Trophic Nutrition in ICU Patients Undergoing High-Flow Oxygen Therapy and/or Noninvasive Mechanical Ventilation: The Nutri-Trophic Study
Abstract
1. Introduction
2. Materials and Methods
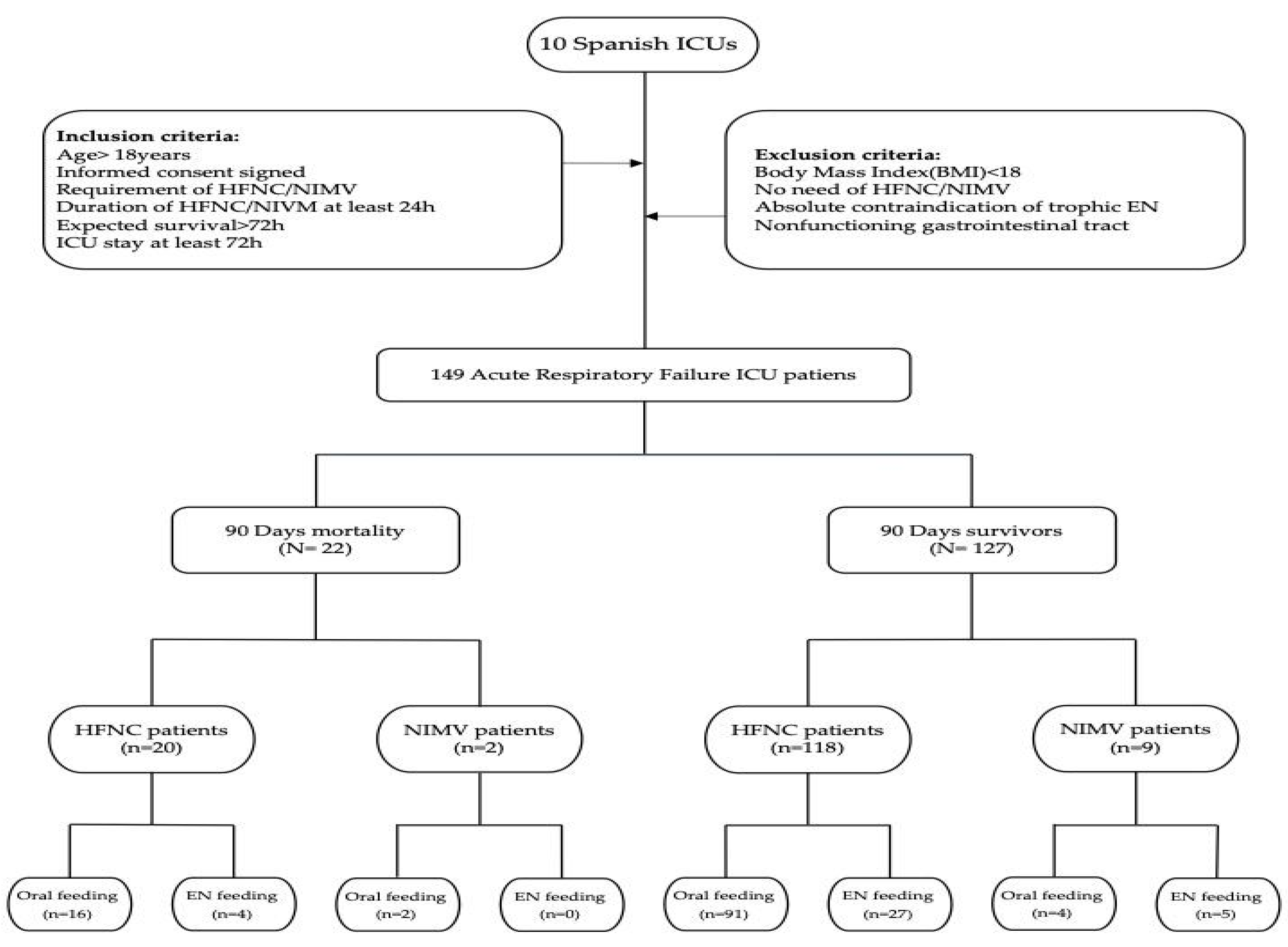
2.1. Study Design (Figure 1)
- Acute respiratory failure, including exacerbation of chronic obstructive pulmonary disease (COPD), in postoperative, immunocompromised patients;
- Prevention of acute respiratory failure in high-risk postextubation patients and initial management of these patients;
- Acute heart failure;
- Obstructive sleep apnea syndrome;
- Thoracic trauma.
