KKISQRYQKFALPQYLKTVYQHQK | MIC (μg/mL) and MBC (μg/mL) | E. coli—MIC 50.0, MBC 100.0; B. subtilis MIC 12.5–25.0, MBC 50.0 | [85] |
| Alpha-S2-Casein | KAMKPWIQPKTKVIPYVRYL | MIC (μM) | C. sakazakii—39.063; L. monocytogenes—39.063 | [87] | |
| Alpha-S2-Casein | KAMKPWIQPKTKVIP | MIC (μM) | C. sakazakii—>1.250; L. monocytogenes—156.25 | [87] | |
| Alpha-S2-Casein | SR9 | KAMKPW | MIC (μg/mL) and MBC (μg/mL) | E. coli—MIC 150.0, MBC 300.0; B. subtilis MIC 150.0, MBC 600.0 | [85] |
| Alpha-S2-Casein | IVLNPWDQVK | MIC (μg/mL) | B. subtilis—1363.0; E. coli NEB 5α—681.0; E. coli ATCC 25922—1363.0 | [75] | |
| Alpha-S2-Casein | SR1 | IQPKTKVIPYVR | MIC (μg/mL) and MBC (μg/mL) | E. coli—MIC 50.0, MBC > 100.0; B. subtilis MIC 50.0, MBC > 100.0 | [85] |
| Alpha-S2-Casein | CR4 | ALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL | MIC (μg/mL) | B. subtilis—10.7; L. innocua—10.7; L. monocytogenes 2310—10.7; S. typhimirium—21.4 | [46] |
| Alpha-S2-Casein | SSSEESII | MIC (mM) | L. innocua ATCC 33090—0.205; Micrococcus luteus ATCC 4698—0.818; E. coli ATCC 25922—0.654; S. enteritidis ATCC13076—1.145 | [93] | |
| Alpha-S2-Casein | KTVDMESTEVFTKKTKLTEEEKNRLNFLKK | MIC (mM) | B. subtilis ATCC6633—3.10–6.67 | [94] | |
| Beta-Casein | YPVEPF | Diffusion assay | Bacillus spp.—NA; L. monocytogenes—NA | [95] | |
| Beta-Casein | Bc3 | EMPFPK | MIC (μg/mL) | E. coli PTCC 1399—60.0; S. aureus PTCC 1431—25.0 | [96] |
| Beta-Casein | Casecidin 17 | YQEPVLGPVRGPFPIIV | MIC (mg/mL) | E. coli DH5a—0.5; E. coli DPC6053—0.4 | [81] |
| Beta-Casein | Casecidin 15 | YQEPVLGPVRGPFPI | MIC (mg/mL) | E. coli DH5a—0.5; E. coli DPC6053—0.4 | [81] |
| Beta-Casein | Bc6 | VLPVPQK | MIC (μg/mL) | E. coli PTCC 1399—45.0; S. aureus PTCC 1431—25.0 | [96] |
| Beta-Casein | Bc8 | VKEAMAPK | MIC (μg/mL) | E. coli PTCC 1399—30.0; S. aureus PTCC 1431—10.0 | [96] |
| Beta-Casein | SR8/Bc5 | AVPYPQR | MIC (μg/mL) and MBC (μg/mL) | (1) E. coli—MIC 100.0, MBC > 100.0; B. subtilis MIC 50.0, MBC > 100.0 | [85] |
| MIC (μg/mL) | (2) E. coli PTCC 1399—40.0; S. aureus PTCC 1431—20.0 | [96] | |||
| Beta-Casein | VPYPQRDMPIQAFL | MIC (μg/mL) | E. coli NEB 5α—493.0 | [75] | |
| Beta-Casein | Bc14 | VLPVPQKAVPYPQR | MIC (μg/mL) | E. coli PTCC 1399—30.0; S. aureus PTCC 1431—10.0–15.0 | [96] |
| Beta-Casein | RINKK | MIC (mM) | E. coli 2.59 | [97] | |
| Beta-Casein | Bc11 | HKEMPFPK | MIC (μg/mL) | E. coli PTCC 1399—50.0; S. aureus PTCC 1431—20.0 | [96] |
| Beta-Casein | Bc12 | EAMAPKHK | MIC (μg/mL) | E. coli PTCC 1399—30.0; S. aureus PTCC 1431—15.0 | [96] |
| Beta-Casein | Bc1 | EAMAPK | MIC (μg/mL) | E. coli PTCC 1399—60.0; S. aureus PTCC 1431—25.0 | [96] |
| Beta-Casein | LGDT2 | VAGTWY | log(N0/Nf) | B. subtilis—2.2 | [98] |
| Beta-Casein | LGDT1 | IPAVFK | log(N0/Nf) | (1) B. subtilis—2.2; S. zooepidemicus—0.4 | [98] |
| MIC (μg/mL) | (2) E. coli—55.0; S. aureus—30.0 | [99] | |||
| Beta-Casein | IDALNENK | log(N0/Nf) | (1) L. monocytogenes—0.72; S. aureus—1.03; E. coli—0.63 | [99] | |
| log(N0/Nf) | (2) E. coli—70.0; S. aureus—35.0 | [100] | |||
| Beta-Casein | LGDT3 | VLVLDTDYK | log(N0/Nf) | B. subtilis—2.4 | [98] |
| Beta-Casein | TPEVDDEALEK | log(N0/Nf) | L. monocytogenes—1.03; S. aureus—1.23; E. coli—0.69 | [100] | |
| Beta-Casein | IRL | log(N0/Nf) | L. ivanovii—NA; E. coli—NA | [101] | |
| Beta-Casein | LGDT4 | AASDISLLDAQSAPLR | log(N0/Nf) | B. subtilis—1.5; S. lentus—1.5; S. zooepidemicus—0.6 | [98] |
