Carrageenan as a Potential Factor of Inflammatory Bowel Diseases
Abstract
1. Introduction–Food Additives Associated with IBD
1.1. Use of Carrageenan and Food Thickeners, Viscosity Modifiers
1.2. Products Containing Carrageenan
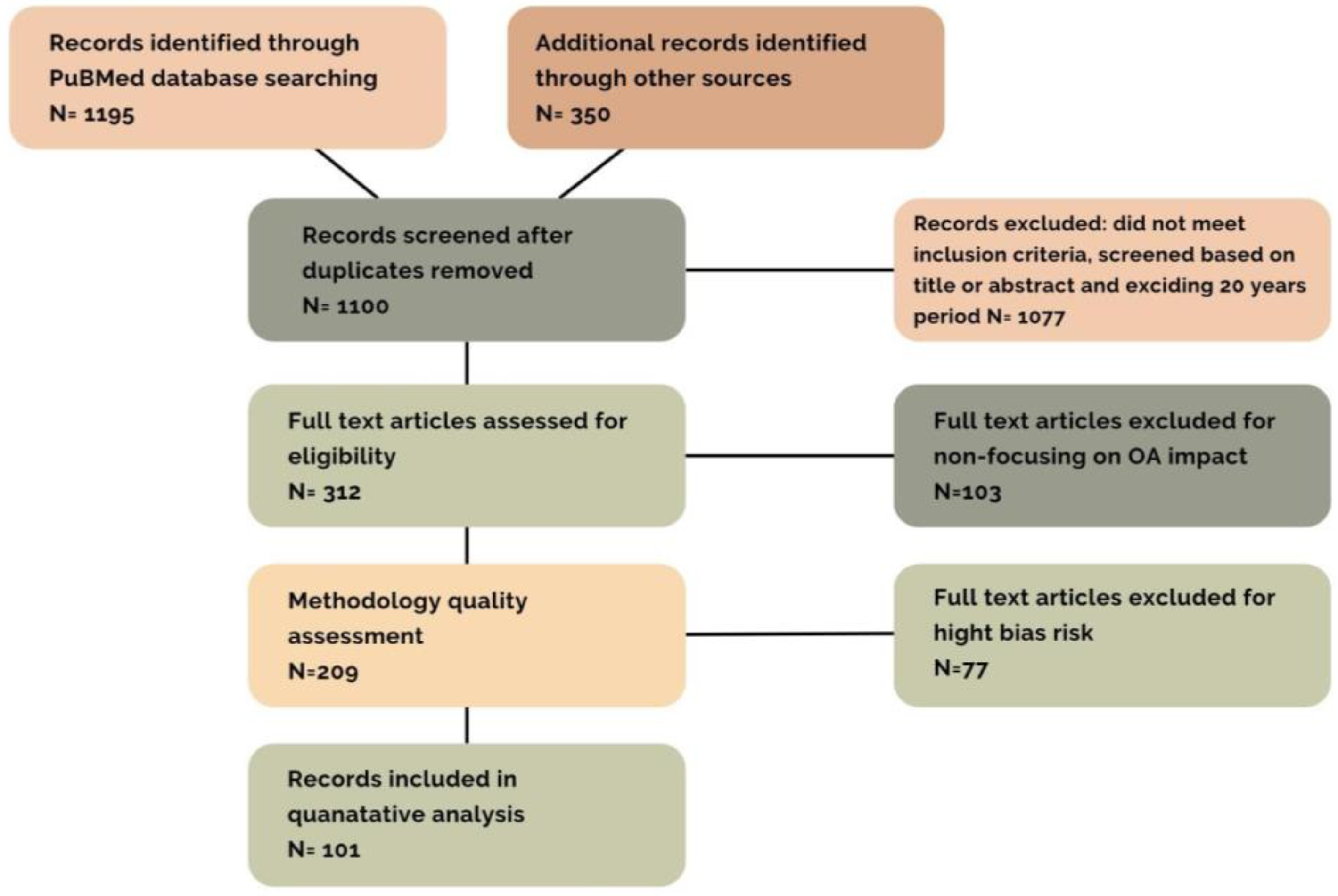
2. Material and Methods
Structure of Basic Research
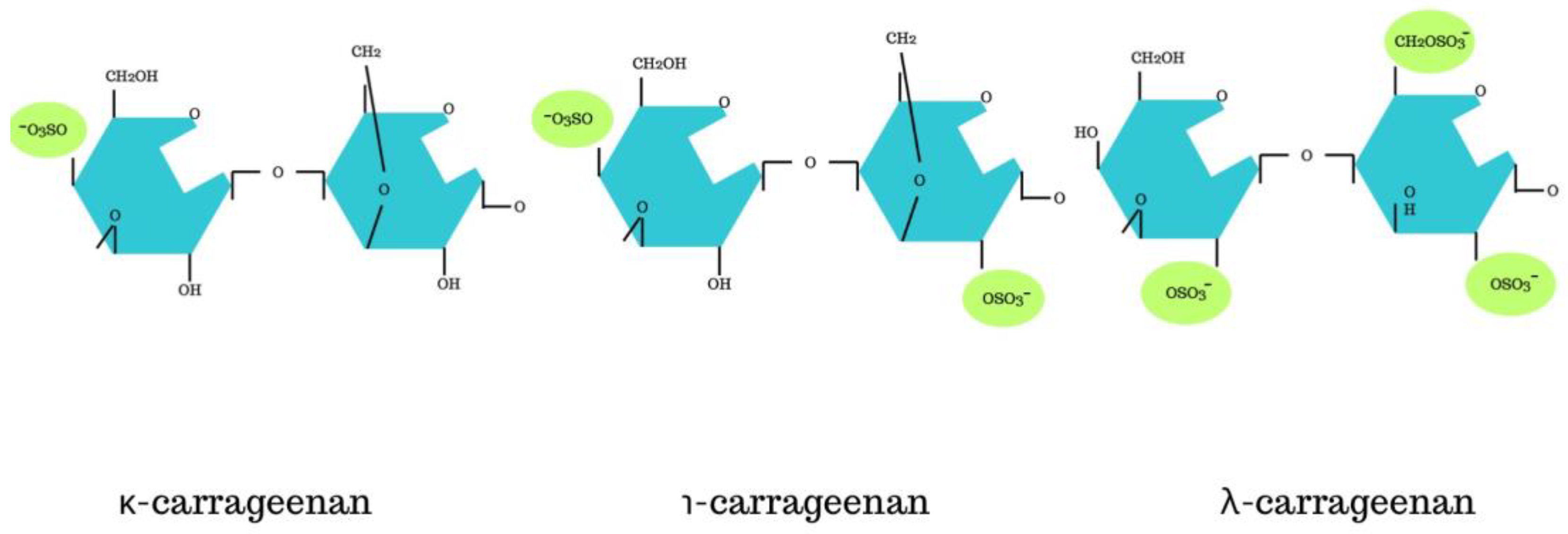
3. Structure of Carrageenan
3.1. Factors Promoting Inflammation through Carrageenan
3.2. Mechanism of Carrageenan Action
3.2.1. Animal Model
3.2.2. Human Model
4. Gut Dysbiosis
5. Associated Symptoms after Carrageenan Consumption
5.1. Alleviation Process of Symptoms