- Age ≥ 18 years;
- Signed informed consent;
- Requirement of HFNC oxygen therapy and/or NIMV;
- Duration of HFNC/NIMV or oxygen therapy ≥ 24 h;
- Expected survival > 72 h;
- ICU stay ≥ 72 h.
- Body mass index (BMI) < 18;
- No requirement of HFNC oxygen therapy and/or NIMV;
- Absolute contraindication to trophic EN (active gastrointestinal bleeding, intestinal obstruction, etc.) or nonfunctioning gastrointestinal tract.
2.2. Trophic Nutrition
2.3. Study Variables
- (a)
- Patients general characteristics:
- -
- Age and sex;
- -
- Weight in kg and height in meters. Body mass index (BMI) in kg/m2;
- -
- Severity scales—Apache-II [18] (first 24 h);
- -
- Admission reasons/patients type—sepsis, cardiac surgery, other surgeries, nonsurgical cardiology, trauma, burns, pneumonia (community-acquired, healthcare-associated, or hospital-acquired), acute respiratory distress syndrome (ARDS), and others;
- -
- Number of days NIMV and/or HFNC therapy received. Number of days ICU and hospital stay.
- -
- 90-day mortality.
- (b)
- Other variables:
- -
- Scores for the Sequential Organ Failure Assessment (SOFA) scale [20]—days 0 and 3;
- -
- Nosocomial infections (tracheobronchitis and ventilator-associated pneumonia (VAP), bacteremia, and urinary tract infections (UTIs)) defined according to the ENVIN-HELICS) criteria [35].
- -
- For other infections, we recorded (if applicable):
- -
- Presence of continuous renal replacement therapy (CRRT);
- -
- Patients receiving acute mucosal gastro-duodenal lesions (AMGDLs) prophylaxis;
- -
- Levels of albumin, prealbumin, retinol, and transferrin upon admission and weekly;
- -
- Maximum bilirubin, AST, ALT, ALP, and GGT levels;
- -
- Patients receiving antimicrobial treatment.
- (c)
- Variables recorded related to trophic EN:
- -
- Number of days of EN with HFNC/VMNI;
- -
- Energy target (kcal);
- -
- Enteral volume administered per day (mL);
- -
- Nutritional calories received per day (kcal/kg/day);
- -
- Enteral caloric intake per day (kcal/kg/day);
- -
- Total caloric intake, all sources, per day (kcal/kg/day);
- -
- Ratio of received calories (%) to target calories (kcal/kg/day);
- -
- Parenteral dextrose intake per day (kcal/kg/day);
- -
- Median (interquartile range (IQR)), minimum and maximum blood glucose per day;
- -
- Patients receiving prokinetics;
- -
- Energy balance;
- -
- Prescribed protein (g/day);
- -
- Daily protein intake (g/kg/day);
- -
- Ratio of received protein (%) to target protein (g/day).
- (d)
- Safety recorded variables:
- -
- Gastric residual (≥ 500 mL/day);
- -
- Abdominal distension;
- -
- Diarrhea;
- -
- Vomiting/regurgitation;
- -
- Broncho-aspiration;
- -
- Nasogastric tube complications;
- -
- Discontinuance/reason trophic EN.
2.4. Outcomes of the Study
- -
- Death (90-day mortality);
- -
- Discontinuation of nutritional therapy;
- -
- Infectious complications.
2.5. Statistical Analysis
2.5.1. Design
- -
- Ratio of received calories (%) to target calories (kcal/kg/day) = (delivered enteral kcal + delivered parenteral kcal / target kcal);
- -
- Ratio of received protein (%) to target protein (g/day) = (gr. delivered enteral proteins + gr. delivered parenteral proteins/grs. target proteins);
- -
- A patient was considered to have infectious complications if and only if he/she presented at least one of the following events: tracheobronchitis, VAP, bacteremia, UTI, or any other infection.
