Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes and Analyses
2.3. Definition of Other Factors
2.4. Postoperative Chemotherapy with S-1
2.5. Postoperative Follow-Up
2.6. Clinicopathological Variables
3. Results
3.1. Patient Background
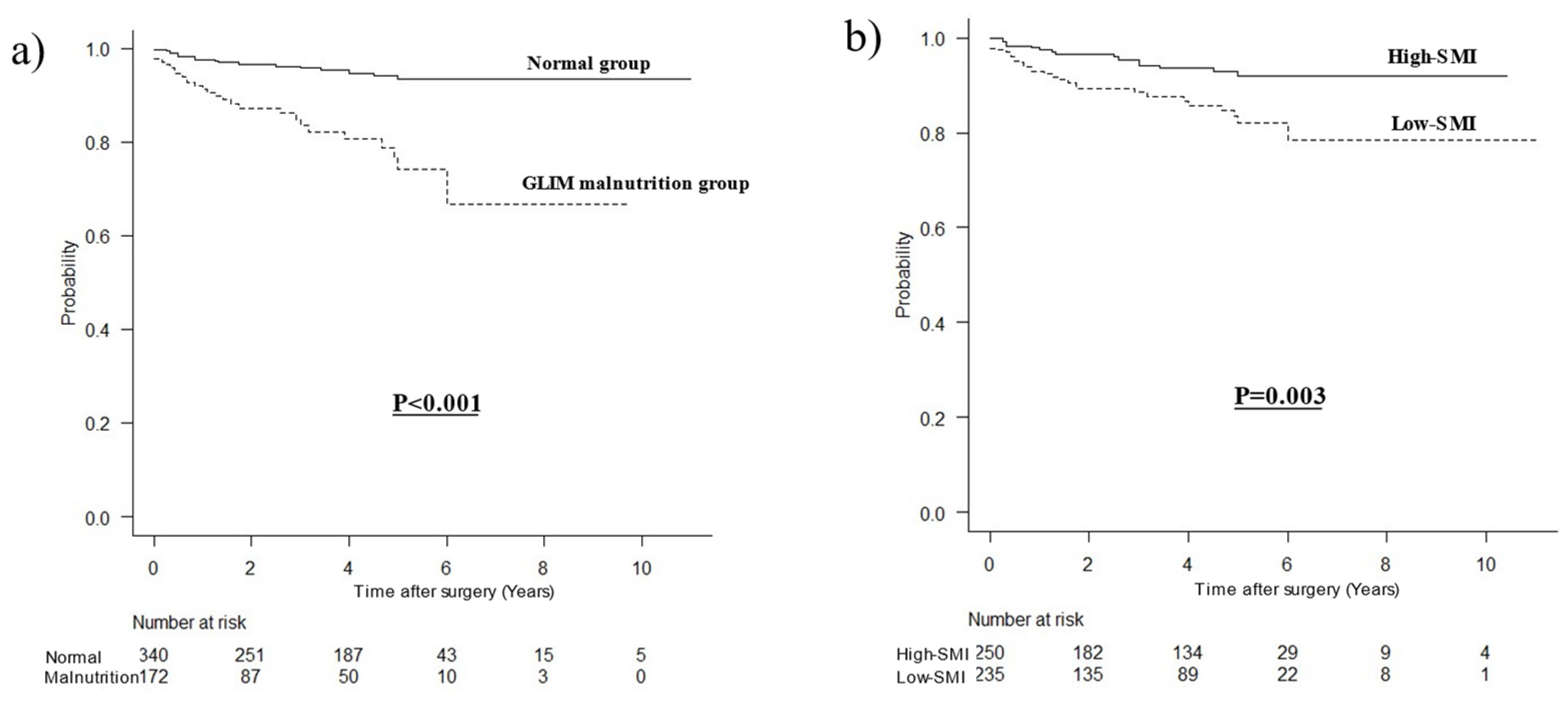
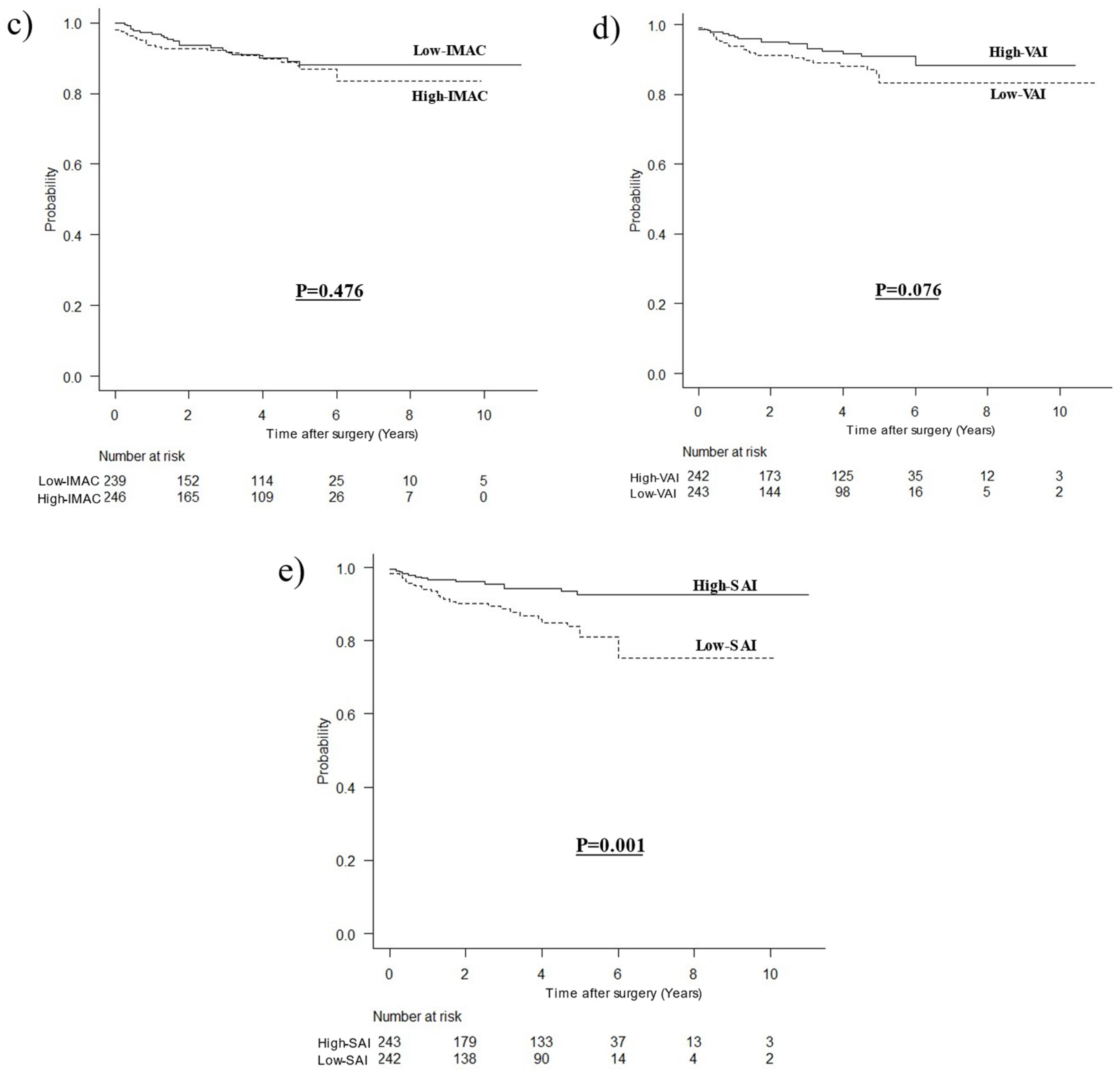
3.2. Comparison of OCS Curves
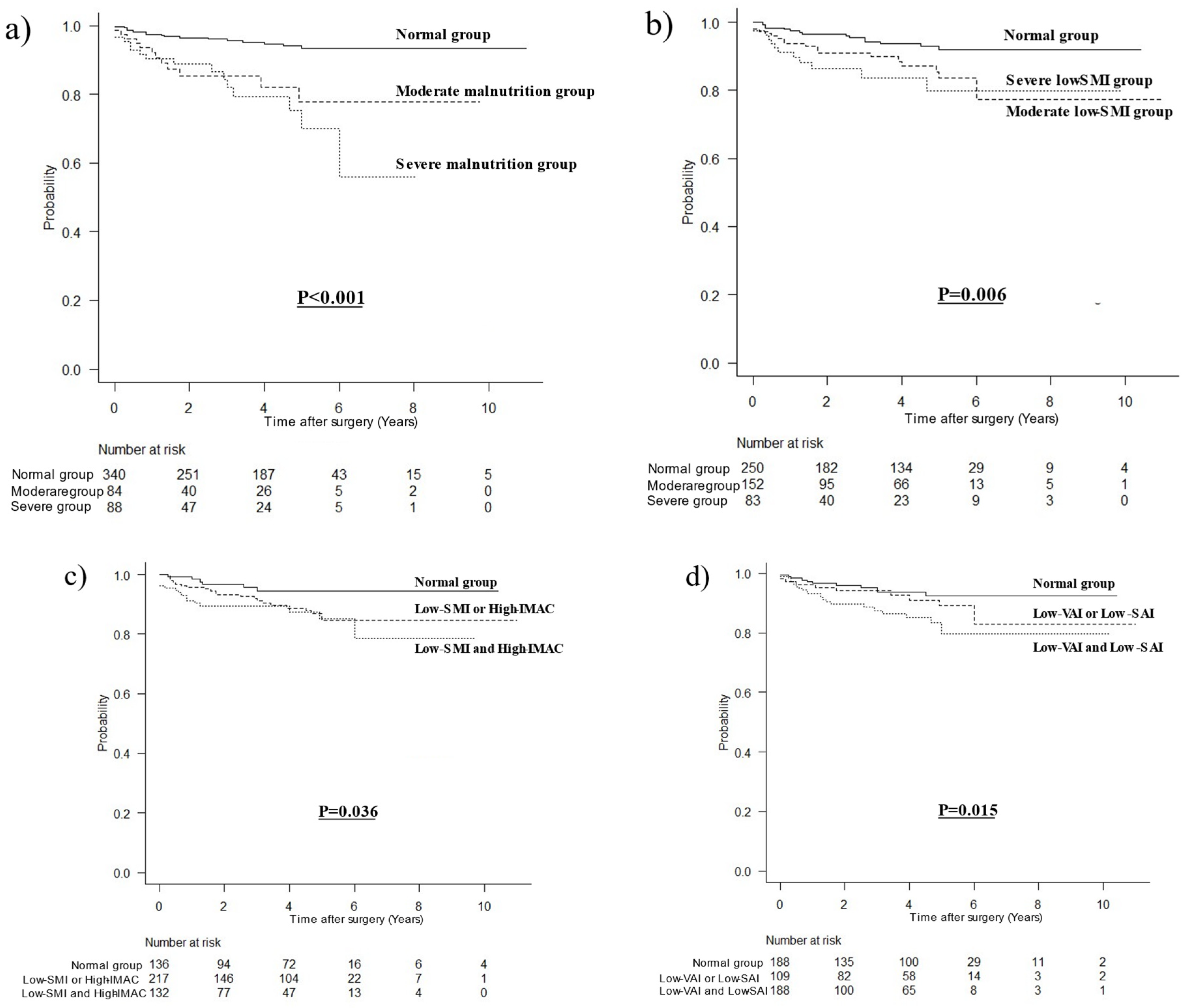
3.3. Stratified Survival Curves for OCS
3.4. Prognostic Factors for OCS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamarajah, S.K.; Bundred, J.; Tan, B.H.L. Body composition assessment and sarcopenia in patients with gastric cancer: A systematic review and meta-analysis. Gastric. Cancer 2019, 22, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2045–2054. [Google Scholar] [CrossRef]
- Matsui, R.; Watanabe, J.; Banno, M.; Inaki, N.; Fukunaga, T. Association of visceral adipose tissue with postoperative outcome in upper gastrointestinal cancer: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar]
- Yang, Z.; Zhou, X.; Ma, B.; Xing, Y.; Jiang, X.; Wang, Z. Predictive value of preoperative sarcopenia in patients with gastric cancer: A meta-analysis and systematic review. J. Gastrointest. Surg. 2018, 22, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Inaki, N.; Tsuji, T. Impact of visceral adipose tissue on compliance of adjuvant chemotherapy and relapse-free survival after gastrectomy for gastric cancer: A propensity score matching analysis. Clin. Nutr. 2021, 40, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Tang, L.; Chen, Y.; Li, Y.L.; Zhang, X.P.; Sun, Y.S. Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1237–1247. [Google Scholar] [CrossRef]