| Kappa-Casein | Kappacin | MAIPPKKNQDKTEIPTINTIASGEPTSTPTTEAVESTVATLEDSPEVIESPPEINTVQVTSTAV | MIC (mg/mL) | (1) E. faecalis—0.64 | [102] |
| MIC (μg/mL) | (2) S. mutans—59.0 | [103] | |||
| Kappa-Casein | YVL | log(N0/Nf) | E. coli—> 6; S. maracescens—3.08; L. innocua—> 6.0; S. carnosus > 6.0 | [97] | |
| Kappa-Casein | IQY | log(N0/Nf) | E. coli—> 6.0; S. maracescens—0.39; L. innocua—0.27; S. carnosus—0.22 | [97] | |
| Kappa-Casein | YYQQKPVA | log(N0/Nf) | E. coli—3.46; S. maracescens—0.04; L. innocua—0.66; S. carnosus—1.14 | [97] | |
| Kappa-Casein | VQVTSTAV | log(N0/Nf) | E. coli—0.10; S. maracescens—0.25; L. innocua—1.99; S. carnosus—1.17 | [97] | |
| Kappa-Casein | VESTVATL | log(N0/Nf) | E. coli—>6; S. maracescens—0.59; L. innocua—1.89; S. carnosus—3.44 | [97] | |
| Kappa-Casein | SR13 | TEAVESTVATL | MIC (μg/mL) | E. coli—50.0; B. subtilis—50.0 | [85] |
| Kappa-Casein | STVATL | log(N0/Nf) | E. coli—0.67; L. innocua—0.42; S. carnosus—0.29 | [97] | |
| Kappa-Casein | PAAVRSPAQILQ | log(N0/Nf) | E. coli—>6.0; S. maracescens—0.20; L. innocua—0.84; S. carnosus—0.97 | [97] | |
| Kappa-Casein | MMK | MIC (μg/mL) | E. coli—125.0; S. aureus—70.0 | [104] | |
| Kappa-Casein | MAIPPKKNQDKTEIPTINT | MIC (mg/mL) | E. coli—0.25 | [105] | |
| Kappa-Casein | IAK | MIC (μg/mL) | E. coli—100.0; S. aureus—60.0; L. casei—70.0; L. acidophilus—70.0 | [103] | |
| Kappa-Casein | FSDKIAK | log(N0/Nf) | E. coli—>6.0; S. maracescens—0.27; L. innocua—>6.0; S. carnosus—>6.0 | [97] | |
| Kappa-Casein | FFSDK | MIC (μg/mL) | E. coli—200; S. aureus—90 | [104] | |
| Kappa-Casein | EIPT | log(N0/Nf) | E. coli—2.59; S. maracescens—1.69; L. innocua—0.88; S. carnosus—0.74 | [97] | |
| Kappa-Casein | AVESTVATLEDSPEVIESPPE | MIC (μg/mL) | S. mutans—59.0 | [103] |
| Product Name | Manufacturer | Type of Food | Peptide Name |
|---|---|---|---|
| Calpico (Europe) or Calpis AMEAL S (Japan) | Calpis Co., Japan | Fermented milk | B ioactive peptides Ile-Pro-Pro (IPP) and Val-Pro-Pro (VPP) from b- and k-CN |
| Capolac | Arla Foods, Denmark | Ingredient | Casein phosphopeptides |
| Casein DP Peptio Drink | Kanebo, Japan | Soft drink | Casein-derived dodecapeptide FFVAPFPEVFGK |
| CE90CPP | DMV, Netherlands | Ingredient | Casein phosphopeptides (CPPs) |
| C12 Peptide | DMV, Netherlands | Ingredient | Casein-derived dodecapeptide FFVAPFPEVFGK |
| Evolus | Valio, Finland | Fermented milk, calcium enriched | C12 |
| Kotsu Kotsu calcium | Asahi, Japan | Soft drink | CPP |
| PeptoPro | DSM Food Specialists, Netherlands | Ingredient | Hydrolyzed casein |
| ProDiet F200 | Ingredia, France | Milk Drink, Confectionary | CPP |
| Tekkotsu Inryou | Suntory, Japan | Soft drink | CPP |
| Peptigen® IF-3080 | Arla Foods, Denmark | Infant formulas | Casein hydrolizates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moita, T.; Pedroso, L.; Santos, I.; Lima, A. Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems. Nutrients 2025, 17, 1615. https://doi.org/10.3390/nu17101615
Moita T, Pedroso L, Santos I, Lima A. Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems. Nutrients. 2025; 17(10):1615. https://doi.org/10.3390/nu17101615
Chicago/Turabian StyleMoita, Tomás, Laurentina Pedroso, Isabel Santos, and Ana Lima. 2025. "Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems" Nutrients 17, no. 10: 1615. https://doi.org/10.3390/nu17101615
APA StyleMoita, T., Pedroso, L., Santos, I., & Lima, A. (2025). Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems. Nutrients, 17(10), 1615. https://doi.org/10.3390/nu17101615