5.2. The Therapeutic Potential of Marine Polysaccharides
6. New Findings
6.1. Carrageenan in Various Dietary Patterns
6.2. The Impact of Carrageenan and the Gut Microbiota
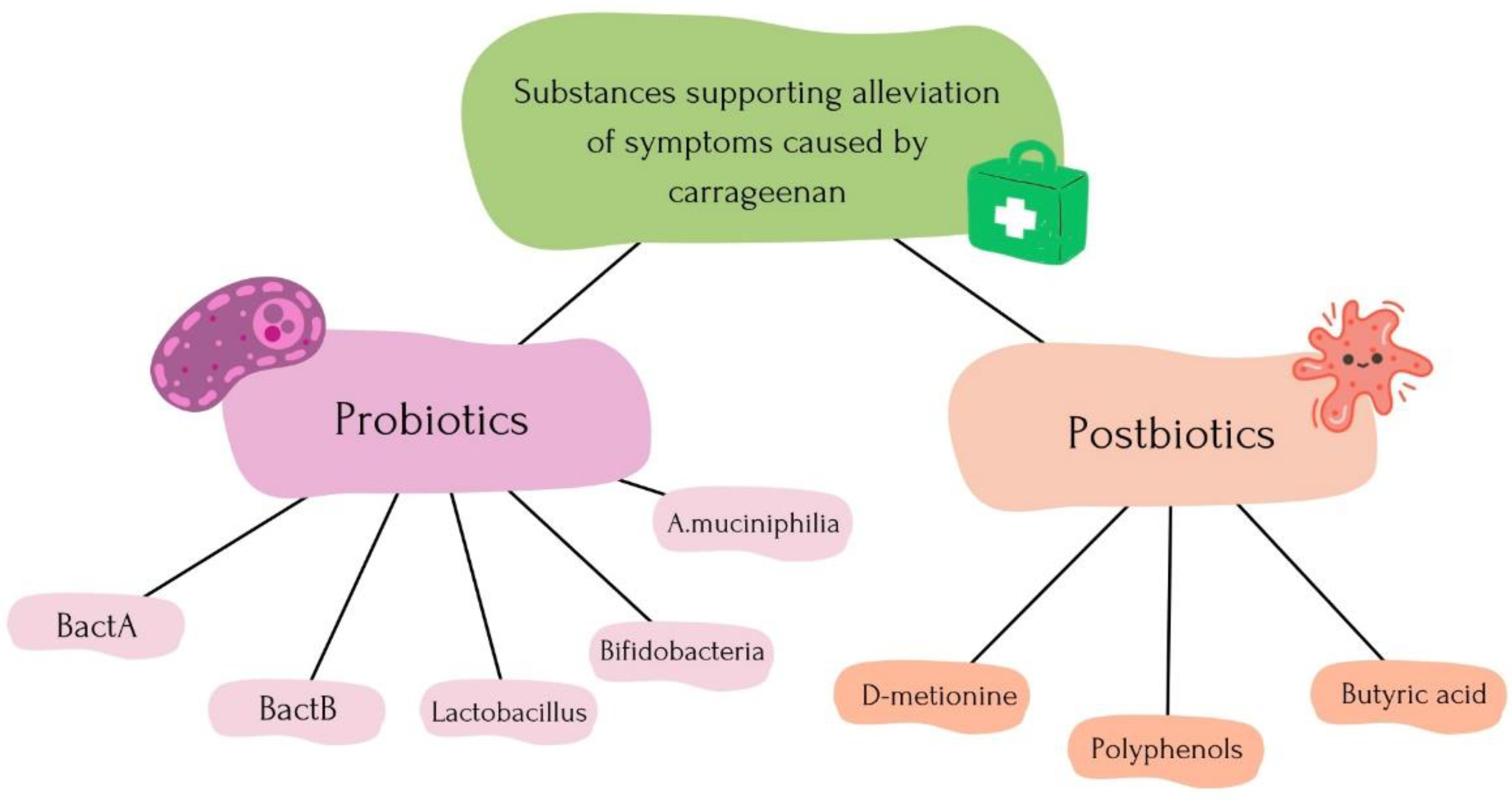
6.3. Probiotic Therapy and Postbiotic Therapy to Alleviate Carrageenan Effects
6.4. Carrageenan Use among People
6.5. New Applications of Marine Algae including Carrageenan
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuang, R.; O’Keefe, S.J.D.; del Aguila de Rivers, C.R.; Koutroumpakis, F.; Binion, D.G. Is Salt at Fault? Dietary Salt Consumption and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 140–150. [Google Scholar] [CrossRef]
- Rogozińska, I.; Wichrowska, D. Most Popular Fixatives Used in Modern Food Technology. Eng. Chem. Appar. 2011, 2, 19–21. [Google Scholar]
- Gultekin, F.; Oner, M.E.; Savas, H.B.; Dogan, B. Food Additives and Microbiota. North. Clin. Istanb. 2019, 7, 192–200. [Google Scholar] [CrossRef]
- Food Additives and Health: Sweeteners, Flavor Enhancers, Emulsifiers, Stabilizers—Allergy. Available online: http://alergia.org.pl/index.php/2017/08/10/dodatki-do-zywnosci-a-zdrowie-slodziki-wzmacniacze-smaku-emulgatory-stabilizatory/ (accessed on 23 January 2024).