2.5.2. Sample Size Calculation
2.5.3. Univariate Analysis
2.5.4. Multivariate Logistic Regression
3. Results
3.1. Demographic and General Data According to Survival (Table 1)
| Overall N = 149 | Survivors N = 127 | Nonsurvivors N = 22 | p-Value | |
|---|---|---|---|---|
| Age (years) | 62.6 ± 13.9 | 61.6 ± 14.0 | 68.2 ± 12.7 | 0.041 |
| Sex male | 102 (68.5) | 88 (69.3) | 14 (63.6) | 0.598 |
| Body mass index (kg/m2) | 28.1 ± 5.6 | 28.3 ± 5.5 | 27.1 ± 5.8 | 0.359 |
| Apache-II score | 15 (10; 20) | 14 (19; 19) | 18 (15; 22) | 0.01 |
| SOFA upon admission | 4 (2; 6) | 3 (2; 6) | 6 (3; 8) | 0.015 |
| SOFA on day three | 3 (2; 6) | 3 (2; 5) | 6 (3; 7) | 0.008 |
| Complications | ||||
| Tracheobronchitis | 12 (8.1) | 7 (5.5) | 5 (22.7) | 0.018 |
| VAP | 10 (6.7) | 7 (5.5) | 3 (13.6) | 0.168 |
| Bacteremia | 12 (8.1) | 5 (3.9) | 7 (31.8) | <0.001 |
| UTIs | 9 (6.0) | 4 (3.1) | 5 (22.7) | 0.004 |
| Other infections | 9 (6.0) | 6 (4.7) | 3 (13.6) | 0.13 |
| CRRT | 12 (8.1) | 5 (3.9) | 7 (31.8) | <0.001 |
| Prophylaxis AGDML | 129 (86.6) | 109 (85.8) | 20 (90.9) | 0.739 |
| Prokinetics | 22 (14.8) | 16 (12.6) | 6 (27.31) | 0.099 |
| Antimicrobial treatments | 81 (54.4) | 61 (48.0) | 20 (90.9) | <0.001 |
| 90-Day mortality | 22 (14.8) | 0 | 22 (100.0) | <0.001 |
| Oral feedings | 110 (76.9) | 92 (72.4) | 18 (81.8) | 0.467 |
| NGFs | 36 (25.2) | 29 (22.8) | 7 (31.8) | 0.72 |
| Diarrhea | 18 (12.1) | 16 (12.6) | 2 (9.1) | 1 |
| Gastric residue > 500 mL (n) | 2 (1.3) | 2 (1.6) | 0 | 1 |
| Vomiting/regurgitation | 1 (0.7) | 1 (0.8) | 0 | 1 |
| Broncho-aspiration | 0 | 0 | 0 | 1 |
| NG tube obstruction | 1 (0.7) | 1 (0.8) | 0 | 1 |
| Abdominal distention | 4 (2.7) | 2 (1.6) | 2 (9.1) | 0.104 |
| NG tube displacement | 4 (2.7) | 4 (3.1) | 0 | 1 |
| EN discontinuation | 10 (6.7) | 7 (5.5) | 3 (13.6) | 0.168 |
| Oxygen therapy type: | 0.666 | |||
| HFNC | 138 (92.6) | 118 (92.9) | 20 (90.9) | |
| NIMV | 11 (7.4) | 9 (7.1) | 2 (9.1) | |
| ICU days | 9 (6; 16) | 9 (6; 14) | 16 (7; 30) | 0.019 |
| Hospital days | 15 (8; 23) | 16 (10; 23) | 14 (7; 26) | 0.802 |
| HFNC days | 3 (2; 4) | 3 (2; 4) | 2 (2; 3) | 0.064 |
| NIMV days | 4 (3; 6) | 4 (3; 6) | 4 (3; 4) | 0.158 |
| Albumin (g/dL) | 3 (3; 3) | 3 (3; 3) | 3 (3; 3) | 0.116 |
| Prealbumin (mg/dL) | 15 (10; 20) | 15 (10; 21) | 15 (8; 18) | 0.558 |
| Retinol (UI) | 4 (2; 5) | 4 (2; 5) | 4 (3; 6) | 0.296 |
| Transferrin (md/dL) | 154 (122; 176) | 158 (127; 177) | 117 (97; 130) | 0.009 |
| Bilirubin (mg/dL) | 0.53 (0.35; 0.78) | 0.49 (0.35; 0.68) | 0.80 (0.52; 1.19) | 0.007 |
| AST (U/L) | 38 (21; 54) | 38 (22; 52) | 36 (18; 76) | 0.896 |
| ALT (U/L) | 37 (20; 58) | 38 (20; 60) | 26 (15; 55) | 0.225 |
| GGT (U/L) | 79 (60; 108) | 73 (38; 138) | 62 (52; 130) | 0.977 |
| ALP (U/L) | 69 (39; 138) | 79 (60; 102) | 82 (61; 121) | 0.707 |
| INR | 1.10 (1.01; 1.19) | 1.10 (1.00; 1.20) | 1.12 (1.06; 1.17) | 0.603 |
| Prothrombin (s) | 13 (12; 15) | 13 (12; 15) | 13 (12; 14) | 0.447 |
| Urea (mg/dL) | 52 (39; 74) | 50 (38; 67) | 74 (51; 89) | 0.009 |
| Creatinine (mg/dL) | 0.79 (0.61; 1.17) | 0.77 (0.61; 1.04) | 1.23 (0.70; 1.47) | 0.042 |
| Daily data * | ||||