- Watanabe, J.; Osaki, T.; Ueyama, T.; Koyama, M.; Iki, M.; Endo, K.; Tatebe, S.; Hirooka, Y. The combination of preoperative skeletal muscle quantity and quality is an important indicator of survival in elderly patients undergoing curative gastrectomy for gastric cancer. World J. Surg. 2021, 45, 2868–2877. [Google Scholar] [CrossRef]
- Nakamura, T.; Hojo, Y.; Kumamoto, T.; Kurahashi, Y.; Ishida, Y.; Shinohara, H. History of the lymph node numbering system in the Japanese Classification of Gastric Carcinoma since 1962. Surg. Today 2022, 52, 1515–1523. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T. Impact of visceral adipose tissue on long-term outcomes after gastrectomy for advanced gastric cancer. Nutrition 2022, 97, 111619. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T. Impact of preoperative muscle quality on postoperative severe complications after radical gastrectomy for gastric cancer patients. Ann. Gastroenterol. Surg. 2021, 5, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.B.; Shi, M.M.; Huang, Z.X.; Zhang, W.T.; Zhang, H.H.; Shen, X.; Chen, X.-D. Impact of malnutrition diagnosed using Global Leadership Initiative on Malnutrition criteria on clinical outcomes of patients with gastric cancer. J. Parenter. Enteral. Nutr. 2021, 46, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric. Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Karayiannis, D.; Bouloubasi, Z.; Poulia, K.A.; Kompogiorgas, S.; Konstantinou, D.; Vougas, V. Global Leadership Initiative on Malnutrition criteria predict pulmonary complications and 90-day mortality after major abdominal surgery in cancer patients. Nutrients 2020, 12, 3726. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T. The impact of the preoperative hand grip strength on the long-term outcomes after gastrectomy for advanced gastric cancer. Surg. Today 2021, 51, 1179–1187. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; Tanaka, C.; et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 2019, 8, 5194–5201. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, Q.; Li, J.; Bai, B.; Li, Z.; Wu, X.; Yu, P.; Li, X.; Yin, J. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Adachi, Y.; Taniguchi, A.; Kimura, Y.; Iitaka, D.; Iwata, G.; Yamaoka, N. Prognostic impacts of categorized postoperative complications in surgery for gastric cancer. Asian J. Surg. 2023, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
| BWL (%) | BMI (kg/m2) | Skeletal Muscle Mass | |
|---|---|---|---|
| Moderate malnutrition | 5–10% within past 6 months prior to surgery or 10–20% beyond 6 months prior to surgery | <20.0 if <70 years old or <22.0 if ≥70 years old | Not available |
| Severe malnutrition | >10% within past 6 months prior to surgery or >20% beyond 6 months prior to surgery | <18.5 if <70 years old or <20.0 if ≥70 years old | Not available |
| Parameters | Cutoff Values | |||
|---|---|---|---|---|
| Male | Female | Prevalence (%) | ||
| Low SMI (cm2/m2) | Moderate | <40.8 | <34.9 | 31.3% |
| Severe | <34.5 | <28.9 | 17.1% | |
| High IMAC | >−0.42 | >−0.32 | 50.7% | |
| Low VAI (cm2/m2) | <35.42 | <26.81 | 50.1% | |
| Low SAI (cm2/m2) | <33.90 | <41.70 | 49.9% | |
| All Patients (n = 512) | |
|---|---|
| Sex | |
| Male | 336 (65.6%) |
| Female | 176 (34.4%) |
| Age (mean ± SD) | 67.93 ± 11.10 |
| BMI (mean ± SD) | 22.75 ± 3.52 |
| Surgical approach | |
| Laparoscopic | 266 (52.0%) |
| Open | 246 (48.0%) |
| Surgical procedure | |
| Distal gastrectomy | 279 (54.5%) |
| Proximal gastrectomy | 25 (4.9%) |
| Total gastrectomy | 208 (40.6%) |
| Lymph node dissection | |
| D1+ | 233 (45.5%) |
| D2 | 279 (54.5%) |
| Clinical stage | |
| II | 163 (31.8%) |