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 2. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubaraca, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and Nutritional Factors in Inflammatory Bowel Diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Cholewiak-Goralczyk, A. Non-Specific Inflammatory Bowel Disease (IBD)—Diet as Support in Treatment. Animal Expert, no. 2. 2017. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-d5ce390c-6e4f-49c3-bbc0-23bf2a0b4250 (accessed on 23 January 2024).
- Hodson, R. Inflammatory Bowel Disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef]
- Lee, D.; Swan, C.K.; Suskind, D.; Wahbeh, G.; Vanamala, J.; Baldassano, R.N.; Leonard, M.B.; Lampe, J.W. Children with Crohn’s Disease Frequently Consume Select Food Additives. Dig. Dis. Sci. 2018, 63, 2722–2728. [Google Scholar] [CrossRef]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dillillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Chang, C.-C.; Nagarajan, D.; Chen, C.-Y.; Chang, J.-S. Algae-derived Hydrocolloids in Foods: Applications and Health-related Issues. Bioengineered 2021, 12, 3787–3801. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Tumor Necrosis Factor α-induced Inflammation Is Increased but Apoptosis Is Inhibited by Common Food Additive Carrageenan. J. Biol. Chem. 2010, 285, 39511–39522. [Google Scholar] [CrossRef]
- Katsoudas, N.; Tavakoli, P.; Wu, N.; Shapiro, A.; Leach, S.T.; Williams, A.J.; Paramsothy, R.; Ghaly, S.; Connor, S.J.; Samocha-Bonet, D.; et al. Dietary Emulsifier Exposure in People with Inflammatory Bowel Disease Compared with Healthy Controls: Is There a Cause for Concern? Inflamm. Bowel Dis. 2024, izad318. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118, 109351. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Lima, A.; Gonçalves, M.M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.; Boavida Ferreira, R.; et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef]
- Dwita, L.P.; Hikmawanti, N.P.E.; Yeni; Supandi. Extract, fractions, and ethyl-p-methoxycinnamate isolate from Kaempferia galanga Elicit anti-inflammatory activity by limiting leukotriene B4 (LTB4) production. J. Tradit. Complement. Med. 2021, 11, 563–569. [Google Scholar] [CrossRef]
- Thirunavukkarasu, K.; Tan, B.; Swearingen, C.A.; Rocha, G.; Bui, H.H.; McCann, D.J.; Jones, S.B.; Norman, B.H.; Pfeifer, L.A.; Saha, J.K. Pharmacological Characterization of a Potent Inhibitor of Autotaxin in Animal Models of Inflammatory Bowel Disease and Multiple Sclerosis. J. Pharmacol. Exp. Ther. 2016, 359, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Harding, A.; Menghini, P.; Himmelman, C.; Retuerto, M.; Nickerson, K.P.; Lam, M.; Croniger, C.M.; McLean, M.H.; Durum, S.K.; et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease-Like Ileitis. Inflamm. Bowel. Dis. 2018, 24, 1005–1020. [Google Scholar] [CrossRef]
- Halmos, E.P.; Mack, A.; Gibson, P.R. Review article: Emulsifiers in the food supply and implications for gastrointestinal disease. Aliment. Pharmacol. Ther. 2019, 49, 41–50. [Google Scholar] [CrossRef]