| Energy target (Kcal) | 1811 (1500; 2180) | 1825 (1500; 2150) | 1775 (1470; 2240) | 0.696 |
| Volume of enteral administration (mL) | 450 (267; 500) | 500 (288; 500) | 335 (228; 438) | 0.01 |
| Enteral intake (Kcal) | 520 (300; 600) | 549 (300; 600) | 396 (277; 480) | 0.02 |
| Ratio of energy intake/target | 0.28 (0.18; 0.36) | 0.29 (0.19; 0.38) | 0.24 (0.17; 0.32) | 0.208 |
| Parenteral dextrose intake (Kcal) | 70 (0; 190) | 46 (0; 180) | 150 (101; 200) | 0.007 |
| Prescribed protein (g/day) | 50 (50; 90) | 50 (50; 92) | 50 (49; 50) | 0.051 |
| Protein intake (g/day) | 50 (25; 50) | 50 (25; 50) | 33 (23; 48) | 0.028 |
| Ratio of protein intake/target | 0.81 (0.49; 1.00) | 0.90 (0.49; 1.00) | 0.65 (0.51; 0.85) | 0.265 |
| Gastric residue (mL) (n) | 69 (50; 128) (n = 26) | 62 (50; 108) (n = 24) | 180 (150; 210) (n = 2) | 0.101 |
| Total kcal intake | 600 (449; 750) | 600 (458; 755) | 571 (397; 705) | 0.281 |
| Caloric intake (Kcal/kg) | 7 (5; 10) | 7 (5; 10) | 7 (6; 9) | 0.83 |
| Ratio of total energy intake/target | 0.32 (0.23; 0.44) | 0.31 (0.23; 0.45) | 0.34 (0.24; 0.42) | 0.944 |
| PN kcal intake (n) | 1322 (439; 1568) (n = 6) | 1518 (1125; 1585) (n = 5) | 160 (160; 160) (n = 1) | 0.143 |
| PN protein intake (g) (n) | 84 (76; 96) (n = 6) | 83 (74; 99) (n = 5) | 84 (84; 84) (n = 1) | 0.77 |
| Propofol kcal intake (n) | 82 (18; 143) (n = 4) | 82 (18; 143) (n = 4) | (n = 0) | |
| Glycemia (mg/dL) | ||||
| Median | 130 (113; 153) | 130 (112; 152) | 134 (123; 161) | 0.378 |
| Minimum | 110 (93; 128) | 110 (94; 127) | 113 (93; 131) | 0.522 |
| Maximum | 153 (130; 189) | 150 (126; 184) | 176 (140; 228) | 0.077 |
3.2. Mortality (Table 1)
3.3. Nutrition-Related Data, Outcomes, and Discontinuance of Nutritional Therapy (Table S1)
3.4. Infectious Complications (Table 4)
| Infectious Complications | |||
|---|---|---|---|
| No N = 112 | Yes N = 37 | p-Value | |
| Age (years) | 62.5 ± 13.8 | 62.8 ± 14.5 | 0.924 |
| Sex male | 74 (66.1) | 28 (75.7) | 0.276 |
| Body mass index (Kg/m2) | 28.1 ± 5.8 | 28.3 ± 4.9 | 0.885 |
| Apache-II score | 14 (10; 19) | 17 (12; 21) | 0.046 |
| SOFA upon admission | 3 (2; 6) | 4 (3; 7) | 0.128 |
| SOFA on day 3 | 3 (2; 5) | 4 (3; 7) | 0.013 |
| Complications | |||
| Tracheobronchitis | 0 | 12 (32.4) | <0.001 |
| VAP | 0 | 10 (27.0) | <0.001 |
| Bacteremia | 0 | 12 (32.4) | <0.001 |
| UTIs | 0 | 9 (24.3) | <0.001 |
| Other infections | 0 | 9 (24.3) | <0.001 |
| CRRT | 4 (3.6) | 8 (21.6) | 0.002 |
| Prophylaxis_AGDML | 94 (83.9) | 35 (94.6) | 0.162 |
| Prokinetics | 12 (10.7) | 10 (27.0) | 0.015 |
| Antimicrobial treatments | 48 (42.9) | 33 (89.2) | <0.001 |
| 90-day mortality | 8 (7.1) | 14 (37.8) | <0.001 |
| Oral feedings | 83 (76.8) | 27 (77.1) | 0.972 |
| NGFs | 27 (25.0) | 9 (25.7) | 0.933 |
| Diarrhea | 15 (13.4) | 3 (8.1) | 0.563 |
| Gastric residue > 500 mL (n) | 2 (1.8) | 0 | 1 |
| Vomiting/regurgitation | 0 | 1 (2.7) | 0.248 |
| Broncho-aspiration | 0 | 0 | 1 |
| NG tube obstruction | 0 | 1 (2.7) | 0.248 |
| Abdominal distention | 4 (3.6) | 0 | 0.572 |
| NG tube displacement | 2 (1.8) | 2 (5.4) | 0.257 |
| EN discontinuation | 9 (8.0) | 1 (2.7) | 0.452 |