| III | 349 (68.2%) |
| Pathological stage | |
| I | 88 (17.2%) |
| II | 176 (34.4%) |
| III | 193 (37.7%) |
| IV | 55 (10.7%) |
| Postoperative chemotherapy | 326 (63.7%) |
| Comorbidity | |
| CKD | 93 (18.2%) |
| COPD | 110 (21.5%) |
| Diabetes | 92 (18.0%) |
| CHF | 28 (5.5%) |
| Preoperative albumin (g/dL) | |
| >3.5 | 409 (82.6%) |
| ≤3.5 | 86 (17.4%) |
| Preoperative CRP (mg/dL) | |
| <0.5 | 423 (82.6%) |
| ≥0.5 | 89 (17.4%) |
| Malnutrition defined by GLIM criteria | |
| Moderate | 84 (16.4%) |
| Severe | 88 (17.2%) |
| SMI (cm2/m2), median (IQR) | 39.08 (33.98–45.33) |
| Low SMI (all patients) | 235 (48.5%) |
| Low SMI (moderate) | 152 (31.3%) |
| Low SMI (severe) | 83 (17.1%) |
| IMAC, median (IQR) | −0.39 (−0.47 to −0.28) |
| High IMAC | 246 (50.7%) |
| VAI (cm2/m2), median (IQR) | 32.46 (16.69–51.02) |
| Low VAI | 243 (50.1%) |
| SAI (cm2/m2), median (IQR) | 36.32 (21.70–53.83) |
| Low SAI | 242 (49.9%) |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Sex | ||||||
| Female | 1 | |||||
| Male | 1.186 | 0.631–2.230 | 0.597 | |||
| Age (years) | ||||||
| <70 | 1 | 1 | ||||
| ≥70 | 3.669 | 1.918–7.020 | <0.001 | 1.709 | 0.820–3.562 | 0.153 |
| Surgical procedure Distal gastrectomy | 1 | |||||
| Total gastrectomy | 0.802 | 0.431–1.493 | 0.487 | |||
| Surgical approach | ||||||
| Laparoscopic | 1 | |||||
| Open | 1.278 | 0.712–2.296 | 0.411 | |||
| Lymph node dissection | ||||||
| D1+ | 1 | 1 | ||||
| D2 | 0.534 | 0.295–0.965 | 0.038 | 0.736 | 0.392–1.382 | 0.34 |
| p stage | ||||||
| <III | 1 | |||||
| >III | 0.854 | 0.465–1.567 | 0.61 | |||
| Postoperative chemotherapy Absent | 1 | 1 | ||||
| Present | 0.204 | 0.107–0.388 | <0.001 | 0.283 | 0.140–0.573 | <0.001 |
| CKD | ||||||
| Absent | 1 | |||||
| Present | 1.406 | 0.696–2.839 | 0.342 | |||