- Martino, J.V.; Van Limbergen, J.; Cahill, L.E. The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation. Front. Pediatr. 2017, 5, 96. Available online: https://www.frontiersin.org/articles/10.3389/fped.2017.00096 (accessed on 23 January 2024). [CrossRef]
- Benard, C.; Cultrone, A.; Michel, C.; Rosales, C.; Segain, J.P.; Lahaye, M.; Galmiche, J.P.; Cherbut, C.; Blottière, H.M. Degraded carrageenan causing colitis in rats induces TNF secretion and ICAM-1 upregulation in monocytes through NF-κB activation. PLoS ONE 2010, 5, e8666. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.A.; Chiang, B.L. Inflammasomes and human autoimmunity: A comprehensive review. J Autoimmun. 2015, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Sołowiej, B. The effect of κ-carrageenan on physicochemical properties of processed cheese analogs. Food Sci. Technol. Qual. 2012, 2, 107–118. [Google Scholar]
- Błaszak, B.B.; Gozdecka, G.; Shyichuk, A. Carrageenan as a functional additive in the production of cheese and cheese-like products. Acta Sci. Pol. Technol. Aliment. 2018, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kozłowicz, K. Characterization of the use of selected cryoprotective substances in freezing and storage of food. Acta Sci. Pol. Tech. Agrar. 2012, 11, 13–24. [Google Scholar] [CrossRef]
- Gustaw, W.; Sołowiej, B.; Jabłońska-Ryś, E.; Zalewska-Korona, M. Selected rheological properties of aqueous dispersions of casein-polysaccharide. Food Sci. Technol. Qual. 2013, 2, 92–105. [Google Scholar]
- Pilarska, A.; Gawałek, J. Hydrocolloids—Substances stabilizing food Functions, modifications and legal conditions. Part I. Przemysł Spożywczy 2016, 70, 36–39. [Google Scholar] [CrossRef]
- Imeson, A.P. 7—Carrageenan and furcellaran. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 164–185. [Google Scholar] [CrossRef]
- FAO; WHO. Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2021, 25, 1172–1186. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- Nastaj, M.; Gustaw, W.; Sołowiej, B. Rheological properties of desserts obtained from whey proteins with the addition of various sweetening substances. Food Sci. Technol. Qual. 2007, 5, 283–291. [Google Scholar]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Kamińska-Dwórznicka, A.; Samborska, K.; Rybak, K. The effect of kappa carrageenan hydrolysates on limiting excessive ice crystal growth in dairy ice creams. Food Sci. Technol. Qual. 2015, 5, 87–98. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological Activities of Carrageenan. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Carbohydrates: Fundamentals and Applications, Part A; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 113–124. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, A.; Li, Y.; Chang, Y.; Xue, C.; Tang, Q. The risk of carrageenan-induced colitis is exacerbated under high-sucrose/high-salt diet. Int. J. Biol. Macromol. 2022, 210, 475–482. [Google Scholar] [CrossRef]
- Mucyna-1 (MUC1) as a Promising Molecular Target in Anti-Cancer Therapy. Available online: https://phmd.pl/resources/html/article/details?id=186807&language=pl (accessed on 1 February 2024).