| Oxygen therapy type: | 0.467 | ||
| HFNC | 105 (93.8) | 33 (89.2) | |
| NIMV | 7 (6.2) | 4 (10.8) | |
| ICU days | 8 (6; 14) | 12 (9; 24) | <0.001 |
| Hospital days | 15 (8; 23) | 19 (10; 24) | 0.387 |
| HFNC days | 3 (2; 4) | 3 (2; 3) | 0.712 |
| NIMV days | 4 (2; 6) | 4 (3; 6) | 0.936 |
| Albumin (g/dL) | 3 (3; 3) | 3 (3; 3) | 0.072 |
| Prealbumin (mg/dL) | 16 (11; 21) | 14 (9; 19) | 0.351 |
| Retinol (UI) | 4 (2; 5) | 3 (2; 5) | 0.478 |
| Transferrin (md/dL) | 158 (122; 178) | 145 (122; 168) | 0.218 |
| Bilirubin (mg/dL) | 0.53 (0.35; 0.70) | 0.54 (0.36; 1.08) | 0.404 |
| AST (U/L) | 36 (21; 56) | 38 (24; 48) | 0.961 |
| ALT (U/L) | 38 (19; 62) | 32 (20; 56) | 0.612 |
| GGT (U/L) | 77 (57; 100) | 89 (66; 136) | 0.041 |
| ALP(U/L) | 69 (40; 142) | 66 (36; 120) | 0.666 |
| INR | 1.10 (1.00; 1.17) | 1.15 (1.06; 1.23) | 0.026 |
| Prothrombin (s) | 13 (12; 14) | 14 (13; 25) | 0.004 |
| Urea (mg/dL) | 52 (38; 71) | 55 (40; 78) | 0.61 |
| Creatinine (mg/dL) | 0.75 (0.60; 1.03) | 1.08 (0.76; 1.49) | 0.009 |
| Daily data * | |||
| Energy target (Kcal) | 1800 (1435; 2100) | 1875 (1580; 2492) | 0.134 |
| Volume of enteral administration (mL) | 457 (250; 500) | 408 (309; 500) | 0.809 |
| Enteral intake (Kcal) | 531 (300; 600) | 480 (371; 600) | 0.987 |
| Ratio of energy intake/target | 0.29 (0.17; 0.38) | 0.25 (0.19; 0.33) | 0.332 |
| Parenteral dextrose intake (Kcal) | 45 (0; 179) | 113 (42; 200) | 0.013 |
| Prescribed protein (g/day) | 50 (50; 94) | 50 (50; 50) | 0.012 |
| Protein intake (g/day) | 50 (25; 50) | 40 (24; 50) | 0.177 |
| Ratio of protein intake/target | 0.83 (0.47; 1.00) | 0.80 (0.61; 1.00) | 0.397 |
| Gastric residue (mL) (n) | 62 (50; 100) (n = 21) | 120 (75; 240) (n = 5) | 0.103 |
| Total kcal intake (Kcal) | 600 (393; 744) | 641 (515; 751) | 0.247 |
| Caloric intake (Kcal/kg) | 7 (5; 10) | 7 (6; 10) | 0.597 |
| Ratio of total energy intake/target | 0.32 (0.22; 0.46) | 0.32 (0.25; 0.40) | 0.878 |
| PN kcal intake (n) | 1518 (1125; 1585) (n = 5) | 210 (210; 210) (n = 1) | 0.38 |
| PN protein intake (g) (n) | 84 (83; 99) (n = 5) | 25 (25; 25) (n = 1) | 0.143 |
| Propofol kcal intake (n) | 82 (52; 112) (n = 2) | 75 (38; 112) (n = 2) | 1 |
| Glycemia (md/dL) | |||
| Median | 130 (111; 152) | 130 (119; 159) | 0.501 |
| Minimum | 110 (94; 127) | 116 (92; 130) | 0.46 |
| Maximum | 154 (130; 182) | 152 (132; 206) | 0.511 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban, A.; Anzueto, A.; Frutos, F.; Alía, I.; Brochard, L.; Stewart, T.E.; Benito, S.; Epstein, S.K.; Apezteguía, C.; Nightingale, P.; et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 2002, 287, 345–355. [Google Scholar] [CrossRef]
- Esteban, A.; Frutos-Vivar, F.; Muriel, A.; Ferguson, N.D.; Peñuelas, O.; Abraira, V.; Raymondos, K.; Rios, F.; Nin, N.; Apezteguía, C.; et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am. J. Respir. Crit. Care Med. 2013, 188, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Gay, P.C. Complications of noninvasive ventilation in acute care. Respir. Care 2009, 54, 246–257; discussion 257–258. [Google Scholar] [PubMed]