| Diabetes | ||||||
| Absent | 1 | |||||
| Present | 1.769 | 0.914–3.427 | 0.091 | |||
| COPD | ||||||
| Absent | 1 | |||||
| Present | 1.735 | 0.910–3.309 | 0.095 | |||
| CHF | ||||||
| Absent | 1 | |||||
| Present | 2.201 | 0.867–5.586 | 0.097 | |||
| GLIM malnutrition | ||||||
| Normal | 1 | 1 | ||||
| Moderate | 2.316 | 1.193–4.496 | 0.013 | 2.100 | 0.904–4.880 | 0.085 |
| Severe | 3.350 | 1.813–6.191 | <0.001 | 3.310 | 1.426–7.682 | 0.005 |
| SMI (cm2/m2) | ||||||
| High | 1 | 1 | ||||
| Low (moderate) | 1.559 | 0.858–2.831 | 0.145 | |||
| Low (severe) | 2.149 | 1.107–4.173 | 0.024 | 1.121 | 0.547–2.297 | 0.756 |
| IMAC | ||||||
| Low | 1 | |||||
| High | 1.238 | 0.688–2.229 | 0.477 | |||
| VAI (cm2/m2) | ||||||
| High | 1 | |||||
| Low | 1.706 | 0.938–3.102 | 0.08 | |||
| SAI (cm2/m2) | ||||||
| High | 1 | 1 | ||||
| Low | 2.698 | 1.431–5.086 | 0.002 | 1.876 | 0.897–3.925 | 0.095 |
| Postoperative complications | ||||||
| Absent | 1 | 1 | ||||
| Total | 3.230 | 1.794–5.815 | <0.001 | |||
| Severe | 4.797 | 2.551–9.022 | <0.001 | 3.353 | 1.707–6.588 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, R.; Inaki, N.; Tsuji, T. Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer. Nutrients 2023, 15, 3182. https://doi.org/10.3390/nu15143182
Matsui R, Inaki N, Tsuji T. Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer. Nutrients. 2023; 15(14):3182. https://doi.org/10.3390/nu15143182
Chicago/Turabian StyleMatsui, Ryota, Noriyuki Inaki, and Toshikatsu Tsuji. 2023. "Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer" Nutrients 15, no. 14: 3182. https://doi.org/10.3390/nu15143182
APA StyleMatsui, R., Inaki, N., & Tsuji, T. (2023). Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer. Nutrients, 15(14), 3182. https://doi.org/10.3390/nu15143182