- Limketkai, B.N.; Zipporah, I.E.; Teuta, G.H.; Parian, A.; Matarese, L.E.; Bracewell, K. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhail, A.A.; Reid, P.E.; Culling, C.F.A.; Dunn, W.L.; Clay, M.G. Studies of the degraded carrageenan-induced colitis of rabbits. II. Changes in the epithelial glycoproteinO-acylated sialic acids associated with the induction and healing phases. Histochem. J. 1984, 16, 555–564. [Google Scholar] [CrossRef]
- Tsuji, R.F.; Hoshino, K.; Noro, Y.; Tsuji, N.M.; Kurokawa, T.; Masuda, T.; Akira, S.; Nowak, B. Suppression of allergic reaction by λ-carrageenan: Toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin. Exp. Allergy 2003, 33, 249–258. [Google Scholar] [CrossRef]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin Ch Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhen, Z.; Niu, T.; Zhu, X.; Gao, Y.; Yan, J.; Chen, Y.; Yan, X.; Chen, H. κ-Carrageenan Enhances Lipopolysaccharide-Induced Interleukin-8 Secretion by Stimulating the Bcl10-NF-κB Pathway in HT-29 Cells and Aggravates C. freundii-Induced Inflammation in Mice. Mediat. Inflamm. 2017, 2017, e8634865. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.-E.; Khafipour, E. Carrageenan Gum and Adherent Invasive Escherichia coli in a Piglet Model of Inflammatory Bowel Disease: Impact on Intestinal Mucosa-associated Microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, H. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Liu, S.; Zhang, Z.; Murielle, D.; Dudeja, P.K.; Michel, G.; Linhardta, R.; Tobacman, J.K. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 2010, 21, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Borthakur, A.; Pant, N.; Dudeja, P.K.; Tobacman, J.K. Bcl10 mediates LPS-induced activation of NF-κB and IL-8 in human intestinal epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G429–G437. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, F.; Mao, H.; Yan, X. Degraded λ-carrageenan activates NF-κB and AP-1 pathways in macrophages and enhances LPS-induced TNF-α secretion through AP-1. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Wang, F.; Chen, H.-M.; Yan, X.-J. κ-carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol. Med. Rep. 2013, 8, 1635–1642. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 973–982. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Anbazhagan, A.N.; Kumar, A.; Dudeja, P.K.; Tobacman, J.K. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Lehto, M.; Mäkelä-Salmi, N.; Hillilä, M.; Groop, P.H.; Salmenkari, H. Randomized controlled pilot study: Effect of carrageenan emulsifier on inflammation and gastrointestinal symptoms in quiescent ulcerative colitis. Food Nutr. Res. 2023, 67, 9575. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, H.G.; Kim, J.; Park, S.H.; Park, J.; Oh, C.G.; Do, K.H.; Lee, S.J.; Park, Y.C.; Ahn, S.C.; et al. Pro-apoptotic action of macrophage inhibitory cytokine 1 and counteraction of activating transcription factor 3 in carrageenan-exposed enterocytes. Toxicol. Lett. 2014, 231, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fahoum, L.; Moscovici, A.; David, S.; Shaoul, R.; Rozen, G.; Meyron-Holtz, E.G.; Lesmes, U. Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function. Mol. Nutr. Food Res. 2017, 61, 1600545. [Google Scholar] [CrossRef] [PubMed]
- David-Birman, T.; Mackie, A.; Lesmes, U. Impact of dietary fibers on the properties and proteolytic digestibility of lactoferrin nano-particles. Food Hydrocoll. 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Chassaing, B.; Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1720–1728. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Wronecki, J. Can the gut microbiota be effectively modified? Gastroenterol. Kliniczna. Postępy Standardy 2018, 10, 123–124. [Google Scholar]
- Gałecka, M.; Basińska, A.M.; Bartnicka, A. The significance of gut microbiota in shaping human health—Implications for the practice of family medicine. Forum Med. Rodz. 2018, 12, 170–182. [Google Scholar]