- Liesching, T.; Kwok, H.; Hill, N.S. Acute applications of noninvasive positive pressure ventilation. Chest 2003, 124, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Contou, D.; Fragnoli, C.; Cordoba-Izquierdo, A.; Boissier, F.; Brun-Buisson, C. Non-invasive ventilation for acute hypoxemic respiratory failure: Intubation rate and risk factors. Crit. Care 2013, 17, R269. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, S.M.; Idone, F.A.; Vaschetto, R.; Festa, R.; Cataldo, A.; Antonicelli, F.; Montini, L.; De Gaetano, A.; Navalesi, P.; Antonelli, M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am. J. Respir. Crit. Care Med. 2014, 190, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, leNGFh of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Cohen, J.; Singer, P. Computerized energy balance and complications in critically ill patients: An observational study. Clin. Nutr. 2006, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.P.; Cayeux, M.C.; Delarue, J.; Berger, M.M. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, K.A. Importance of enteral feeding in maintaining gut integrity. Tech. Gastrointest. Endosc. 2001, 3, 2–8. [Google Scholar] [CrossRef]
- Deitch, E.A. Role of the gut lymphatic system in multiple organ failure. Curr. Opin. Crit. Care 2001, 7, 92–98. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2022, 46, 12–41, Erratum in JPEN J. Parenter. Enter. Nutr. 2022, 46, 1458–1459. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respir. Care 2016, 61, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Montejo, J.C.; Miñambres, E.; Bordejé, L.; Mesejo, A.; Acosta, J.; Heras, A.; Ferré, M.; Fernandez-Ortega, F.; Vaquerizo, C.I. Gastric residual volume during enteral nutrition in ICU patients: The REGANE study. Intensive Care Med. 2010, 36, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Elke, G.; Weimann, A. Nutrition in the Intensive Care Unit—A Narrative Review. Nutrients 2021, 13, 2851. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; Offner, P.J.; Moore, E.E.; Biffl, W.L. NISS predicts postinjury multiple organ failure better than the ISS. J. Trauma Inj. Infect. Crit. Care 2000, 48, 624–627; discussion 627–628. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Reintam Blaser, A.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N. Engl. J. Med. 2015, 372, 2398–2408, Erratum in N. Engl. J. Med. 2015, 373, 1281. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y.; González, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C.; Ichai, C.; Orban, J.-C.; Groeneveld, A.B.J. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Lowen, C.C.; Rg, M. The 2016 ESPEN Arvid Wretlind lecture: The gut in stress. Clin. Nutr. 2018, 37, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Moukarzel, A.A.; Bhuta, S.; Belle, M.; Ament, M.E.; Eckhert, C.D.; Hollander, D.; Gornbeln, J.; Kopple, J.D.; Vijayaroghavan, S.R. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J. Parenter. Enter. Nutr. 1995, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Besanko, L.K.; Burgstad, C.; Bellon, M.; Holloway, R.H.; Chapman, M.; Horowitz, M.; Fraser, R.J.L. Delayed enteral feeding impairs intestinal carbohydrate absorption in critically ill patients. Crit. Care Med. 2012, 40, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, H.; Hong, Z.; Wang, C.; Zheng, T.; Ren, Y.; Chen, K.; Liu, S.; Wang, G.; Gu, G.; et al. Early enteral nutrition preserves intestinal barrier function through reducing the formation of neutrophil extracellular traps (NETs) in critically ill surgical patients. Oxid. Med. Cell. Longev. 2020, 2020, 8815655. [Google Scholar] [CrossRef]
- Tian, F.; Heighes, P.T.; Allingstrup, M.J.; Doig, G.S. Early enteral nutrition provided within 24 hours of ICU admission: A meta-analysis of randomized controlled trials. Crit. Care Med. 2018, 2018, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Allingstrup, M.J.; Kondrup, J.; Wiis, J.; Claudius, C.; Pedersen, U.G.; Hein-Rasmussen, R.; Bjerregaard, M.R.; Steensen, M.; Jensen, T.H.; Lange, T.; et al. Early oal-directed nutrition versus standard of care in adult intensive care patients: The single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017, 43, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.L.; Chapple, L.S.; Chapman, M.J.; Moran, J.L.; Peake, S.L. Protein delivery and clinical outcomes in the critically ill: A systematic review and meta-analysis. Crit. Care Resusc. 2017, 19, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Estudio Nacional de Vigilancia de Infección Nosocomial en UCI (ENVIN-HELICS). Manual de Definiciones y Términos. Available online: http://hws.vhebron.net/envin-helics/Help/Manual_2017.pdf (accessed on 16 April 2015).
- R Package, version 4.2.1; R Development Core Team: Vienna, Austria, 2022.
- Casaer, M.P.; Wilmer, A.; Hermans, G.; Wouters, P.J.; Mesotten, D. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: A post hoc analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Verbruggen, S.; Casaer, M.P.; Gunst, J.; Wouters, P.J.; Hanot, J.; Guerra, G.G.; Vlasselaers, D.; Joosten, K.; Van den Berghe, G. Efect of early supplemental parenteral nutrition in the paediatric ICU: A preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir. Med. 2017, 5, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fetterplace, K.; Gill, B.M.T.; Chapple, L.S.; Presneill, J.J.; MacIsaac, C.; Deane, A.M. Systematic review with meta-analysis of patient-centered outcomes, comparing international guideline-recommended enteral protein delivery with usual care. JPEN J. Parenter. Enter. Nutr. 2020, 44, 610–620. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fu, P.K.; Chao, W.C.; Wang, W.N.; Chen, C.H.; Huang, Y.C. Full Versus Trophic Feeds in Critically Ill Adults with High and Low Nutritional Risk Scores: A Randomized Controlled Trial. Nutrients 2020, 12, 3518. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012, 307, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Montejo, J.C.; Grau, T.; Acosta, J.; Ruiz-Santana, S.; Planas, M.; García-De-Lorenzo, A.; Mesejo, A.; Cervera, M.; Sánchez-Alvarez, C.; Núñez-Ruiz, R.; et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit. Care Med. 2002, 30, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Rattanachaiwong, S. To eat or to breathe? The answer is both! Nutritional management during noninvasive ventilation. Crit. Care 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Antonini, M.V.; Sica, A.; Dell’Amore, C.; Martino, C.; Gamberini, E.; Bissoni, L.; Circelli, A.; Bolondi, G.; Santonastaso, D.P.; et al. Infection-related ventilator-associated complications in critically ill patients with trauma: A retrospective analysis. Antibiotics 2023, 12, 176. [Google Scholar] [CrossRef] [PubMed]