- Nagao-Kitamoto, H.; Shreiner, A.B.; Gillilland, M.G.; Kitamoto, S.; Ishii, C.; Hirayama, A.; Kuffa, P.; El-Zaatari, M.; Grasberger, H.; Seekatz, A.M.; et al. Functional Characterization of Inflammatory Bowel Disease-Associated Gut Dysbiosis in Gnotobiotic Mice. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 468–481. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial Tight Junctions in Intestinal Inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Bloom, S.M.; Bijanki, V.N.; Nava, G.M.; Sun, L.; Malvin, N.P.; Donermeyer, D.L.; Dunne, W.M., Jr.; Allen, P.M.; Stappenbeck, T.S. Commensal Bacteroides Species Induce Colitis in Host-Genotype-Specific Fashion in a Mouse Model of Inflammatory Bowel Disease. Cell Host Microbe 2011, 9, 390–403. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Q.; Zhang, G.; Ma, C.; Dai, X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. J. Funct. Foods 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome-A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kanarek, E.; Sowińska, A.; Cukrowska, B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 2021, 13, 756. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1183–1194. [Google Scholar] [CrossRef]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. ROS in Gastrointestinal Inflammation: Rescue or Sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.13428 (accessed on 8 February 2024). [CrossRef] [PubMed]
- Li, J.; Li, Y.X.; Chen, M.H.; Li, J.; Du, J.; Shen, B.; Xia, X.M. Changes in the phosphorylation of claudins during the course of experimental colitis. Int. J. Clin. Exp. Pathol. 2015, 8, 12225–12233. [Google Scholar] [PubMed]
- Ahmad, T.; Ishaq, M.; Karpiniec, S.; Park, A.; Stringer, D.; Singh, N.; Ratanpaul, V.; Wolfswinkel, K.; Fitton, H.; Caruso, V.; et al. Oral Macrocystis Pyrifera Fucoidan Administration Exhibits Anti-Inflammatory and Antioxidant Properties and Improves DSS-Induced Colitis in C57BL/6J Mice. Available online: https://www.mdpi.com/1999-4923/14/11/2383 (accessed on 8 February 2024).
- Ikeda, Y.; Tsuji, A.; Matsuda, S. Gut Protective Effect from Newly Isolated Bacteria as Probiotics against Dextran Sulfate Sodium and Carrageenan-Induced Ulcerative Colitis. Microorganisms 2023, 11, 1858. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.J.; Câmara, N.O.S.; Sales-Campos, H. Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease—An Overview of Human Studies. Front. Pharmacol. 2024, 9, 430081. Available online: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2018.01571 (accessed on 8 February 2024). [CrossRef] [PubMed]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut-Liver-Brain Axes? Int J Mol Sci. 2023, 24, 3900. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Matsuda, S. Gut Protective Effect from D-Methionine or Butyric Acid against DSS and Carrageenan-Induced Ulcerative Colitis. Molecules 2023, 28, 4392. [Google Scholar] [CrossRef] [PubMed]
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory Lymphocytes and Intestinal Inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.D.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25, 4. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.-O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.S.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 3. [Google Scholar] [CrossRef]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. Biomed. Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. 3 Biotech 2022, 12, 154. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shang, X.; Chen, P.; Huang, X. How does carrageenan cause colitis? A review. Carbohydr. Polym. 2023, 302, 120374. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Wojciechowska, A.; Portmann, R.; Shpigelman, A.; Lesmes, U. The impact of food-grade carrageenans and consumer age on the in vitro proteolysis of whey proteins. Food Res. Int. 2020, 130, 108964. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komisarska, P.; Pinyosinwat, A.; Saleem, M.; Szczuko, M. Carrageenan as a Potential Factor of Inflammatory Bowel Diseases. Nutrients 2024, 16, 1367. https://doi.org/10.3390/nu16091367
Komisarska P, Pinyosinwat A, Saleem M, Szczuko M. Carrageenan as a Potential Factor of Inflammatory Bowel Diseases. Nutrients. 2024; 16(9):1367. https://doi.org/10.3390/nu16091367
Chicago/Turabian StyleKomisarska, Paulina, Anan Pinyosinwat, Mutaz Saleem, and Małgorzata Szczuko. 2024. "Carrageenan as a Potential Factor of Inflammatory Bowel Diseases" Nutrients 16, no. 9: 1367. https://doi.org/10.3390/nu16091367
APA StyleKomisarska, P., Pinyosinwat, A., Saleem, M., & Szczuko, M. (2024). Carrageenan as a Potential Factor of Inflammatory Bowel Diseases. Nutrients, 16(9), 1367. https://doi.org/10.3390/nu16091367