| Variables * | Coefficient (SE) | p-Value ** | BIC *** | OddsRatio (95% CI) |
|---|---|---|---|---|
| (Intercept) | −6.447 (1.649) | - | - | - |
| Age, per year | 0.056 (0.023) | 0.007 | 119.2 | 1.057 (1.011; 1.106) |
| Bacteremia | 2.183 (0.763) | 0.004 | 120 | 8.869 (1.990; 39.531) |
| CRRT | 2.100 (0.752) | 0.006 | 119.5 | 8.168 (1.871; 35.667) |
| Variables * | Coefficient (SE) | p-Value ** | BIC *** | Odds Ratio (95% CI) |
|---|---|---|---|---|
| (Intercept) | −7.956 (1.785) | - | - | - |
| SOFA upon admission, per unit | 0.379 (0.168) | 0.016 | 49.7 | 1.461 (1.051; 2.032) |
| Urea, per mg/dL | 0.029 (0.008) | <0.001 | 61.8 | 1.029 (1.013; 1.045) |
| Variables * | Coefficient (SE) | p-Value ** | BIC *** | Odds Ratio (95% CI) |
|---|---|---|---|---|
| (Intercept) | −1.872 (0.321) | - | - | - |
| CRRT | 1.801 (0.667) | 0.005 | 157.9 | 6.054 (1.639; 22.37) |
| ICU days (per day) | 0.044 (0.016) | 0.004 | 158.6 | 1.045 (1.013; 1.078) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reta-Pérez, O.; Colmenero-Ruiz, M.; Hernández-Socorro, C.R.; Saavedra, P.; Maichle, S.F.; Portugal, E.; Cerezo-Arias, M.; Sánchez Alés, L.; Martínez-Carmona, J.F.; Mateu-Campos, L.; et al. Trophic Nutrition in ICU Patients Undergoing High-Flow Oxygen Therapy and/or Noninvasive Mechanical Ventilation: The Nutri-Trophic Study. Nutrients 2024, 16, 1366. https://doi.org/10.3390/nu16091366
Reta-Pérez O, Colmenero-Ruiz M, Hernández-Socorro CR, Saavedra P, Maichle SF, Portugal E, Cerezo-Arias M, Sánchez Alés L, Martínez-Carmona JF, Mateu-Campos L, et al. Trophic Nutrition in ICU Patients Undergoing High-Flow Oxygen Therapy and/or Noninvasive Mechanical Ventilation: The Nutri-Trophic Study. Nutrients. 2024; 16(9):1366. https://doi.org/10.3390/nu16091366
Chicago/Turabian StyleReta-Pérez, Olivia, Manuel Colmenero-Ruiz, Carmen Rosa Hernández-Socorro, Pedro Saavedra, Silmary F. Maichle, Esther Portugal, Mariola Cerezo-Arias, Laura Sánchez Alés, Juan F. Martínez-Carmona, Lidon Mateu-Campos, and et al. 2024. "Trophic Nutrition in ICU Patients Undergoing High-Flow Oxygen Therapy and/or Noninvasive Mechanical Ventilation: The Nutri-Trophic Study" Nutrients 16, no. 9: 1366. https://doi.org/10.3390/nu16091366
APA StyleReta-Pérez, O., Colmenero-Ruiz, M., Hernández-Socorro, C. R., Saavedra, P., Maichle, S. F., Portugal, E., Cerezo-Arias, M., Sánchez Alés, L., Martínez-Carmona, J. F., Mateu-Campos, L., Lorencio-Cárdenas, C., García-Miguélez, A., Sosa-Durr, M., San Martín-Bragado, M., & Ruiz-Santana, S. (2024). Trophic Nutrition in ICU Patients Undergoing High-Flow Oxygen Therapy and/or Noninvasive Mechanical Ventilation: The Nutri-Trophic Study. Nutrients, 16(9), 1366. https://doi.org/10.3390/nu16091366






